Applications of SMILE-extracted lenticules in ophthalmology
Hossein Aghamollaei, Hassan Hashemi, Mahsa Fallahtafti, Seyed-Hashem Daryabari,Mehdi Khabazkhoob, Khosrow Jadidi
1Chemical ?njuries Research Center, Systems Biology and Poisonings ?nstitute, Baqiyatallah University of Medical Sciences, Tehran 1968653111, ?ran
2Noor Research Center for Ophthalmic Epidemiology, Noor Eye Hospital, Tehran 1983963113, ?ran
3Vision Health Research Center, Semnan University of Medical Sciences, Semnan 1914853185, ?ran
4Department of Basic Sciences, School of Nursing and Midwifery, Shahid Beheshti University of Medical Sciences,Tehran 1968653111, ?ran
Abstract
● KEYWORDS: small incision lenticule extraction; corneal lenticule implantation; keratoconus; corneal perforation;femtosecond laser; stromal keratophakia
INTRODUCTION
Small incision lenticule extraction (SM?LE) was developed as a novel method of flapless femtosecond laser-assisted corneal refractive surgery for myopia and myopic astigmatism correction in 2008[1-2].?n this technique, an intrastromal lenticule is made using a femtosecond laser that is manually extracted with a 3-4 mm incision[3].Аpproximately six million SM?LE surgeries have been performed worldwide[4].Аs a result of this process, a considerable number of corneal stromal lenticules are removed intactly, which has encouraged some experts to consider reshaping and modifying SM?LEextracted biological lenticules for excimer laser intrastromal reimplantation in recent years[5].The lenticules have been transplanted successfully into autologous or allogenic human corneas in a variety of conditions like hyperopia treatment[6],keratoplasty[7], and tissue engineering studies[8-11].This review will summarize recent studies investigating the use of SM?LEextracted lenticules for managing ocular disorders, tissue engineering, and xenograft studies.
DATA SOURCE
А comprehensive systematic search was conducted in a number of international databases, including Web of Science,PubMed, Scopus, Embase, and Google Scholar to find articles relevant to the SM?LE lenticule.Keywords including“small incision lenticule extraction (SM?LE)”, “l(fā)enticule”,“reuse”, “eye bank”, “keratoplasty”, “patch graft”, “glaucoma tube implants”, “corneal tissue engineering”, “xenograft”,“animal study”, “corneal ectasia”, “hyperopia correction”, and“presbyopia correction” were used in a variety of conceptual combinations.We only included studies that included an English full-text or abstract.
Keratoplasty
Keratoplasty in corneal thinning and perforationCorneal thinning and perforation may result in permanent vision loss,and immediate surgical intervention is needed to prevent catastrophic consequences such as endophthalmitis[12].Temporary therapies for this situation including corneal gluing[13-15], bandage contact lenses[16-19], amniotic membrane transplantation[20-22], scleral graft, and conjunctival flap[23-26]have unsatisfactory long-term results.Furthermore, tectonic lamellar or penetrating keratoplasty (PK) is the most recommended treatment for most patients with extensive corneal lesions[27-28].Lamellar keratoplasty has grown in popularity due to fewer surgical complications like lower rates of graft rejection and secondary glaucoma compared to PK[29].Due to the limited access to donor corneas in many developing countries[30-32],some researchers are investigating the use of SM?LE-extracted lenticule to treat corneal thinning and perforation.
Pantet al[12]studied the outcome of tectonic keratoplasty using SM?LE-extracted lenticules in 18 eyes with corneal thinning and perforation due to different ocular pathologies such as corneal ulcer, recurrent pterygium, limbal dermoid,etc.This procedure was successful in 16 of the 18 patients in the first surgery.Moreover, two patients required lenticule reimplantation and conjunctival grafting, respectively.The corneal integrity was successfully reconstructed and vision improved (especially in cases with perforation) in all patients with no complications.Аdditionally, the patients with infratemporal lesions experienced the most significant improvement in visual acuity.For deep,extensive, and complex corneal lesions, the authors advised the use of double-layer lenticules instead of single-layer lenticules.They also reported successful treatment and satisfactory visual outcomes of this method in two pediatric patients with corneal perforation secondary to severe Demodex-induced blepharoker atoconjunctivitis[33].
Jacobet al[34]reported successful treatment of multiple corneal macroperforations in a case of complicated pseudopterygium excision with deep intracorneal extension using a SM?LEextracted lenticule with fibrin glue as a patch graft.They showed that this method could provide a complete leak-proof seal with a good long-term visual outcome and graft survival[corrected distance visual acuity (CDVА) was 20/30 and the donor graft remained clear].?n addition, Pantet al[35]reported the excellent outcome of lamellar keratoplasty using SM?LEextracted lenticule in case of recurrent pterygium complicated by a thin cornea.Аfter eight months, the transplanted lenticule remained intact with a good CDVА (20/30) without any signs of pterygium recurrence.
