Two-dimensional dumbbell silicene as a promising anode material for(Li/Na/K)-ion batteries
Man Liu(劉曼), Zishuang Cheng(程子爽), Xiaoming Zhang(張小明), Yefeng Li(李葉楓), Lei Jin(靳蕾),Cong Liu(劉叢), Xuefang Dai(代學芳), Ying Liu(劉影),§, Xiaotian Wang(王嘯天), and Guodong Liu(劉國棟),
1State Key Laboratory of Reliability and Intelligence of Electrical Equipment,and School of Materials Science and Engineering,Hebei University of Technology,Tianjin 300130,China
2School of Physical Science and Technology,Southwest University,Chongqing 400715,China
Keywords: dumbbell silicene,density functional theory,anode materials,ion batteries
1.Introduction
With the widespread use of mobile electronic devices and energy vehicles, there has been widespread interest in developing batteries with high capacity, long cycle life and excellent stability.[1,2]As a sustainable and clean energy source,rechargeable lithium-ion batteries (LIBs) are one of the most efficient portable and rechargeable energy devices in storage technology.[3–5]Although LIBs play a major role in the chemical energy storage market, current LIBs technology can no longer fully meet the growing demand for green energy due to the low lithium content in the earth’s crust,limited energy storage capacity, and high cost effectiveness.People are already desperately looking for other battery systems that can replace LIBs.Sodium ion batteries(SIBs)and potassium ion batteries(PIBs) are considered as reliable alternatives to LIBs due to their similar chemistry, abundant reserves and low cost.One of the hot research topics now is to develop good sodium and potassium ion batteries.[6–9]However, the current electrode materials have limited the further improvement of battery performances because of their poor electrical properties,mechanical properties and restricted working environment.Therefore,it is especially necessary to promote the research and development of new electrode materials of the next generation.[10,11]
Since 2004,when graphene was exfoliated from graphite by mechanical exfoliation,[12]the two-dimensional (2D) materials have become a hot research topic due to their unique structures and electrochemical properties.Owing to the advantages of high specific surface area, extensive ion embedding channels, low ion diffusion barrier and suppression of dendritic growth, 2D materials have been considered as very promising electrode materials for ion batteries.[13–18]With the continuous improvement of synthesis methods,various anode materials for ion batteries have been developed,like graphite,h-BN, silicon materials, TMDs, MXenes, and so forth.[19–32]However,current battery designs also face several challenges,such as better cycling performance,higher power density and energy density,and better safety.Therefore,there is an urgent demand for the development of anode materials with better performances.It is well known that elemental silicon, which is abundant and non-toxic, has been a hot topic of research.Thus,many researchers have turned their attention to 2D silicon materials.It has been reported that when silicon atoms are adsorbed on LB silicon,a more stable dumbbell-like structure is formed.[33,34]More importantly,the formation of dumbbell(DB)silicene on the Ag(110)surface has been experimentally confirmed.[35]
Based on the superior application prospects of silicene materials, we investigate the feasibility of DB silicene as an anode material for alkali metal ion batteries.In current work,based on the first-principles calculations,the stabilities of DB silicene structure are confirmed by phonon spectra andab initiomolecular dynamics simulations.The adsorption energies of six different adsorption sites are calculated to obtain the most stable adsorption sites for DB silicene.We recognize that the adsorption energies of all adsorption sites are negative,indicating that DB silicene is able to achieve stable Li,Na,and K adsorption, and considerable charge transfer is observed during this period.In addition,we investigate the migration paths and diffusion barrier energies of Li/Na/K ions on the monolayer.We find that the minimum diffusion barriers of Li, Na,and K ions are much lower than many classical 2D electrodes with high storage capacity.This paper also explores the capacity limit of this silicene material to accommodate ions,calculating the open circuit voltage and theoretical capacity.Finally,the paper also compares the calculation results of two different van der Waals correction methods,DFT-D3 and DFT-D2.All results indicate that two-dimensional DB silicene is a very promising candidate anode material for alkali metal ion batteries.
2.Computational details
In our work, we have performed the first-principles calculations using the Viennaab initiosimulation package(VASP)[36]based on the density functional theory (DFT).[37]For the exchange–correlation potential,we applied the generalized gradient approximation (GGA) of the Perdew–Burke–Ernzerhof (PBE) functional.[38,39]All atomic positions were fully relaxed during the calculations, and the cutoff energy,force and energy convergence criteria were chosen as 500 eV,0.01 eV·?A-1and 10-6eV,respectively.A vacuum space with a thickness of 20 ?A was built in the bare silicene to avoid interaction between two single monolayers.Moreover, we have taken into account the long-range van der Waals interactions in the calculations by using the DFT-D3 and DFT-D2 methods.[40,41]In the adsorption calculations, the Brillouin zone was sampled with a 5×5×1 Monkhorst–Packk-point mesh for the geometrical optimization and with a 7×7×1kmesh for electronic structure calculations.To investigate the dynamical stability of this silicene, the phonon spectra were calculated using the PHONOPY package.[42,43]Theab initiomolecular dynamics(AIMD)simulations using the canonical ensemble conditions (NVT) were carried out to evaluate the thermal stability of DB silicene.[44]The climbing-image nudged elastic band(CI-NEB)method was used to obtain the diffusion barrier height during the ion diffusion process.[45,46]
3.Results and discussion
3.1.Structure,stability and electronic properties of DB silicene
Figure 1(a)shows a schematic diagram of the optimized structure of the unit cell of DB silicene.The cell is a hexagonal lattice consisting of 10 Si atoms with two different types of Si,quadruple coordination and triple coordination,and whose space group isP6/mmm.After full geometrical optimization,the lattice constant is 7.43 ?A,the buckling height is 2.71 ?A,and the Si–Si bond length is about 2.35 ?A.Based on the optimized structure, we investigate the kinetic stability of DB silicene.The resulting phonon spectra are shown in Fig.1(b).Apparently,no imaginary frequency can be found in the phonon spectra, proving that DB silicene is kinetically stable.To determine its thermal stability,ab initiomolecular dynamics simulations(AIMD)are performed at temperatures of 300 K and 500 K.Figures 1(c)and S1 of supporting information show the variation of the total energy with the AIMD step at 300 K and 500 K.The structural stability is good within the narrow energy fluctuation range and no structural reconstruction occurs.The results of both phonon dispersion spectra and molecular dynamics calculations indicate that DB silicene has good stability.Figure 1(d)shows the band structure of DB silicene.It is a semiconductor with an indirect band gap of 0.25 eV,which is consistent with the previous work.[47]

