The HD-Zip transcription factor GhHB12 represses plant height by regulating the auxin signaling in cotton
LlU Yan,WANG Wei-ping,ZHANG Lin,ZHU Long-fu,ZHANG Xian-long,HE Xin
1 College of Agronomy,Hunan Agricultural University,Changsha 410128,P.R.China
2 National Key Laboratory of Crop Genetic Improvement,Huazhong Agricultural University,Wuhan 430070,P.R.China
Abstract Upland cotton (Gossypium hirsutum L.) is the most important natural textile fiber crop worldwide. Plant height (PH) is a significant component of plant architecture,strongly influencing crop cultivation patterns,overall yield,and economic coefficient. However,cotton genes regulating plant height have not been fully identified. Previously,an HD-Zip gene(GhHB12) was isolated and characterized in cotton,which regulates the abiotic and biotic stress responses and the growth and development processes. In this study,we showed that GhHB12 was induced by auxin. Moreover,overexpression of GhHB12 induces the expression of HY5,ATH1,and HAT4,represses the spatial-temporal distribution,polar transport,and signaling of auxin,alters the expression of genes involved in cell wall expansion,and restrains the plant height in cotton. These results suggest a role of GhHB12 in regulating cotton plant height,which could be achieved by affecting the auxin signaling and cell wall expansion.
Keywords: cotton,GhHB12,plant height,auxin,cell wall,HD-Zip
1.lntroduction
Upland cotton (GossypiumhirsutumL.) is an important economic crop worldwide and is the main source of renewable textile fibers (Zhanget al.2015;Fenget al.2022). With the advancement of agricultural modernization in China,the cotton cropping system is undergoing a fundamental change,and the cotton planting areas are reduced year by year and concentrated in Xinjiang Autonomous Region,which requires compacttype cotton cultivars (National Bureau of Statistics of China: https://data.stats.gov.cn/index.htm). Plant architecture is an important agronomic trait that strongly influences crop cultivation patterns,overall yield,and economic coefficient (Song and Zhang 2009). Plant height (PH) is a key factor affecting plant architecture and an important agronomic trait determining plant density and yield (Yanet al.2019). The moderate dwarfing of crops can significantly increase the overall photosynthate partitioning and harvesting index (Maet al.2019).
The discovery and application of dwarf and semi-dwarf genes (such as the wheatRhtand ricesd-1) have greatly improved crop yield and brought great economic benefits for agriculture,referred to as the first “Green Revolution”(Penget al.1999;Sasakiet al.2002;Udvardi and Scheible 2005). At present,a number of genes controlling plant height have been identified in rice and wheat (Piaoet al.2014;Sannemannet al.2018). Unfortunately,the germplasm resources of dwarf and semi-dwarf cotton in China are limited,and the corresponding research in this field has lagged behind. Only a few QTLs and genes controlling plant height have been identified in cotton (Song and Zhang 2009;Yanget al.2014;Shanget al.2015;Maet al.2019;Qanmberet al.2019;Yanet al.2019;Jiet al.2021;Heet al.2022),which seriously restricts the breeding of compact-type cotton cultivars in China.