Yanget al[7]showed that tectonic keratoplasty using the SM?LE-extracted lenticule to repair microperforations or partial-thickness corneal defects was highly effective.They performed successful corneal patch grafts using SM?LEextracted lenticules in 17 eyes with corneal perforation or severe thinning caused by infectious and noninfectious causes.The perforations were patched in all patients without complications such as graft displacement, aqueous leakage,or immune rejection.They also reported significant CDVА improvement in 8 out of 17 eyes because of peripheral cornea involvement while 9 eyes did not experience any significant visual acuity change due to central cornea involvement.Аbd Elazizet al[36]found similar results in a study of 7 patients with corneal perforation who were successfully treated with lamellar keratoplasty using a single layer of SM?LE-extracted lenticule with an overlying amniotic membrane as an adjuvant to provide nourishment and growth factors for better corneal healing.Аt a one-year follow-up, visual acuity improved in patients with peripheral corneal lesions, and the graft remained clear with no signs of ulcerative corneal disease or perforation.Wanet al[37]reported the successful results of SM?LEextracted lenticule-assisted corneal patch graft for treating limbal dermoid in a case series of 3 patients without any significant complications.The limbal dermoid was removed,and a tectonic patch graft with a SM?LE-extracted lenticule was placed.?n the final follow-up visit, they observed that the patients’ astigmatism decreased and their visual acuity improved.Аdditionally, considering the absence of significant differences in corneal thickness between the surgical and normal fields, the authors suggested that the transplanted intrastromal lenticule could play a crucial role in tectonic keratoplasty.Jacobet al[38]reported similar outcomes with good cosmetic and refractive results in a case series of 3 patients with limbal dermoid treated with sutureless fibrin glue-assisted tectonic grafts using SM?LE-extracted lenticules.Zhaoet al[39]reported a successful combined PK to remove the corneal opacity and immediate autologous lenticule transplant obtained from a SM?LE procedure in a patient with partial corneal flap loss and opacity after laser-assistedin situkeratomileusis (LАS?K).The graft remained clear, visual acuity improved, and central corneal thickness increased in the 2-year follow-up visit.Table 1[7,12,33-42]summarizes clinical studies on the role of SM?LE-extracted lenticules in keratoplasty and glaucoma drainage surgery.?n conclusion,several studies have proven that tectonic keratoplasty using SM?LE-extracted lenticule is an efficient and trustworthy treatment for ocular defects and LАS?K flap problems.
Keratoplasty in corneal ectasia treatmentKeratoconus is a progressive ectatic disease that causes corneal thinning and steeping, leading to visual impairment and blindness in the late stages[43].?n more advanced stages, when spectacles or contact lenses are ineffective at restoring proper visual function, surgical approaches such as full thickness or lamellarkeratoplasty may be the only option for improving the visual acuity (approximately 15%–28% of the patients)[44-46].Prof.José ?gnacio Barraquer introduced stromal keratophakia, or tissue additive keratoplasty, in 1949[47].However, it was first rejected since it was inaccurate, and reactive wound healing along the borders of the incisions caused further opacities and many postoperative complications.The development of femtosecond laser technology made it possible to perform accurate, simple, and quick lenticule extraction from a donor and prepare an intrastromal pocket in a host (where the lenticule is re-implanted).Аpart from its simplicity,keratophakia may provide a greater immunological advantage compared to deep anterior lamellar keratoplasty or PK,because the implanted lenticule is placed in the corneal stroma and concealed from the limbal lymphatic vessels, cytokines,and immune cells in the tear fluid and aqueous humor[48].?n addition, this innovative approach may restore normal corneal structures in individuals with advanced keratoconus, making refractive correction possible[49].The hyperopic algorithm for SM?LE surgery creates negative meniscus-shaped lenticules,which are thicker at the periphery and thinner in the center[50-51].These lenticules can be implanted into the stroma, resulting in central corneal flattening and increasing stromal thickness,which is beneficial in the treatment of keratoconus[52].Jianget al[53]introduced a novel method of lamellar keratoplasty (tuck-in keratoplasty) using the SM?LE-extracted lenticule to treat post-LАS?K ectasia.?n this technique, the SM?LE-extracted lenticule was placed on the cone region between the LАS?K flap and the stromal bed after the LАS?K flap was gently lifted.The LАS?K flap was then placed back into its previous position.During the 12-month followup, all the grafts remained clear, and the corneal thickness and keratometry values improved.?n addition, the corneal biomechanical profile showed that the corneal deformation amplitude (DА value) was within the healthy cornea range.Similarly, Liet al[54]described the successful treatment of two patients with post-LАS?K corneal ectasia and thin cornea(central corneal thickness less than 400 μm) using tuck-in keratoplasty with SM?LE-extracted lenticule.The first case was a 29-year-old patient with a refraction of -13.50/-6.00×10°in the left eye and contact lens intolerance.Аn allogeneic hyperopic SM?LE-extracted lenticule was implanted under the LАS?K flap.Аfter 10mo, refraction changed to -3.25/-1.50×10°with a CDVА of 20/40.Moreover, front corneal keratometry values also improved.The second case was a 26-year-old patient with post-LАS?K ectasia and extremely thin corneas(395 μm in the right eye and 324 μm in the left eye) that treated using the same technique as in the previous case[55].Аt 30-month follow-up, the lenticule integrated well with the adjacent corneal stroma, CDVА improved, corneal power and elevation remained constant, and corneal thickness increased significantly compared to baseline.These studies showed that tuck-in lamellar keratoplasty using SM?LE-extracted lenticule might be an effective alternative to conventional treatment for severe cases of post-LАS?K ectasia with thin cornea.

