Effect of mono-/divalent metal ions on the conductivity characteristics of DNA solutions transferring through a microfluidic channel
Jie Zhu(朱潔), Jing Xue(薛菁), Wei Zhao(趙偉), Chen Zhang(張琛),Xiaoqiang Feng(馮曉強), and Kaige Wang(王凱歌)
State Key Laboratory of Cultivation Base for Photoelectric Technology and Functional Materials,Key Laboratory of Optoelectronic Technology of Shaanxi Province,National Center for International Research of Photoelectric Technology&Nano-functional Materials and Application,Institute of Photonics and Photon-Technology,Northwest University,Xi’an 710069,China
Keywords: microfluidics, interaction between deoxyribonucleic acid (DNA) and metal ions, conductivityvoltage relations,asymmetric electrodes
1.Introduction
It is of vital importance for life science research,clinical medicine,new drug development and human health,to study,analyze and comprehend the basic life process at the level of a single molecule.Deoxyribonucleic acid(DNA),as the most important genetic material for eukaryotic organisms,is an essential factor to maintain the propagation and development of species.DNA molecules are responsible for a variety of life functions,[1–4]which are always inseparable from the participation of metal ions.That is,organic life relies on inorganic elements to perform various life processes.[5,6]The study of interactions between DNA and metal ions at the single molecule level has profound meaning.
DNA molecules have a delicate and complex spatial structure, which is crucial for maintaining the structure and function of organisms.Under physiological PH conditions,DNA is a negatively charged polymer,which needs to be combined with metal ions (e.g., Na+, K+, Mg2+, Ca2+, etc.) to stabilize its spatial structure and maintain normal functions.In addition,metal ions are essential for a variety of cellular responses and various metabolic and physiological functions in life.For instance,a slight deviation of metal ion concentration from the normal level could lead to malfunction of diseases of living organisms,such as the lack of Mg2+or Ca2+results in cardiovascular disease and osteoporosis,and Na+,K+,Mg2+,Ca2+play crucial roles as neurotransmitters in cells.[7,8]However, excess metal ionsin vivoare toxic to life, such as an overabundance of Cu2+and Fe2+in patients with hepatolenticular degeneration and thalassemia.Moreover, some metal ions also affect the spatial structure of DNA.[9,10]
Since the 1990s numerous single-molecule research techniques have been developed,[11–13]and the electrokinetic(EK)method is one of the most effective tools for manipulating and analyzing DNA molecules.[14]However, the conductivity of DNA is quite low,e.g.,the genome of bacteriophage,λ-DNA is almost an insulator with one-dimensional conductivity less than~10-16S/cm, whose length is 48502 base pair, it is a commonly used substrate for restriction endonucleases and generating DNA size marker.[15]It is found that DNA modification with metal ions effectively enhanced the manipulation of DNA molecules.[16]The interaction between metal ions and DNA molecules changes their configuration and conformation,thus affecting the conductivity of DNA molecules.Previous studies have found that there are several factors that affect the electrical characteristics of mixed solutions of DNA and metal ions as they pass through a micro-/nanochannel,such as the concentration and PH values of the electrolyte, the material and inner diameter of the micro-/nanochannel, etc.[17–19]Further research indicates that the interaction between DNA molecules and metal ions promotes or inhibits the concentration or mobility of the charged particles in the mixed solution,leading to different electrical characteristics,[20]however,how the interaction works remains unclear.
In this investigation, we systematically studied the conductivity-voltage relationship of theλ-DNA solutions with different metal ions as they were electrically driven by a low voltage (≤100 mV) through the microchannel.The interaction betweenλ-DNA and metal ions with different valance states and concentrations was theoretically explained, which provided information for exploring the dynamics of DNA molecules in an electric field.Also, explorations of the effect of different (monovalent/divalent) metal ions onλ-DNA solution brought insight into manipulatingλ-DNA molecules through the EK method, which is of great importance for the electrophoresis-based DNA detection.We hoped this research is helpful for the precise manipulation of single biological molecules with micro-/nanofluidic devices.