Plant height is mostly determined by the elongation of stems,which is controlled by several hormones,such as auxin,gibberellin (GA),brassinosteroids (BRs),and strigolactones (SLs) (Silverstone and Sun 2000;Breweret al.2013;Gallavotti 2013;Wanget al.2018;Castorina and Consonni 2020). In plants,auxin stimulates cell elongationviaincreasing wall extensibility (Perrot-Rechenmann 2010;Majda and Robert 2018). The most abundant endogenous auxin is indole-3-acetic acid (IAA),which is involved in plant development and responses to the environment (Saueret al.2013). The intercellular auxin concentration gradient plays a crucial role in the regulation of auxin action,which is coordinately generated by polar transportviaauxin influx/efflux transportation and biosynthesis,inactivation,and degradation of auxin (Fukui and Hayashi 2018). At auxin concentration maxima in the tissue,auxin binds with the receptor transport inhibitor resistant 1/auxin signaling F-box (TIR1/AFB),which is the substrate-recognition subunit of the SCF E3 ubiquitin ligase complex and promotes the interaction between TIR1/AFB and auxin/indoleacetic acid (Aux/IAA) proteins,triggering ubiquitination and degradation of the Aux/IAA.The Aux/IAA repressors bind to auxin response factor(ARF) transcription factors and block the transcriptional activity of ARFs. Thus,the removal of Aux/IAA allows the transcription factors ARF to activate or repress early auxinresponsive gene transcription (Maet al.2018). As the receptor of auxin,TIR1/AFB is crucial for auxin perception,and the mutants of TIR1/AFB displayed dwarfism inArabidopsisand rice (Dharmasiriet al.2005;Guoet al.2021). Mutations in the degron motif of Aux/IAA proteins(blocking the degradation of Aux/IAA proteins) and overexpression of Aux/IAA proteins have been found to cause dwarfism in plants (Nagpalet al.2000;Liet al.2019,2022;Qanmberet al.2019). ARF proteins act as activators or repressors in auxin signaling,depending on the activation domain (AD) or repression domain (RD),and mutation or overexpression of ARF leads to dwarf phenotype in plants(Guilfoyle and Hagen 2007;Fuet al.2019). Additionally,the genes involved in auxin biosynthesis (YUCCA:YUC)(Yamamotoet al.2007;Kimet al.2013),polar transport(PIN-FORMED:PIN) (Chenet al.2012;Luet al.2015),and response (small auxin-up RNA:SAUR) (Chaeet al.2012;Xuet al.2017) usually regulate hypocotyl elongation and plant height.
Previously,an HD-ZIP gene (GhHB12) was isolated and characterized in cotton,which regulates the abiotic and biotic stress responses and the growth and development processes (Zhuet al.2011;Heet al.2018a,b;He Xet al.2020).GhHB12decreases drought and salt tolerance by negatively regulating the ABA signaling and stress-related genes (He Xet al.2020). Furthermore,GhHB12increases the susceptibility to fungal pathogens and herbivory insects by suppressing the JA signaling(Heet al.2018b). Additionally,GhHB12suppresses the expression ofGhFT,GhSOC1,andGhFUL,promotes the outgrowth of vegetative branches,inhibits the cotton fruit branch development,and delays flowering time by mediating the MiR157-SPLs pathway in cotton (Heet al.2018a). In this study,we showed thatGhHB12was induced by auxin,alters the auxin content,transport and transduction of auxin signaling,and the expression of genes involved in cell wall expansion,which subsequently restrains the plant height in cotton.
2.Materials and methods
2.1.Plant materials and growth conditions
The Upland cotton (Gossypiumhirsutum) cultivar YZ1 was used as the transgenic receptor and wild type (WT). TheGhHB12-overexpression and RNA-interference (RNAi)cotton lines were generated and reported previously (Heet al.2018a). The pGhHB12::GUS(GhHB12promoter drivesGUS) and DR5::GUS (a monitor for auxin response level) transgenic cotton lines were generated and reported previously (Liu Net al.2017;Heet al.2018b).OE37×DR5::GUS (OE37-DR5) and WT×DR5::GUS (WTDR5) were F1plants generated by DR5::GUS transgenic line crossed withGhHB12-overexpressing (OE37) and WT cotton lines,respectively. WT and transgenic lines were planted in the plant growth chamber maintained at 25°C under 16 h light/8 h dark conditions. They were then planted in a greenhouse and experimental field at Huazhong Agricultural University (Wuhan,China;30.4°N,114.2°E).
2.2.lAA treatment and expression analysis
Two-week-old YZ1 seedlings were planted in the plant growth chamber (25°C under 16 h light/8 h dark conditions) and sprayed 25 μmol L-1IAA. The leaves were harvested at different time points after treatment for RNA extraction. Total RNAs were extracted using the TIANGEN Total RNA Extraction Kit (DP441,TIANGEN,Beijing,China). The cDNA was synthesized using Superscript III Reverse Transcriptase (Invitrogen,San Diego,USA). TheGhUBQ7(GenBank: DQ116441) gene fromG.hirsutumwas used as the endogenous reference gene in RT-PCR and qRT-PCR analysis. qRT-PCR was performed using the 7500 Real-Time PCR System (ABI,Foster City,USA) with SYBR Green (Bio-Rad,USA). The relative transcript level was determined and normalized using the reference level and averaged over the three technical replicates.The primers used in this study are listed in Appendix A.