Fig.1.(a)Top and side views of optimized structure of DB silicene.(b)Phonon spectra of DB silicene.(c)Total potential energy fluctuation of DB silicene at 300 K in AIMD simulations.(d)Band structure of DB silicene.
3.2.Adsorption of Li/Na/K ions on DB silicene
Higher density of active sites and suitable adsorption energies between ions and monolayer are the two main factors affecting the performances of electrode materials.Therefore,the adsorption energies of single Li/Na/K ions on DB silicene have been investigated in this paper.The calculation substrate is used a 2×2 supercell of DB silicene.To find their most stable adsorption sites, we consider six typical adsorption sites,as shown in Fig.2(a),which are denoted as sites S1–S6.Geometric relaxation shows that site S6 is not a stable adsorption site for either Li/Na/K ions and the adsorbed ions would migrate to other sites.Sites S3 and S5 are also unstable for K ions and the ions at these two sites would migrate smoothly to the vacant sites,which means that the vacant sites are very favorable for adsorption.We calculate the adsorption energy(Ead)by using
whereESi(DB)andELi/Na/K+Si(DB)are the total energies of Si(DB)before and after Li/Na/K adsorption.ELi/Na/Kis the energy per atom in the bulk Li/Na/K metal.The calculated results show that all adsorption energies are negative, with site S5 having the lowest adsorption energy for Li and Na and site S1 having the lowest adsorption energy for K.Specifically,the adsorption energies of the most stable adsorption sites are-0.838 eV for Li ions,-0.845 eV for Na ions,and-1.496 eV for K ions.Figure 2(b)shows the DOS of DB silicene before and after Li/Na/K ions adsorbed at the most stable sites to investigate the electronic structure changes.It can be found that after the adsorption of Li/Na/K ions by DB silicene,the system conductivity is enhanced and shows a metallic state,which can ensure good conductivity of the battery during operation.