2.Experimental setup
The experimental setup is shown in Fig.1, containing a potentiostat (EA 162, eDAQ, Australia), a micro-current detector (e-corder 401, eDAQ, Australia), a homemade sample reservoir, and a computer to control the equipment and process experimental data.The reservoir consists of two symmetric chambers defined as cis and trans respectively, and only connect by the capillary(3 mm in length, 5μm in diameter).Due to the performance of the polytetrafluoroethylene(PTFE),the sample reservoir possesses excellent insulation, corrosion resistance,and high-temperature resistance.
A three-electrode measurement system was used to improve the accuracy of potential control.All the electrodes in this research were made of platinum, and the working electrode (W) and the reference electrode (R) were inserted into the cis chamber,while the counter electrode(C)was dipped in the trans chamber.Notably, the surface area of the counter electrode (cathode) was 20 times than that of the working electrode (anode).Duanet al.found that utilization of this three-electrode system reduces the recovery time in the current response of the detector,and improves the stability of the measurements.[17,18,21]Moreover, this integrated system was fixed in a grounded Faraday cage to shield external electromagnetic interference.The potentiostat could provide external voltages ranging from-10 V to 10 V with an accuracy of 1 μV, which was connected to the electrodes to provide stable and accurate voltage.The micro-current detector, whose optimal detecting accuracy is up to femtoampere order, was used to monitor the responding current in the microchannel of different solutions with a sampling frequency of 2 kHz.The current signal was transferred to the professional analysis software e-DAQ chat in the computer in real-time for further processing and analysis.
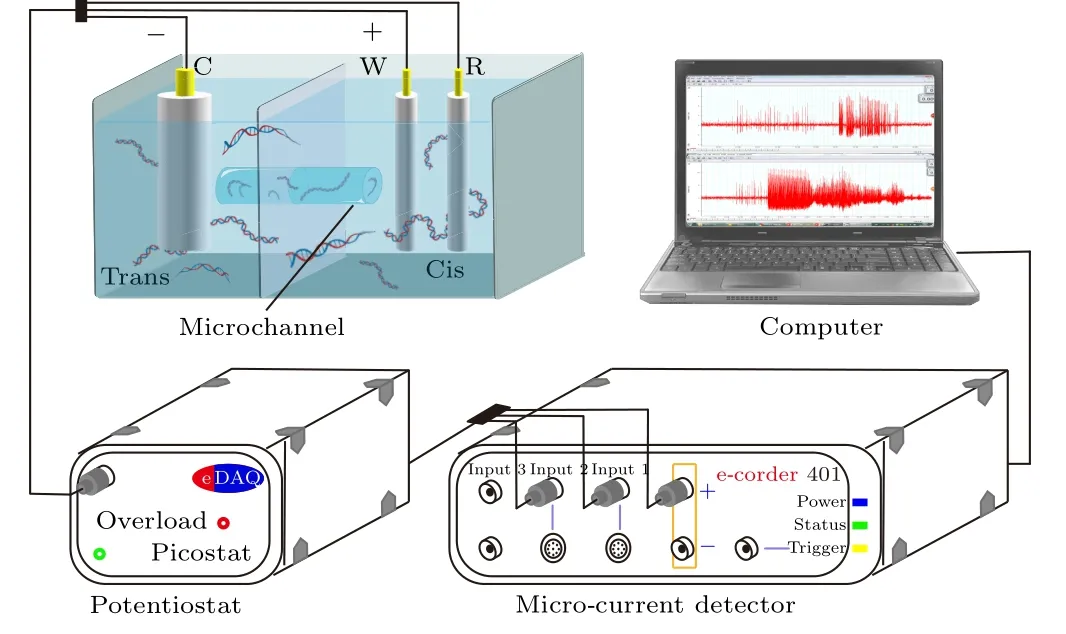
Fig.1.Schematic illustration of the experimental setup.