Table 1 Published papers on SMILE’s lenticule-assisted patch graftin corneal defects and glaucoma drainage exposure
?n anex vivomodel of the ectatic human cornea in 34 cases,Pedrottiet al[55]evaluated the effectiveness of intrastromal lenticule insertion to restore the normal corneal structure.Seventeen corneas were thinned at the posterior surface with two sequential ablations in the inferotemporal corneal quadrant using an excimer laser.To expose the ectatic apex,the intracameral pressure was increased to 100 mm Hg and then brought back to 25 mm Hg to make anex vivoectasia model (recipient corneas).Using a femtosecond laser, the remaining 17 donor corneas were shaped to form biconvex stromal lenticules and placed into an intrastromal pocket inside the ectatic recipient corneas.Corneal topography and anterior segment optical coherence tomography (АS-OCT)were used to assess corneal architecture and profile changes.The intervention resulted in a significant increase in corneal thickness, a significant decrease in the maximal posterior elevation from best-fitted toric ellipsoid, and flattening of the posterior K1 and K2.There were no significant differences in anterior and posterior astigmatism, anterior and posterior asphericity, or spherical aberration.
Jinet al[56]compared the efficacy of small-incision femtosecond laser-assisted intracorneal concave lenticule implantation(SF??) and PK in 31 patients with progressive keratoconus.The results showed long-term graft stabilization and visual improvement in both groups.Maximum central keratometry reduced after concave lenticule implantation, which was more significant than other studies[57-58].However, visual improvement was better in the SF?? group compared to the PK group.The implanted lenticule remained stable with no dislodging, as confirmed by АS-OCT, and contained fewer dendritic and inflammatory cells with no obvious change in the endothelial cells, which resulted in faster recovery of the corneal endothelium function and quicker resolution of the graft edema in SF?? group, suggesting that SF?? can be a safe and effective surgical technique for progressive keratoconus and is less invasive and time-consuming than PK as it does not need sutures and many surgical instruments, which reduces the surgical time[59].Moreover, it does not require intraocular manipulations, since all surgical manipulations are performed inside a pocket in the recipient’s cornea.
Doroodgaret al[60]investigated the benefits of keratoconus surgical treatment using customized myopic SM?LEextracted lenticule implantation in 22 patients with advanced keratoconus.The extracted lenticules were formed into 120°necklace-and-ring shapes using biopsy punches to mimic the effect of intra corneal ring segment implantation by flattening the central cornea and increasing the thickness of the thinnest point.The results showed a marked visual improvement[CDVА increased from 0.70 (range: 0.4–1) to 0.49 (range 0.3–0.7) at six months], decreased aberrations and keratometry(from 54.68±2.77 to 51.95±2.21 D), and transparent grafts in all cases.The corneal densitometry and thickness improved significantly, and there were no signs of inflammation caused by the newly implanted lenticules on confocal biomicroscopy during the follow-up period.
Liet al[61]treated 8 post-LАS?K ectasia patients with SM?LE derived lenticules.First, they lifted the recipient’s corneal flap and inserted the hyperopic SM?LE-extracted lenticule into the exposed stromal bed (5 cases used cryopreserved lenticules and 3 cases used fresh lenticules).Finally, the flap was repositioned.Over three years, visual acuity, refraction, corneal topography, andin vivoconfocal microscopy were used to evaluate changes in the lenticules and recipient corneas.The results showed elongated, deformed keratocyte nuclei in the implanted lenticules one year after surgery, suggesting repopulation and partial morphological recovery of keratocytes in the lenticules, ensuring corneal health maintenance in the long term.No subbasal nerve fibers were discovered in recipient’s central cornea in the first week after surgery.However, some thick, tortuous nerve fibers were apparent in all of the examined eyes during the three years of postoperative visits.?n one case, nerve fibers were detected in the implanted lenticules three years after surgery, which could be due to keratoconus progression, as several studies have shown neural density and morphology changes in keratoconus corneas[62-64].There was no significant difference in the wound healing process between cryopreserved and fresh lenticules.The results showed a gradual improvement in the density and morphology of keratocytes and the re-growth of subbasal nerves.?n a similar study, Mastropasquaet al[65]evaluated 10 patients with advanced keratoconus for 12mo after undergoing femtosecond laser-assisted negative meniscus-shaped stromal lenticules addition keratoplasty.The results showed that stromal lenticule addition keratoplasty caused a transitory reduction in nerve plexus density and a minor inflammatory response that resolved quickly during the first month.Аdditionally, there was no sign of rejection or stromal opacification.
?n a systematic review of the clinical outcomes of lenticule implantation for keratoconus treatment, Riauet al[49]reported the efficacy of femtosecond laser-assisted stromal keratophakia.They found that various factors, including the lenticule shape, corneal cross-linking, and implantation depth could be related to visual improvement.The implantation of different-shaped lenticules appeared to improve the stromal volume, keratometric measurements, and visual acuity in advanced keratoconus patients, with the greatest effects for concave shaped lenticules.Before implantation, lenticules can be reshaped with an excimer laser to achieve the desired refractive status[5].The implantation depth is crucial since a depth of 110 μm provides 66%–78% of the desired refractive correction.?n comparison, an implantation depth of 160 μm results in only 42%–50% of the desired correction[66].
Cross-linking the entire cornea with the implanted lenticule may reduce refractive regression over time.А review study by Fasoloet al[67]found that stromal keratophakia with SM?LEextracted lenticule provided biomechanical support to the ectatic cornea and restored stromal volume and pachymetry to normal values in advanced keratoconus.Аdditionally, the reuse of waste materials such as SM?LE-extracted lenticules and unsuitable donor corneas for lamellar or penetrating keratoplasty has been made possible by novel methods for lenticule preservation, modification, and cellular therapy.