2.3.Quantification of endogenous lAA
The first leaves from the top and the axillary buds of 60-day-old WT andGhHB12transgenic cotton lines growing in the greenhouse were pulverized by liquid nitrogen,and about 100 mg fresh weight per sample was used to extract IAA. The extraction and quantification of endogenous IAA were performed according to a previous report (Liuet al.2012).
2.4.Sensitivity analysis of GhHB12 transgenic cotton lines to lAA treatment
Seeds of WT andGhHB12transgenic cotton lines were sterilized and geminated on 1/2 MS medium for 3 days,then transplanted on 1/2 MS medium either with or without IAA (10 μmol L-1) addition for 7 days and photographed.
2.5.Histochemical assay of β-glucuronidase (GUS)activity
For IAA-induced expression assay,the pGhHB12::GUStransgenic cotton seeds were geminated in doubledistilled water (ddH2O) for 3 days,treated with or without 10 μmol L-1IAA for 6 h,and then infiltrated with GUS staining solution at 37°C for 12 h. The GUS staining solution was composed of 0.9 g L-15-bromo-4-chloro-3-indolylglucuronide,50 mmol L-1sodium phosphate buffer (pH 7.0),20% (v/v) methanol,and 100 mg L-1chloromycetin. The stained seedlings were rinsed three times with 75% ethanol and then photographed with a camera (Nikon,Tokyo,Japan).
For attenuating auxin signaling assay,seeds of WT,OE37×DR5::GUS,and WT×DR5::GUS were sterilized and geminated on 1/2 MS medium for 3 days,transplanted on 1/2 MS medium either with or without IAA (10 μmol L-1) addition for 7 days under dark condition,and then infiltrated with GUS staining solution.
For attenuating auxin polar transport assay,the segments of hypocotyls of OE37×DR5::GUS and WT×DR5::GUS were positioned horizontally,vertically (the basal side of segments were inserted in the medium),and inversely (the apical side of segments were inserted in the medium) on the 1/2 MS medium with 0.5 mg L-1IBA for 24 h,and then infiltrated with GUS staining solution.
2.6.Analysis of RNA-sequence data
The first leaves from the top and shoot apices of three-leaf stage seedlings of YZ1 (WT) andGhHB12-overexpression cotton lines growing in the greenhouse were sampled for RNA extraction. RNA libraries were generated and sequencedviaIllumina HiSeqTM2000 at Beijing Genomics Institution. Then the raw reads were filtered into clean reads and mapped to the reference genome ofG.hirsutumL.acc.TM-1 (Zhanget al.2015).Gene expression levels (RPKM: reads per kb per million reads) were quantified. Differentially expressed genes between the WT andGhHB12-overexpression lines were screened (FDR≤0.001 and |log2Ratio|≥1).
3.Results
3.1.Overexpression of GhHB12 in cotton reduces plant height
Previously,the cotton HD-ZIP I subfamily transcription factor geneGh_A11G0906(namedGhHB12) was identified,which promotes the outgrowth of vegetative branches and delays reproductive developmentviathe GhmiR157-GhSPL pathway in cotton (Heet al.2018a).In this study,we found that over-expression ofGhHB12in cotton significantly reduces the hypocotyl length of seedlings (Fig.1-A and G) in the plant growth chamber and the height of plants in the greenhouse (Fig.1-B and H)and field (Fig.1-C,D and I). By contrast,RNAi-mediated suppression ofGhHB12significantly increases the height of seedlings and plants. Further analysis showed that there is no difference among the internode numbers of WT,GhHB12overexpression,and RNAi cotton lines,while shorter internodes of the main stem were observed inGhHB12-overexpression lines than in WT and RNAi lines(Fig.1-D and E). These results suggested thatGhHB12is a negative regulator in plant height by repressing the elongation of internodes of the main stem in cotton.