Fig.2.(a)Six possible adsorption sites for Li/Na/K ions on the surface of DB silicene(S1–S6).(b)Density of states(DOS)of DB silicene before and after Li/Na/K adsorptions.
In order to gain insight into the interaction mechanism between Li/Na/K and DB silicene and to understand more clearly the adsorption process of metal ions on DB silicene, we investigated the charge density difference (CDD) of individual Li/Na/K ion adsorption on DB silicene.The equation for the charge density difference is defined as follows:
whereρLi/Na/K+Si(DB)denotes the charge density of the adsorption systems,ρSi(DB)denotes the charge density of DB silicene,andρLi/Na/Kdenotes the charge density of the Li/Na/K atoms.The calculated CDD maps of Li/Na/K ions adsorption at their most stable sites are shown in Fig.3.The blue and yellow areas represent the consumption and accumulation of charge, respectively.The presence of mostly blue areas above the Li/Na/K ions means that a large number of charge is transferred from the ions to the monolayer surface.Bader charge analysis shows that the transferred charge is 0.885e/atom/0.853e/atom/0.868e/atom for Li/Na/K,respectively, indicating that the ions are chemisorbed onto DB silicene surface.These results indicate that DB silicene can be highly feasible as electrode materials for ion batteries.
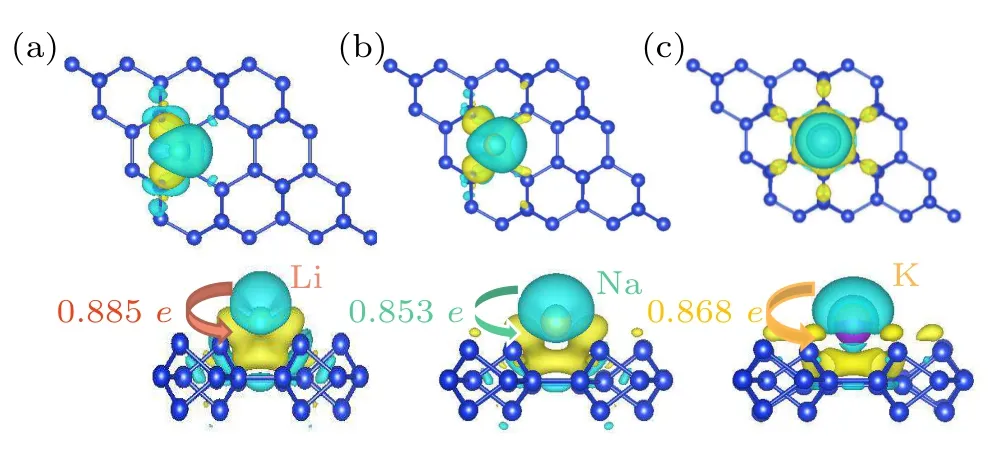
Fig.3.Maps of charge density difference of DB silicene with(a)Li,(b)Na,and(c)K adsorption as well as the amounts of charge transfer.
3.3.Diffusion of metal ions on the surface of DB silicene
It is well known that the ions diffusion potential on the surface of electrode materials is a key factor affecting the charging and discharging rates of batteries.On DB silicene surface, we analyze several possible paths for ions diffusion between the most stable adsorption sites,as shown in Fig.4(a).Considering the structural symmetry and the most stable adsorption sites, the four most probable Li/Na ions diffusion paths are chosen, which are named as P-a, P-b, P-c, and Pd.The two most probable K ion diffusion paths are selected,which are named as P-a and P-b.As shown in Fig.4(b),these optimal migration paths for Li/Na/K ions are P-b/P-c/Pa with the lowest diffusion potentials of 0.032 eV, 0.055 eV,and 0.21 eV, respectively.These values are better than or comparable to many other 2D electrode materials, such as Mo2P[48](0.050 eV for Li),GeS[49](0.236 eV for Li,0.090 eV for Na),MoN2[50](0.432 eV for Li,0.56 eV for Na,0.49 eV for K), MnSb2S4[51](0.28 eV for Li), GeP3[52](0.50 eV for Li), Ti2P[53](0.016 eV for Li, 0.012 eV for Na) and CP6[54](0.26 eV for K).The above results suggest that the Li/Na/K ions diffusion on the surface of DB silicene is highly desirable and imply that it is suitable material for ions diffusion with high charge/discharge rates.