The details of experimental conditions are listed in Table 1.λ-DNA solutions with four kinds of metal ions that commonly exist in life,i.e.,Na+,K+,Mg2+,Ca2+,were studied.Tris(hydroxymethyl)aminomethane buffer solution with a concentration of 0.01 M was used as a solvent to mimic the physiological environment, and the concentration ofλ-DNA was 0.018 nM.In the mixed solutions ofλ-DNA and metal ions,the concentration of metal ions was set in three groups as 0.01 M,0.05 M,and 0.25 M,respectively.The applied external voltage ranged from 0 mV to 100 mV with an interval of 10 mV,for the characteristic potential difference in normal life phenomena is generally on the order of 10 mV.The temperature was kept at 25?C throughout the experiments excluding the influence of temperature fluctuation.The conductivity(G)of each kind of solution was acquired by differentiating the measured current value with respect to the external voltage.To ensure the reliability of the results, the measurements of responding current signals were repeated 5 times at each external voltage in each solution, and took the average of these 5 calculated conductivities as the final result.After completing each round of measurement,the microchannel system and electrodes were thoroughly cleaned for later use.

Table 1.Experimental conditions.
3.Results
3.1.The G–U relations of the λ-DNA solution
Firstly,the conductivity characteristics of theλ-DNA solution(0.018 nM)are investigated.Figure 2 shows the conductivity(G)–voltage(U)relations of theλ-DNA solution.As a non-Newtonian fluid, theG–Urelations of theλ-DNA solution follow nonlinear relationships,and the conductivity of theλ-DNA solution generally increases with the external voltage.

Fig.2.The G–U relations of the λ-DNA solution.
3.2.The G–U relations of λ-DNA-M+solutions
Figure 3 shows theG–Urelations ofλ-DNA solutions with different monovalent metal ions, i.e., Na+, K+,as the concentration of metal ions is 0.01 M, 0.05 M and 0.25 M, respectively.Figure 3(a) depicts theG–Ucurves ofλ-DNA-Na+solutions.It can be seen that as the external voltage increases, the conductivity ofλ-DNA-Na+solutions (with Na+concentration is 0.01 M and 0.05 M) decreases at the beginning but increases asU> 20 mV.When Na+concentration is 0.25 M, the conductivity always increases with the external voltage.Moreover, the conductivity ofλ-DNA-Na+solution increases with Na+concentration asU ≥30 mV, and the conductivity of the mixed solution with Na+concentration of 0.05 M is the biggest one in this research whenU<30 mV.Similar results are presented in Fig.3(b)forλ-DNA-K+solutions,and a little difference is that the conductivity ofλ-DNA-K+solutions increases with K+concentration asU ≥20 mV,and the conductivity of the mixed solution with K+concentration of 0.05 M is the biggest one in this research whenU<20 mV.
3.3.The G–U relations of λ-DNA-M2+solutions
Figure 4 shows theG–Urelations of theλ-DNA solutions with different concentrations (0.01 M, 0.05 M or 0.25 M) of divalent metal ions, i.e., Mg2+, Ca2+.Figure 4(a) shows theG–Urelations of theλ-DNA-Mg2+solutions.As the external voltage increases,the conductivity ofλ-DNA-Mg2+solutions(with Mg2+concentration is 0.01 M,0.05 M and 0.25 M)decreases at the beginning but increases asU> 10 mV,U>30 mV,U>40 mV,respectively.In addition,the conductivity ofλ-DNA-Mg2+solutions decreases with Mg2+concentration asU ≥40 mV,and the conductivity of the mixed solution with Mg2+concentration of 0.05 M is the biggest one in this research whenU<40 mV.
In theλ-DNA-Ca2+solutions with Ca2+concentrations of 0.01 M, 0.05 M, and 0.25 M, as shown in Fig.4(b), the conductivity of the mixed solutions increases with the external voltage asU>10 mV,U>20 mV,U>30 mV, respectively.And the conductivity ofλ-DNA-Ca2+solutions decreases with Ca2+concentration asU ≥20 mV,and the conductivity of the mixed solution with Ca2+concentration of 0.25 M is the biggest one in this research whenU<20 mV.

Fig.3.The G–U curves of λ-DNA solutions with different monovalent metal ions.(a)λ-DNA-Na+ solutions.(b)λ-DNA-K+ solutions.