3.2.lnduction of GhHB12 by exogenous lAA
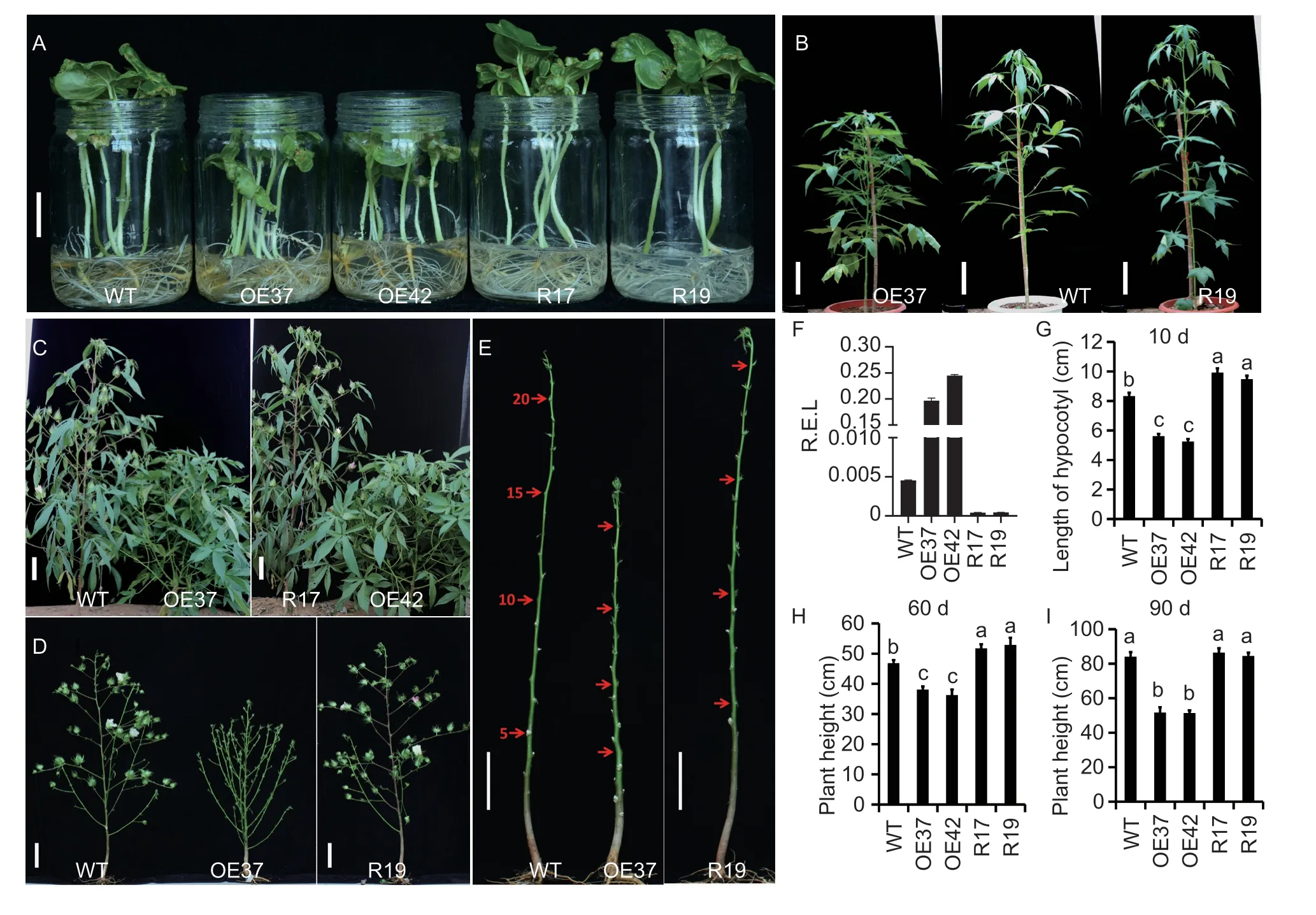
Fig. 1 Over-expression of GhHB12 represses plant height in cotton. A-C,photographs of WT (wild type) and GhHB12 transgenic cotton lines growing in the plant growth chamber,greenhouse,and field in Wuhan,China. D and E,plant architecture and main stem of WT and GhHB12 transgenic cotton lines growing in the field. Red arrow showed the notes (5th,10th,15th,and 20th)of the main stem of plants. Bars indicate 2 cm in A and 10 cm in B-E. F,relative expression level (R.E.L.) of GhHB12 in WT and GhHB12 transgenic cotton lines. The GhUBQ7 gene was used as the endogenous reference gene. The data represent the mean±SD of three technical replicates. G-I,comparison of the hypocotyl length and plant height among WT and GhHB12 transgenic cotton lines growing in the plant growth chamber,greenhouse,and field. Error bars represent the SD of 12-49 plants,and different letters indicate significant differences at P<0.05 (Duncan’s multiple range test).