Fig.4.(a) Potential Li/Na/K ions diffusion paths and (b) the corresponding ions diffusion profiles on DB silicene as well as the size of the minimum diffusion barriers.
3.4.Storage capacity and open circuit voltage
In practical applications, the ion storage capacity and open circuit voltage of electrode materials are the key parameters for evaluating the battery performances.Here,we still use a 2×2 supercell of DB silicene as substrate.To explore the maximum storage capacity of Li/Na/K ions on DB silicene,the ions are added group by group onto its both surfaces until the substrate could not adsorb more ions.We can use the average adsorption energy(Eave)to determine whether the adsorption limit has been reached,which is defined as follows:
whereELiXSi/NaXSi/KXSiare the total energies of the adsorption systems with different concentrations of Li/Na/K ions.TheX1andX2are the concentrations of adsorbed ions (X2>X1).ELi/Na/Kis consistent with that in Eq.(1).It is well known that when the average adsorption energy value is positive,it means that the substrate is not able to adsorb more ions.We consider the possible conformations at different adsorption concentrations and find the conformation that can be stabilized by calculating the adsorption energy, as shown in Fig.S2 of supporting information.Figures 5–7 show the most stable conformations of lithium ion, sodium ion and potassium ion at different concentrations, respectively.It can be found that the metal ions can be adsorbed on the upper and lower surfaces of DB silicene and almost no structural deformation occurs.From Fig.8, the highest Li/Na/K concentrations can reach 0.75, 0.65, and 0.75, respectively.Correspondingly, chemical compositions of DB silicene fully adsorbed Li/Na/K are Li0.75Si(DB),Na0.65Si(DB),and K0.75Si(DB),respectively.Therefore, the theoretical specific capacity (C) of DB silicene can be calculated by the following equation:
whereXmdenotes the maximum concentration of adsorbed metal ions,Fdenotes the Faraday constant(26801 mAh/mol),andMSi(DB)is the relative molecular mass of DB silicene.The calculated maximum theoretical specific capacities of Li,Na and K are 716 mAh/g, 622 mAh/g, and 716 mAh/g, respectively.These capacities are better than or comparable to many other 2D anode materials, as shown in Fig.9, such as MoN2[50](432 mAh/g for Li,864 mAh/g for Na,432 mAh/g for K), Mn2C[55](888 mAh/g for Li, 444 mAh/g for Na),VS2[56](466 mAh/g for Li), Ti3C2[57](320 mAh/g for Li,192 mAh/g for K), GaN[58](938 mAh/g for Li, 625 mAh/g for Na), TiS2[59](479 mAh/g for Na), Sr2N[60](283 mAh/g for Na), ReS2[61](428 mAh/g for Na), boron phosphide[62](570 mAh/g for K), Ti2CP2[63](711 mAh/g for Na/K),Ti2CSi2[63](327 mAh/g for Na/K), SiGe[64](532 mAh/g for K), VS2[65](466 mAh/g for K) and VO2[66](131 mAh/g for K).
It is well known that ion battery anode materials need relatively low OCV values, and these smaller average voltages can obtain higher energy density, effectively avoid the generation of metal dendrites, and ensure high stability and safety of batteries.Furthermore, the voltage range of 0.1 V–1 V is essentially preferred for anode materials and is considered to be the best form for maximum energy density.[67]
As for DB silicene, the typical half-cell reaction can be defined as follows:
In general,the open circuit voltage(OCV)of DB silicene can be calculated by the following equation:
whereESi(DB)andELi/Na/Kare consistent with that in Eq.(1),ELixSi/NaxSi/KxSiare the total energies of the systems after adsorption of Li/Na/K ions,andxis the number of adsorbed ions.As shown in Fig.8,it can be clearly seen that the OCV values are basically in our desired voltage range (0.1 V–1 V) when different numbers of Li/Na/K ions are adsorbed.Besides,the values of average open circuit voltages of LIBs/SIBs/PIBs are calculated to be 0.42 V, 0.41 V, and 0.60 V, respectively.Therefore, our results suggest DB silicene as anode material facilitates the high energy density of the batteries and reduces the formation of dendrites.
Finally, two different van der Waals correction methods,DFT-D3 and DFT-D2, are compared in this work.As shown in Table 1, the results of DFT-D2 are slightly different from those of DFT-D3.The Li/Na/K ions diffusion behaviors and the maximum ions adsorption systems obtained by DFT-D2 are shown in Figs.S3 and S4 of supporting information.One noteworthy point is that the DFT-D2 would overestimate the storage capacity of the electrode material,making the calculation falsely high.In addition,we find that the storage capacity calculation result in previous work(1002 mAh/g for Li)[68]of DB silicene using van der Waals correction with vDW-DF2 is in between.