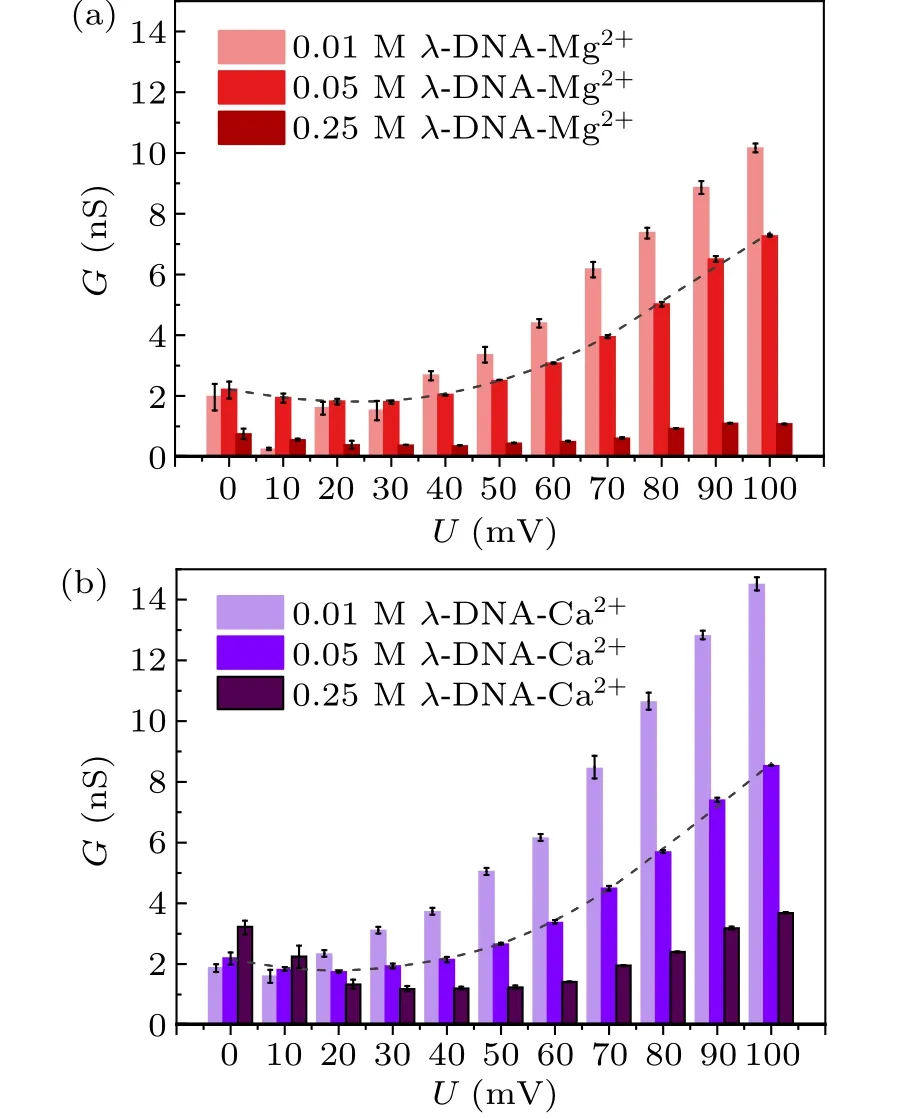
Fig.4.The G–U curves of λ-DNA solutions with different divalent metal ions.(a)λ-DNA-Mg2+ solutions.(b)λ-DNA-Ca2+ solutions.
It can be found from Figs.3 and 4 that the conductivities ofλ-DNA solutions with metal ions basically have a tendency to reduce firstly and then increase as the external voltage increases.And the conductivity ofλ-DNA solutions with monovalent metal ions is always higher than that with divalent metal ions,and when the external voltage reaches a certain threshold(turning point of theG–Ucurve),the conductivity ofλ-DNA solutions with monovalent metal ions increases with the concentration of metal ions, while that ofλ-DNA solutions with divalent metal ions decrease.
Comparing Figs.2–4,it is worth noting that the conductivities ofλ-DNA solutions with high-concentration(0.25 M)divalent metal ions are less than that of theλ-DNA solution.As Fig.5 shows, the conductivity of theλ-DNA-Mg2+solution is less than that of theλ-DNA solution whenU ≥10 mV.And the conductivity of theλ-DNA-Ca2+solution is less than that of theλ-DNA solution whenU ≥20 mV.That is to say,in theλ-DNA solutions with a high concentration of divalent metal ions the conductivity of the mixed solutions is weaker than that of theλ-DNA solution when the external voltage reaches a threshold.