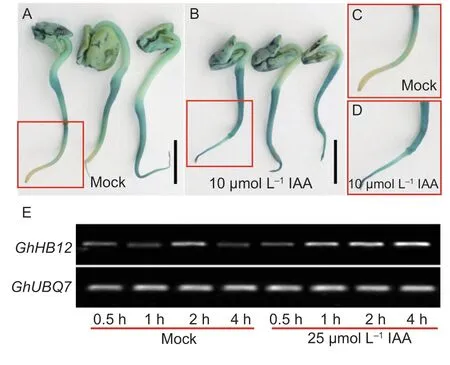
Fig. 2 Induction of GhHB12 by exogenous indole-3-acetic acid(IAA). A-D,GUS staining in the germinated pGhHB12::GUS transgenic cotton seedlings treated with (B and D) or without IAA (A and C). Bars indicate 1 cm in A and B. The size of C and D is 1.5 cm×1.5 cm. E,RT-PCR analysis of GhHB12 in YZ1 leaves treated with or without IAA. The GhUBQ7 gene was used as the endogenous reference gene.
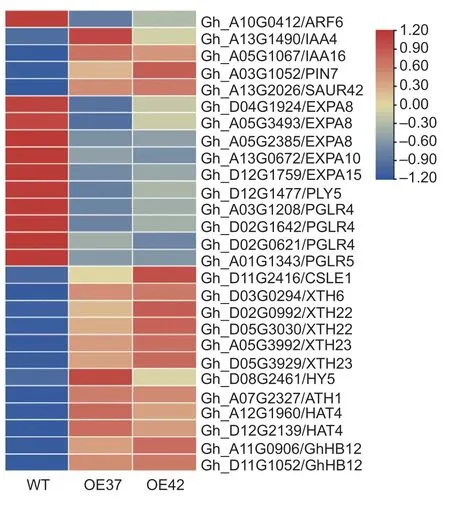
Fig. 3 Heat maps of differentially expressed genes (DEGs)involved in auxin signaling,shade avoidance,and cell wall expansion between wild type (WT) and GhHB12-overexpressing lines. ARF,auxin response factor;IAA,Aux/IAA protein;PIN,auxin efflux carrier family protein;SAUR,small auxin up RNA;EXPA,expansin A;PLY,pectate lyase;PGLR,polygalacturonase;CSLE,cellulose synthase-like protein E;XTH,xyloglucan endotransglycosylase;HY5,ELONGATED HYPOCOTYL 5;ATH1,Arabidopsis thaliana homeobox 1;HAT4,class II homeodomain-leucine zipper transcription factor ATHB2.