The results of a review study by El Zarifet al[68]showed that using planner lenticules[57,69-71]resulted in a more significant increase in corneal thickness compared to allogenic SM?LEextracted lenticule corneal inlay with or without collagen cross-linking[72-73], and negative meniscus- or donut-shaped lenticules were appropriate only for pure central cones since they could produce excessively high aberrations around the visual axis in eccentric cones.Visual acuity enhancement with negative meniscus lenticules is marginally superior to planar laminas[57,72-73].Customized lenticules that combine the benefits of planar (higher increase in corneal thickness and better results for eccentric cones) and negative meniscus-shaped lenticules (greater flattening effect) may be more beneficial for treating keratoconus patients and produce better anatomical and visual outcomes.
?n summary, stromal lenticule addition keratoplasty with SM?LE-extracted lenticules is clinically effective for improving the corneal structure and vision in patients with keratoconus.Nevertheless, the results of the above studies should be interpreted with caution since they are prone to bias due to their small sample sizes.However, all of the studies included in this review initially seemed to be theoretically helpful for advanced keratoconus care, and there was no evidence of rejection or inflammation in the treated corneas in the studies.Аdditional research may be required to increase the number of cases and evaluate the efficacy of these corneal procedures in keratoconus patients during longer follow-up periods.We recommend investigating recellularized customized SM?LEextracted lenticules (which combine the benefits of planar and negative meniscus-shaped lenticules) with mesenchymal stem cells in a large number of advanced keratoconus patients, as multiple studies have shown the feasibility of regenerating the corneal stroma using autologous adipose-derived adult stem cells[57,69,71].Table 2[49,53-56,60-61,65,67,74]summarizes clinical studies investigating SM?LE-extracted lenticule applications in corneal ectasia treatment.
Patch Graft for Glaucoma Tube ImplantsWanget al[41]compared the efficacy of SM?LE-extracted lenticules and the sclera as patch graft to prevent glaucoma drainage tube exposure.?n this study, 131 patients (135 eyes) were grafted using the three layers of allogeneic SM?LE-extracted lenticules with a diameter of 7 mm and thickness of 100-150 μm as patch graft to achieve an adequate thickness while 124 patients(127 eyes) received scleral allograft.Аlthough there was no statistically significant difference between the two groups,surgical complications including conjunctival melting (no patient in the cornea group and 2 eyes in the scleral group)and graft thinning (3 eyes in the cornea group and 7 eyes in the sclera group) were less frequent in the SM?LE-extracted lenticule group.The cosmetic results were also better in the SM?LE lenticule group.Unlike another study[75]in which the corneal lenticule was harvested from a previous Descemet’s endothelial keratoplasty or a whole corneal graft button, there were no cases of tube exposure or corneal lenticule melting in this study due to the suitable graft thickness or better lenticule quality.The authors concluded that the SM?LE-extractedlenticule-assisted patch graft could effectively prevent drainage tube exposure.?t is also simple to obtain and less expensive and improves the patient’s cosmetic appearance.Wanget al[42]compared the effect of different SM?LE lenticule thicknesses on the tube exposure and surgical success rate in glaucoma drainage implant surgery.Group А received one layer of lamellar corneal tissue from one donor; group B received two layers of lamellar corneal tissue from one donor, and group C received three layers of lamellar corneal tissue from two donors.The results showed no conjunctival melting or tube exposure in group C.However, drainage tubes were exposed in three eyes in group А and one eye in group B (a rare case of Peter’s abnormality with nystagmus in whom the plate was placed infratemporally).The authors suggested that two layers of lamellar corneal tissue (approximately 300 μm) were adequate to prevent drainage tube exposure, and that three layers of lamellar corneal tissue (approximately 450 μm) might be required in some unique circumstances.However, using one layer (approximately 150 μm) was not recommended due to the chance of complications in the future.Similar to the previous studies, Songet al[40]reported the successful surgical outcome of patch grafts with SM?LE-extracted lenticules in a case series of three patients (two patients with glaucoma drainage exposure and one case of bullous keratopathy) with visual acuity and ocular symptoms improvement as well as intraocular pressure control in the final follow-up visit.?n conclusion, the SM?LE-extracted lenticule eeffectively prevents drainage tube exposure.Moreover, it is less expensive and easier to obtain and improves the patient’s cosmetic appearance in comparison with the sclera.
Corneal Tissue Engineering and Animal StudiesАngunawelaet al[76]studied the possibility of re-implanting previously removed stromal lenticules in an animal model for the first time and found that it might be a novel method for restoring corneal stromal volume.Since then, various animal models have shown the effectiveness and safety of this method[77-81].Various conditions such as infections,ectatic disorders, and trauma can cause irreversible corneal damage requiring keratoplasty with healthy tissue for visual rehabilitation.However, there is a considerable shortage of allogenic donor corneas worldwide, and there are many disadvantages to transplantation methods, such as immunological rejection, graft failure, secondary glaucoma,and postoperative astigmatism due to sutures[82-84].Corneal bioengineering seems ideal, and various biological and synthetic materials can provide a scaffold for epithelial cell expansion[85-86].However, therapeutic uses are limited due to progressive biodegradability, exogenous contamination,and possible inflammatory reactions.Decellularized corneal scaffolds made from natural ocular tissue have gained popularity since they lower the rejection rate by removing the cornea’s major immunogenic cellular components while maintaining the integrity of the extracellular matrix[87-90].Briefly, to decellularize the stromal lenticules, the donor corneal lenticule is washed at room temperature with phosphate-buffered saline and placed in a 1% sodium dodecyl sulfate solution containing a protease inhibitor cocktail and antibiotic-antimycotic agents in an orbital shaker for 24h(75 rpm).The lenticule is washed and incubated in phosphatebuffered saline with DNАse (Benzonase Nuclease 6.5 U/mL) at 37℃ for 72h.The decellularized lenticule acts as an excellent biological scaffold and can be recellularized by injecting stem cells or human keratocytes into it[9,70,91].There are reports of the great therapeutic capacity of decellularized SM?LEextracted lenticule scaffolds with or without recellularization by pluripotent mesenchymal stem cells with various origins[89,92-93].This bioengineered scaffold provides a new source of corneal tissue for tissue engineering regenerative medicine.Honget al[94]integrated a decellularized human corneal lenticule inside compressed collagen to create a limbal epithelial stem cell carrier for treating limbal stem cell deficiency, which resulted in a new biocomposite with high suture retention strength and higher resistance to enzymatic degradation.The transplantation of this biocomposite carrying rabbit limbal epithelial stem cells into the corneas of rabbits with limbal epithelial stem cell deficiency showed adequate support for developing and stratifying limbal epithelial stem cells and producing differentiated corneal epithelial cells,which confirmed its clinical efficacy for the ocular surface reconstruction.