Fig.5. G–U relations of λ-DNA solutions with or without Mg2+,Ca2+.
TheG–Urelations ofλ-DNA solutions with metal ions are affected by the experimental conditions and equipment,and also significantly influenced by the interactions between DNA and metal ions.The conductivity of solutions is determined by the properties of charged particles (e.g., DNA and metal ions) in solutions.And the electrophoretic mobility of particles is related to the radius and charge of itself and the strength of the external voltage.Inλ-DNA solutions with metal ions, the interaction betweenλ-DNA and metal ions varies for the type of metal ions and applies different effects on DNA structure,thus resulting in changes in the mobility of charged particles in the solution, and showing different electrical characteristics.
4.Discussion
4.1.Effect of interactions between metal ions and DNA
Interactions between DNA and different metal ions are quite complex and have varied effects on the configuration and conformation of DNA molecules,thus resulting in diverse characteristics of the mixed solutions of DNA and metal ions.It is essentially due to the distinct interaction mechanisms betweenλ-DNA and different metal ions that the conductivity ofλ-DNA with mono-/divalent metal ions features quite differently.The conductivity ofλ-DNA solutions with metal ions is mainly determined by the charges, concentration, and electrophoretic mobility of the charged particles in solutions.Interactions betweenλ-DNA molecules and metal ions alter the conformation or even break the strands ofλ-DNA,which causes changes in the properties of charged particles in the mixed solutions thus affecting the conductivity.Theλ-DNA solutions with or without metal ions are non-Newtonian fluids,and theirI–Ucurves follow the nonlinear relationship[20]
whereIis the steady current,I0is the initial current as the applied external voltage is 0,Uis the applied external voltage.The coefficientA(q,b,C)=∑i q2i biCiis determined by the charge, mobility and ionic concentration of ions in solutions, andqi,bi,Ciare the charges, mobility, and ionic concentration of thei-th ion,respectively.The exponentkcan be used to evaluate the sensitivity of the solutions’current variety to the applied voltage.According toG= dI/dUand Eq.(1),the conductivity of the mixed solutions ofλ-DNA and metal ions follows:
whereG0is a constant related to the electrical properties of the solution.
The DNA molecule usually exists in a physiological environment in a structure of a double helix, resembling a ladder that’s been twisted along its length as Fig.6 shows, and in the mixed solutions, the DNA molecule is considered as a negatively charged polyelectrolyte and attracts surrounding metal ions.Deoxyriboses and bases in DNA molecules contain oxygen and nitrogen, and phosphoric acid is negatively charged.Positively charged metal ions prefer binding in these sites.Metal ions bond directly with the oxygen atom of the phosphate group and the hydroxyl group of ribose, and the oxygen and nitrogen atoms on the bases, or cooperation of the atoms aforementioned.Moreover, metal ions can bind to DNA through their coordinated water molecules in an indirect way.[22–24]Besides, bindings between metal ions andπelectrons of the atoms on the bases disturb the stability of DNA structure and even lead to a break of DNA chains.[25]
Metal ions with different valences have distinct binding preferences for DNA molecules.[26,27]In the mixed solutions ofλ-DNA and monovalent metal ions,metal ions prefer binding to the adenine–thymine (A–T) riched segments at minor grooves, and the width of the minor groove gets narrowest when the metal ions are connected to the phosphate groups.The structure of theλ-DNA molecule has been compressed enhancing its particle property,and the mobility of the charged species improves to show a higher conductivity.Besides,when the concentration of the metal ions further increases,the insertion in bases destroys the hydrogen bonds between base pairs,resulting in a break ofλ-DNA chains.[26]The conductivity ofλ-DNA solutions with monovalent metal ions increases as more charged species have been released into the solution.Unlike the monovalent metal ions, bivalent metal cations tend to bind to the guanine–cytosine(G–C)riched segments at major grooves accompanied by the bending of DNA strands or even the agglomeration of DNA.Moreover, divalent metal ions interact with DNA molecules most frequently via water molecules in their first solvation shell,[26]which improves the particle properties of DNA and enhances the mobility of particles in the mixed solutions leading to an increase in the conductivity.