Fig.5.Top and side views of DB silicene adsorbed with different concentrations of Li ions.

Fig.6.Top and side views of DB silicene adsorbed with different concentrations of Na ions.

Fig.7.Top and side views of DB silicene adsorbed with different concentrations of K ions.

Fig.8.The OCV and the average adsorption energy(Eave)for the interaction of(a)Li-ion,(b)Na-ion,and(c)K-ion on DB silicene as a function of Li/Na/K ions concentrations.

Fig.9.Comparison of the storage capacity and diffusion barrier between DB silicene and typical 2D anode materials,where panel(a)is for Li,panel(b)is for Na and panel(c)is for K.

Table 1.Adsorption sites, adsorption energies (Ead), diffusion barriers,and capacities(Cm)of dumbbell(DB)silicene as anode material for Li/Na/K ions batteries.
4.Conclusion
We have systematically investigated the performances of DB silicene as potential LIBs/NIBs/PIBs anode material by the first-principles calculations.The results show that Li/Na/K ions can be stably adsorbed on the surface of DB silicene.Besides, the DB silicene has excellent metallic conductivity after adsorption of metal ions,making it an ideal electrode material.More importantly, the diffusion results show that the lowest diffusion barriers are 32 meV for Li, 55 meV for Na,and 210 meV for K, which are comparable to or much lower than those of most high-capacity 2D electrode materials.The calculated OCV values are in the range of 0.34 V–0.59 V for Li, 0.38 V–0.48 V for Na, and 0.41 V–1.00 V for K.Finally,DB silicene has high ion storage capacities of 716 mAh/g for Li, 622 mAh/g for Na, and 716 mAh/g for K.Thus, the DB silicene is an excellent LIBs/NIBs/PIBs anode material with good electrical conductivity,high storage capacity and fast ion diffusion ability.
Acknowledgments
Project supported by the National Natural Science Foundation of China (Grant No.12274112), the Overseas Scientists Sponsorship Program of Hebei Province of China(Grant No.C20210330),and the State Key Laboratory of Reliability and Intelligence of Electrical Equipment of Hebei University of Technology(Grant No.EERI PI2020009).
- Chinese Physics B的其它文章
- Dynamic responses of an energy harvesting system based on piezoelectric and electromagnetic mechanisms under colored noise
- Intervention against information diffusion in static and temporal coupling networks
- Turing pattern selection for a plant–wrack model with cross-diffusion
- Quantum correlation enhanced bound of the information exclusion principle
- Floquet dynamical quantum phase transitions in transverse XY spin chains under periodic kickings
- Generalized uncertainty principle from long-range kernel effects:The case of the Hawking black hole temperature