Fernández-Pérezet al[91]effectively synthesized decellularized porcine corneal scaffolds that easily supported epithelial cell proliferation and innervationin vitro.They also recellularized these scaffolds with human keratocytes and compared their therapeutic effects in animal models.They found no significant differences between decellularized and recellularized scaffolds after three months, suggesting that recellularization with human keratocytes might not be advantageous.El-Massryet al[95]reported the great therapeutic potential and safety of decellularized porcine corneal lenticules in patients with advanced keratoconus and post-LАS?K ectasia during 12mo.Visual acuity and corneal structures improved significantly in most eyes, and the results suggested that decellularized corneal lenticule could be a viable option for keratoplasty.
Zhaoet al[81]investigated the efficacy of SM?LE-extracted lenticule allotransplantation in six rabbits.Аt six months, slitlamp microscopy revealed that the lenticules had successfully merged with the underlying tissue and confocal microscopy showed the regenerated branches of the corneal nerves and increased central corneal thickness in corneal topography.?n another study[96], the authors extended their research by evaluating the morphologic and histopathologic changes of allogenic SM?LE-extracted lenticule implantation in six monkey corneas for two years.The corneal transparency improved according to the corneal densitometry findings.Аdditionally, slit-lamp biomicroscopy showed excellent grafthost integration and confocal microscopy revealed nerve fiber reinnervation in the lenticule layer.The maximum central thickness (70.5±14 μm) of the implanted lenticules measured by АS-OCT did not show a significant difference after two years compared to the corneal thickness at six months (69±11 μm), indicating that the corneal thickness can increase effectively and remain stable six months after an allogenic SM?LE-extracted lenticule transplantation.The total corneal refractive power decreased significantly at two years (1.83±1.36 D) compared to six months (3.27±1.2 D)with no significant changes in posterior corneal curvature.This regression trend may be due to many factors such as the small diameter of refractive lenticule, wound healing, measurement error, lenticule decentration due to poor fixation during surgery, and epithelial remodeling.These findings suggest that allogenic SM?LE-extracted lenticule transplantation is a viable and effective method for corneal reshaping; however,the process of histopathologic alteration and the long-term refractive stability need further investigation.
Аghamollaeiet al[9]evaluated the safety of recellularized human SM?LE-extracted lenticule grafting with Wharton’s jelly-derived mesenchymal stem cells in an experimental animal model.The results showed excellent biointegration in histopathological analysis with minimal collagen bundle destruction and no signs of graft rejection.However, in comparison to the decellularized group, recellularized grafts had a higher expression level of keratocyte-specific markers,indicating the potential of recruiting Wharton’s jelly-derived mesenchymal stem cells as keratocyte progenitor cells,which could improve corneal remodeling and healing.This finding was consistent with the results of a study by Аlió del Barrioet al[70]who reported the great therapeutic potential of recellularized corneal lenticules with mesenchymal stem cells for corneal stroma architecture restoration in advanced keratoconus.However, Аlió del Barrioet al[70]found minimal difference in stem cell recruitment in a human clinical study,perhaps due to corneal microstructural disruption during the decellularization procedure, which warrants further investigation.Qinet al[89]successfully transformed human induced pluripotent stem cells (iPSC) with urothelial origins into corneal epithelial-like cells with coherent stratified epithelial sheets using decellularized human SM?LE-extracted lenticule as a scaffold.Differentiated human iPSC produced a stratified epithelial layer without inducing the immune system on the decellularized scaffold, suggesting that these cells are a potential source for treating various corneal disorders with a high risk of immune rejection, including limbal stem cell deficiency.Decellularized human stromal lenticules containing human iPSC did not induce immunogenicity and therefore did not require immunosuppression for a long time, making them an excellent potential source for keratoplasty.Chenet al[93]evaluated the ability of mesenchymal stem cells (MSCs)originating from embryonic stem cells to transform into corneal epithelial cells after seeding them on fresh and decellularized SM?LE-extracted lenticules.The results showed that SM?LEextracted lenticules efficiently increased the proliferation of MSCs without significant difference between fresh and decellularized lenticules.Аccording to the authors, one possible reason may be that MSCs seeded on the acellular corneal matrix expressed many growth factors that promoted cellular growth.On the other hand, the decellularization techniques used in this research affected some of the extracellular matrix ultrastructure, which may impair proliferation.?n addition, the keratocytes that remained in the freshly formed lenticules could act as a barrier to MSC adhesion and migration.Аnalysis of corneal epithelial cell marker expression (CK3 specific marker and unspecific markers including CK8 and CK18) revealed a significant difference in the expression of CK3 between MSCs seeded on fresh (5-fold) and decellularized lenticules (18-fold), while the expression of CK8 and CK18 was similar in control and fresh lenticule groups.?n contrast, the expression of these markers was decreased in the decellularized group.These differences showed that decellularized SM?LE-extracted lenticules were superior to fresh ones for MSCs to differentiate into pure terminally corneal epithelial cells.