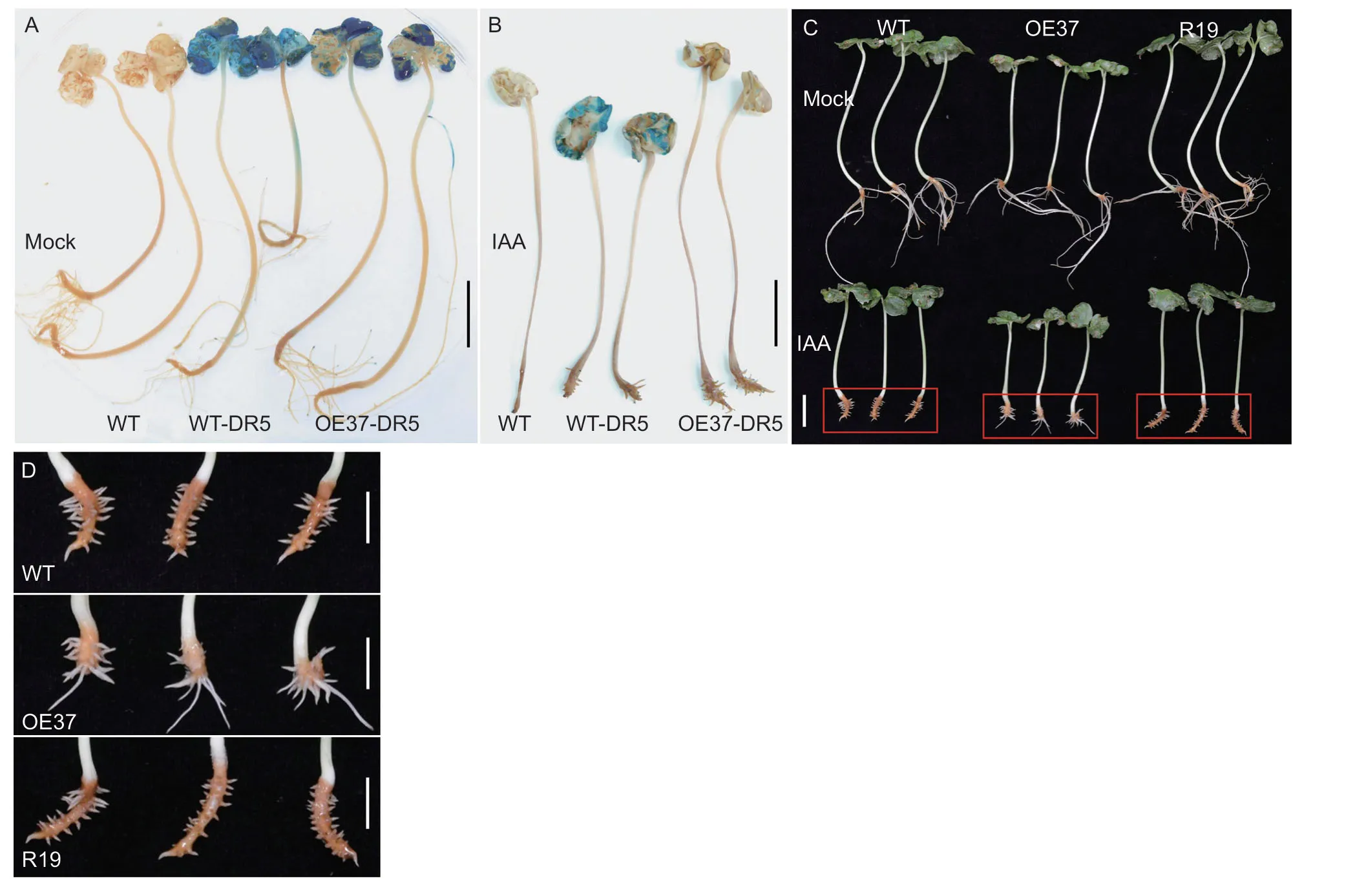
Fig. 4 GhHB12 represses the auxin signaling response. A and B,GUS staining in WT (wild type),WT-DR5 (hybrid plants of WT and DR5::GUS transgenic cotton lines),OE37-DR5 (hybrid plants of GhHB12-overexpressing lines and DR5::GUS transgenic cotton lines) treated without indole-3-acetic acid (IAA) (A) or with IAA (B). C and D,photographs of WT and GhHB12 transgenic cotton lines treated with IAA or without IAA. Bars indicate 2 cm in A-C;bars indicate 1 cm in D.