Fig.6.Interactions between DNA and metal ions.[28,29] (a) Schematic structure of DNA double helix showing minor and major grooves.In DNA,guanine–cytosine(G–C)and adenine–thymine(A–T)pairs are connected through hydrogen bonds.Metal ions can bind to the sites on the same or the opposite strands,and also in a complex form with bases.The binding of metal ions can result in DNA strand break.(b)Hexahydrated divalent metal cation binding to DNA through hydrogen bonding.(c) Molecular structures of magnesium nucleic bases with hydrogen bonded.
In addition, molecular dynamics simulation results indicate that the ratio of the neutralized charge to the original charge on theλ-DNA molecule isγ=1-1/Zξwhen there is only one type of counterion with the valance ofZin the solution, andξis the Manning coefficient.[30]λ-DNA molecules usually exist in the B-type structure at 25?C andξ= 4.2.As only monovalent metal ions are added to theλ-DNA solution,Z= 1 andγ= 0.76, that is, 76% of charges onλ-DNA molecules have been neutralized inλ-DNA solutions with Na+or K+, there are only 24% of charges onλ-DNA molecules retained.Similarly, residual charges onλ-DNA molecules inλ-DNA solutions with Mg2+or Ca2+are 12%.The charges ofλ-DNA molecules inλ-DNA solutions with monovalent metal ions are higher than that ofλ-DNA solutions with divalent metal ions.
4.2.Effect of DNA charge reversal
It is notable that different from cases inλ-DNA solutions with monovalent metal ions,the conductivity ofλ-DNA solutions with Mg2+or Ca2+decreases with the metal ion concentration.The concentration ofλ-DNA(48502 bp)is 0.018 nM carrying negative charges of around 1.75μM in this research,which is much less than the positive charges carried by divalent metal ions.Therefore, the decrease of the conductivity inλ-DNA solutions with divalent metal ions is not only caused by the neutralization betweenλ-DNA and metal ions.A possible explanation is that the coordinated water molecules of divalent metal ions interact with the oxygen atoms on the phosphate group ofλ-DNA forming a solvation shell, which reduces the repulsive force betweenλ-DNA molecules and enhances the particle property ofλ-DNA molecules.Thus, the mobility of charged species inλ-DNA solutions with divalent metal ions increases and makes the conductivity of the mixed solution greater than that of theλ-DNA solution.[31]When the concentration of divalent metal ions continues to increase,the radius of theλ-DNA-metal ions complex increases leading to weakened mobility of the particles.Therefore, the conductivity ofλ-DNA solutions with divalent metal ions decreases with metal ions concentration.
Besides, inλ-DNA-Mg2+andλ-DNA-Ca2+solutions,the hydrated metal ions adsorbed around theλ-DNA and shielded the charge ofλ-DNA gradually as the concentration of divalent metal ions further increases.The electrostatic repulsion betweenλ-DNA molecules decreased, resulting in the condensation of DNA molecules and the decrease of the species mobility in the solution.Bestemanet al.[32]found that multivalent metal ions formed a strongly associated fluid structure on the surface of DNA, which attracts more metal ions adsorbed on DNA molecules.When the total charge of the metal ions adsorbed on DNA molecules was greater than the charge of DNA in absolute value,the sign of the total net charge of DNA reversed.In this research,when the concentration of divalent metal ions exceeded a threshold(e.g.,0.25 M),the total charge of the counter ions adsorbed onλ-DNA was greater than the charge of DNA in absolute value,and the total net charge of someλ-DNA molecules reversed from negative to positive.There existed both negatively charged and positively chargedλ-DNA molecules in the solution at that time, which further intensifies the condensation ofλ-DNA molecules, causing a significant decrease in the species mobility.Thus,the conductivity of the mixed solutions decreased rapidly,even smaller than that of theλ-DNA solution.