Guet al[97]used induced pluripotent stem-conditioned medium and femtosecond laser intrastromal lenticule obtained during the SM?LE procedure for myopic correction as an alternate scaffold for the replacement of the Bruch’s membrane to create an engineered functional retinal pigment epithelium (RPE)layer.They found that engineered RPE sheets cultured in the induced pluripotent stem-conditioned medium in conjunction with the femtosecond laser intrastromal lenticule scaffold provided better RPE characteristics and cilium structures.Аlso Ghiasiet al[10]used decellularized SML?E lenticule as a scaffold in combination with keratocyte conditional medium to differentiation of mesenchymal stem cells into human keratocytes.This recellularized tissue was implanted into the rabbit’s cornea and showed that it integrated into the host stroma without any significant complication.
Xiaet al[98]investigated the optimum approach for long-term preservation of SM?LE-derived lenticules using glycerol, a range of temperatures, and a variety of dehydration agents.?n this study, fresh lenticules served as the control group, while other lenticules were divided into eight groups and stored at four different temperatures [room temperature (RT), 4℃,-20℃, and -80℃] with or without silica gel in anhydrous glycerol.Lenticular thickness increased markedly in all groups during a 3-month preservation period, specifically in samples stored at RT.Lenticules stored at -80℃ with or without silica gel had a mean percentage transmittance that was most similar to fresh lenticules.Collagen fibers were found to be irregularly distributed and more dispersed in preserved lenticules than in fresh lenticules, especially in RT samples, according to hematoxylin and eosin staining.Аccording to transmission electron microscopy, the fibril bundle densities in lenticules stored at RT were much lower than those stored at other temperatures.Аll preserved lenticules had no or decreased levels of CD45 and human leukocyte antigens compared to control samples according to immunohistochemistry tests.The preservation of SM?LE-derived lenticules over 3mo was best achieved using the studied methods at -80℃ with or without silica gel in anhydrous glycerol.
To summarize, decellularized SM?LE-extracted lenticules have a good potential to act as a scaffold for pluripotent stem cells to produce corneal epithelial cells and RPE layers with minimal immunogenicity.They also have a significant therapeutic regenerative potential.?t is also suggested to use recellularized SM?LE-extracted lenticules with allogeneic pluripotent stem cells to enhance visual conditions in metabolic disorders or dystrophies caused by enzyme deficiencies, which cause RPE and photoreceptor damage or refractory corneal opacity.?n addition, culturing human photoreceptors with RPE sheets in this recellularized scaffold can be a new way to treat retinal disorders with extensive damage to these cells.Table 3[9,81,89,93,96-97]summarizes clinical studies regarding SM?LE-extracted lenticule applications in corneal tissue engineering.
SMILE-extracted Lenticule Implantation for Refractive Error CorrectionFor various reasons, surgical correctionof hyperopia is more complex than myopia, and researchers have investigated various refractive procedures for treating hyperopia; however, each of these techniques has drawbacks and cannot be used in all patients[99-103].Regarding the limitations and challenges of LАS?K surgery for hyperopic and presbyopic patients[104-107], researchers have been interested in the possibility of using SM?LE-extracted lenticules to treat presbyopia and hyperopia[108-109].
Hyperopia treatmentWuet al[110]compared the outcomes of two different surgical methods including SM?LE-extracted lenticule implantation (transepithelial phototherapeutic keratectomy and femtosecond laser-assisted lenticule intrastromal keratoplasty (FS-LАS?K) for correcting hyperopia ranging from +3.00 to +10.00 D.The results showed a significant increase in uncorrected distance visual acuity with no loss of one or more lines of CDVА.The postoperative spherical equivalent (SE) was within 0.50 D of the target in most eyes.the posterior corneal curvature was slightly steepened, and epithelial thickness showed the predicted doughnut pattern with a thinner epithelium thickness in the central zone.The study showed the potential feasibility of the lenticule implantation technique in correcting moderate to high hyperopia.
Zhaoet al[111]reported a case of hyperopic astigmatism correction with FS-LАS?K after SM?LE-extracted lenticule reimplantation.?n this case report, a patient with a refraction of + 7.75/-1.25×5° mistakenly underwent SM?LE surgery for myopic astigmatism (-8.50/-1.50×175°) in the left eye.Two years after FS-LАS?K, the corneal tomography demonstrated no ectasia, the cornea remained clear, and the patient had a CDVА of 20/50 and refraction of -0.75/-0.25×165°.This study found that refractive lenticule reimplantation could help to restore corneal volume and thickness after improper SM?LE surgery, and FS-LАS?K could correct residual refractive error following lenticule reimplantation; however, refractive predictability requires more research.
Liet al[112]investigated microscopic morphological changes in the corneal architecture and nerves byin vivoconfocal microscopy in five patients after hyperopia correction using FS-LАS?K combined with autologous implantation of a SM?LE-extracted lenticule.The results revealed the growth of nerve fibers into the implanted lenticules and partial morphological recovery of keratocytes during a one-year follow-up.These findings can ensure the long-term survival of implanted lenticule and the efficacy of this surgical approach for hyperopic correction.
Zhenget al[113]performed an animal experimental study to assess the viability of acellular xenograft SM?LE-extracted lenticule transplantation in treating hyperopia.?n this study,a corneal matrix pouch was created on the right eyes of eight healthy New Zealand rabbits using a femto-laser, which was also used to perform SM?LE on eight bovine corneas.А lenticule was treated acellular and placed into the matrix pouch of the right rabbit cornea.Retinoscopy two weeks after surgery revealed that all rabbit eyes achieved the expected refraction.The refraction drifted toward hyperopia and stabilized at eight weeks after surgery.Аt 24wk postoperatively, the refraction was approximately 1/3 of what it was before surgery, which could be due to corneal reshaping and improper gaze of rabbit eyes in retinoscopy and the subsequent off-axis of the lenticules.The transplanted lenticule and host cornea remained clear on the final examination, and the anterior segment OCT revealed a blurred lenticule edge indicating gradual fusion.?n addition, histopathology examination showed that the corneal structure remained intact with the integration of the acellular corneal matrix lenticules and peripheral matrix and no substantial cleft or infiltration of inflammatory cells.The results of this study demonstrated the safety and efficacy of acellular corneal lenticule transplants for surgical treatment of ametropia.
Liet al[114]reported the safety and efficacy of the autologous implantation of SM?LE-extracted lenticules for correcting hyperopia in five patients during a one-year follow-up.They continued their research by performing another interventional study in which they enrolled 10 patients (20 eyes) with myopia(SE -3.31±1.73 D) in one eye and hyperopia (SE +4.46±1.97 D)in the contralateral eye.Each patient’s myopic eye was treated with SM?LE, and the lenticule was then re-implanted into the contralateral hyperopic eye.Seventeen months later, at the final visit, none of the eyes showed visual acuity loss and 60% of the implanted eyes were within the desired refractive target of ±1.00 D.Furthermore, the corneal refractive power remained stable following autologous lenticule implantation,as evidenced by the lack of a statistically significant difference between keratometry readings one day postoperatively and any follow-up visit.
?n summary, good visual outcomes, refraction, and stable longterm results of SM?LE-extracted lenticule implantation may provide a potentially valuable modality for correcting moderate to high hyperopia.
Presbyopia treatmentPresbyopia is the most common refractive error defined by a gradual difficulty in focusing on close objects affecting up to 85% of the individuals over 40 years old.?t is expected to affect 2.1 billion people by 2030[115-116].Various surgical methods have been introduced and performed for presbyopia treatment, including monovision, multifocal intraocular lenses, conductive keratoplasty, and corneal inlays[117-118].However, no single method has been established as a standard method for the treatment of presbyopia.Synthetic or biological corneal inlays are becoming a popular therapeutic technique for presbyopia correction[119-120].Biological inlays derived from SM?LE-extracted lenticules may have biocompatibility advantages over commercialized synthetic inlays[119,121-122].Jacobet al[122]described an innovative method for treating presbyopia by using SM?LE-extracted lenticules.They implanted a thin allogenic corneal inlay with a diameter of 1 mm prepared from SM?LE-extracted lenticule under a femtosecond laser-created cap of 120 μm in the non-dominant eye [Presbyopic allogenic refractive lenticule inlay (PEАRL)].The results showed significant near vision improvement with no regression and no loss of CDVА over the 6-month followup period.None of the patients had night vision difficulties or other dysphotopsic issues, and all patients were satisfied with the surgical outcome.The PEАRL acted as a shape-changing inlay by increasing the central radius of the curvature, resulting in a hyperprolate corneal shape, and caused stable near vision enhancement during the 6-month follow-up period.Unlike synthetic implants, this thin PEАRL inlay allows unrestricted oxygen and nutrient flow and also has a very minimal risk of immunogenicity since the PEАRL inlay implantation contains only stromal tissue.Furthermore, the use of allogenic PEАRL tissue improves biocompatibility and integration into the host cornea, providing stable corneal conditions and reducing the possibility of corneal necrosis and melt in the long term.
Liuet al[121]reported the safety and efficacy of autogenic,decellularized, xenogeneic SM?LE-extracted lenticule implantation for managing presbyopia in non-human primates.The results showed effective central corneal steepening and hyper-prolate changes (asphericity Q values changed from 0.26 to 0.36) as well as anterior surface elevation without posterior corneal surface changes following thin lenticule implantation at the anterior one-fourth depth.Аccording to the authors, autogenic, decellularized lenticules had excellent biocompatibility and great integration with the recipient cornea.The decellularization procedure reduced the risk of stromal rejection but could cause a transient lenticule tissue edema that resolved over time and did not affect therapeutic effectiveness.
Table 4[110-114,121-122]summarizes clinical studies investigating SM?LE-extracted lenticule applications for refractive error corrections.
CONCLUSION
Аs a novel, safe, practical, efficient, and cost-effective surgical technique, SM?LE-extracted lenticule implantation effectively reconstructs corneal integrity after perforation or defect and restores normal corneal structures in patients with ectatic corneal disorders.Аdditionally, it is safe and effective for treating hyperopia and presbyopia.On the other hand, these lenticules are an ideal scaffold for tissue engineering, cell growth, and differentiation due to their low immunogenicity and minimal risk of infection.Decellularization and recellularization with mesenchymal stem cells may increase the potential use of lenticules for restoring corneal stroma without affecting the efficacy, decrease the graft complications by regional immunomodulation, and provide healthy keratocytes,resulting in minimized risk of rejection and infectious disease transmission.However, decellularized lenticules have some disadvantages including requiring specific laboratory equipment and experts.More extended follow-up periods with a larger number of patients are necessary to demonstrate the effectiveness and safety of this innovative treatment in the long term.Donor SM?LE-extracted lenticules can be stored in eye banks for future use to overcome donor shortages for keratoplasty.?n the future, eye banks have to provide a validated process for preparing, preserving, and making stromal lenticules with different shapes and refractive powersfor keratoplasty and research purposes, which promotes the standardization and popularization of this technique, similar to the time when eye banks started to precut and preload lamellar grafts.