4.3.Effect of the electrode polarization
Due to charge separation, there is a potential difference between the Pt electrodes and the interface of the mixed solutions forms an electric double layer(EDL),which could be evaluated by theζpotential.[33–36]The three-electrode system we used in this investigation has an area ratio of the cathode to the anode of 20:1, that is, the area of the cathode is much larger than that of the anode.Therefore, the potential of the cathode that charges accumulate on the surface of electrodes forming EDLs is higher than that of the anode, and a shielding electric fieldEsis generated in the opposite direction to the external fieldE.Thus, the initial electric field direction is opposite to the external electric field.As shown in Fig.7,with the increasing of the external voltage,the direction of the total electric field is finally in keeping withEand the steady current value turns from negative to positive.

F fie ig ld.)7..Competition between E and Es (?E denotes the total electric
Besides,the thickness of the EDL is related to the concentration and valence of species in the solution,and evaluated by the Debye length
whereε=ε0εris the electric permittivity of the solution,ε0andεrare the vacuum and relative permittivities,respectively,kBis the Boltzmann constant,Tis the temperature,eis the electric charge,qiandCiare the charges and the ionic concentration of thei-th ion,respectively.It can be seen that increasing the concentration of species thinned the EDL, and theζpotential is also reduced,leading to a reduction ofEs.
Inλ-DNA solutions with monovalent metal ions (Na+,K+), more charged species are released after the break ofλ-DNA chains as the metal ions concentration increases.And the potential of the electrodes reduced,for the increase of species concentration,so doesEs.Therefore,the absolute value of theI0inλ-DNA-Na+andλ-DNA-K+solutions decreases with the concentration of metal ions.In contrast,inλ-DNA-Mg2+andλ-DNA-Ca2+solutions, the increase of Mg2+or Ca2+leads to a decrease of species in the solutions with shielding electric field strength increases.Hence, theI0inλ-DNAMg2+andλ-DNA-Ca2+solutions gets more negative as metal ions concentration increases.On the other hand, the external field is weaker than the shielding electric fieldEsin the initial stage.However, as the applied external voltage increases the external field gradually increases and overcomesEs.Therefore, the net potential difference between the electrodes decreases first and then increases with increasing voltage, and the conductivity of the mixed solution follows the same variation.
5.Conclusion
In order to reveal the effect of metal ions on the conductivity characteristics of DNA molecular solution as it passes through a microchannel,the conductivity–voltage relations ofλ-DNA solutions with different metal ions (different valent states and concentrations)are systematically investigated.
In the mixed solutions ofλ-DNA and metal ions,monovalent metal ions(Na+,K+)render the compression of DNA structure at low concentrations, which enhances the mobility of species in DNA solutions.As the concentration of Na+or K+increases, the break of DNA chains results in an increase in species concentration in the solution.All of that helps to improve the conductivity of theλ-DNA solutions.
On the other hand, divalent metal ions (Mg2+, Ca2+)mainly interact with DNA through coordinated water molecules to form a solvation shell.The adsorbed hydrated metal ions shield the charge of DNA molecules and reduce the repulsion force between them,which enhances the mobility of species when the concentration of metal ions is low, and the conductivity of the solution is improved.As divalent metal ions concentration increases, the mobility of charged species in the solution is reduced,for the reason of enlarged complex radius and effect of DNA charge reversion,thus the conductivity of theλ-DNA solutions is weakened.In a word, divalent metal ions strengthen the conductivity ofλ-DNA solutions at low concentrations,but weaken it at high concentrations.
This investigation is meaningful for designing electrophoresis-based DNA detection devices and improving the accuracy of detection and manipulation of a single biomacromolecule.
Acknowledgments
Project supported by the National Natural Science Foundation of China(Grant Nos.62275216 and 61775181),the Innovation Capability Support Program of Shaanxi Province of China(Grant Nos.S2018-ZC-TD-0061 and TZ0393),and the National Key Scientific Instrument and Equipment Development Projects of China(Grant No.51927804).
- Chinese Physics B的其它文章
- Optimal zero-crossing group selection method of the absolute gravimeter based on improved auto-regressive moving average model
- Deterministic remote preparation of multi-qubit equatorial states through dissipative channels
- Direct measurement of nonlocal quantum states without approximation
- Fast and perfect state transfer in superconducting circuit with tunable coupler
- A discrete Boltzmann model with symmetric velocity discretization for compressible flow
- Dynamic modelling and chaos control for a thin plate oscillator using Bubnov–Galerkin integral method