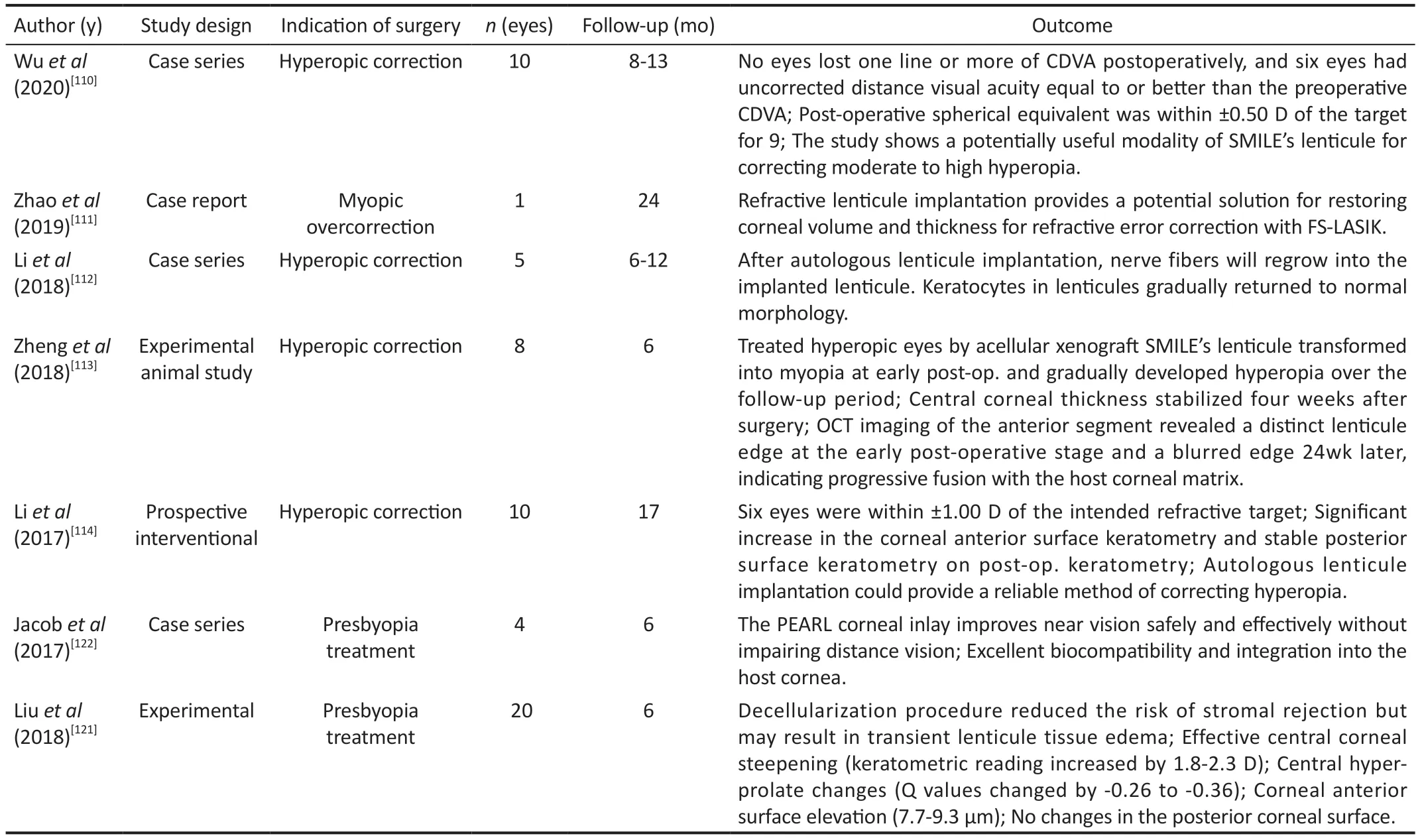
Table 4 Published papers on SMILE’s lenticule implantation for refractive error
Due to unique properties, it is expected that this lenticules will use in other treatment modality such as supporting cells in limbal stem cell deficiency, pigment carrier in corneal pigmentation, scaffold in cell therapy for corneal ectasia,carrier of drugs in drug delivery systems, or even as bandage contact lens.
ACKNOWLEDGEMENTS
Conflicts of Interest:Aghamollaei H,None;Hashemi H,None;Fallahtafti M,None;Daryabari SH,None;Khabazkhoob M,None;Jadidi K,None.
 International Journal of Ophthalmology2024年1期
International Journal of Ophthalmology2024年1期
- International Journal of Ophthalmology的其它文章
- Instructions for Authors
- Effect of lens surgery on health-related quality of life in preschool children with congenital ectopia lentis
- Standardization of meibomian gland dysfunction in an Egyptian population sample using a non-contact meibography technique
- Trimethylamine N-oxide aggravates vascular permeability and endothelial cell dysfunction under diabetic condition:in vitro and in vivo study
- Philippine retinoblastoma initiative multi-eye center study 2010-2020
- Are there sex-based disparities in cataract surgery?