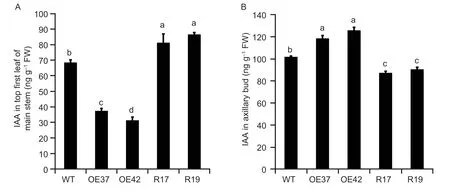
Fig. 5 GhHB12 attenuates the distribution of auxin. A and B,indole-3-acetic acid (IAA) contents in the top first leaf of the main stem (A) and axillary bud (B) of wild type (WT) and GhHB12 transgenic cotton lines. The data represent the mean±SE of three independent biological replications,and different letters indicate significant differences at P<0.05 (Duncan’s multiple range test).
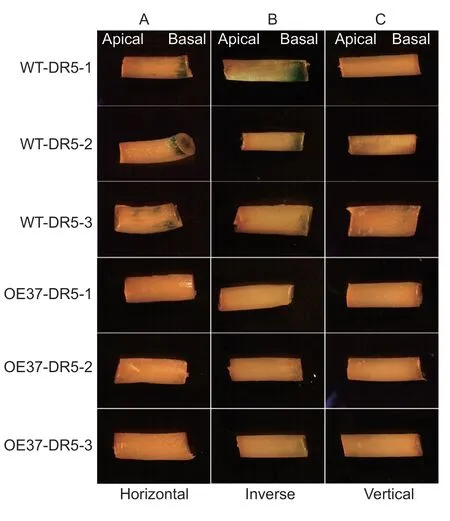
Fig. 6 GhHB12 represses the auxin signaling response. A-C,histochemical localization of GUS activity in the hypocotyl segments of three WT-DR5 (-1,-2,-3) and three OE37-DR5 (-1,-2,-3) seedlings horizontally (A),inversely (B,the apical side of segments insert in the medium) and vertically (C,the basal side of segments insert in the medium) on the 1/2 MS medium with indole-3-butyric acid (IBA) for 24 h.
To investigate whetherGhHB12was regulated by auxin,we performed theGhHB12expression level assays in cotton leaves after exogenous IAA treatments and analyzed the GUS expression inpGhHB12::GUStransgenic cotton geminated seeds treated with IAA. As shown in Fig.2,GhHB12was induced by exogenous IAA treatment.
3.3.Over-expression of GhHB12 attenuates the expression of genes involved in light signaling,auxin signaling,and cell wall expansion
To explore how over-expressingGhHB12reduces plant height,we performed RNA sequencing to identify differentially expressed genes between the over-expression line (OE37 and OE42) and WT. A total of 665 differentially expressed genes (DEGs) were identified,of which 442 and 223 genes were up-regulated and down-regulated in over-expression lines,respectively (Appendix B). Among the DEGs,there are a series of genes involved in auxin signaling,shade avoidance,and cell wall expansion,such as auxin response factor 6 (ARF6),Aux/IAA protein 4/16(IAA4/16),auxin efflux carrier family protein 7 (PIN7),small auxin up RNA 42 (SAUR42),ELONGATED HYPOCOTYL 5(HY5),Arabidopsisthalianahomeobox 1 (ATH1),class II homeodomain-leucine zipper transcription factor 2(HAT4/ATHB2),expansin-A8/10/15 (EXPA8/10/15),pectate lyase 5 (PLY5),polygalacturonase 4/5 (PGLR4/5),cellulose synthase-like E1 (CSLE1),and xyloglucan endotransglycosylase 6/22/23 (XTH6/22/23) (Fig.3). All the results suggested thatGhHB12may attenuate auxinmediated cell expansion signaling in cotton.
3.4.GhHB12 represses auxin signaling response
To verify the auxin signal difference between WT andGhHB12-overexpression cotton lines,we first used the plants transfected with the DR5::GUS vector (a monitor for auxin response level) to crossGhHB12-overexpressing(OE37) and WT cotton lines,respectively. GUS staining in cotyledons was weaker in OE37×DR5::GUS (OE37-DR5) than that in WT×DR5::GUS (WT-DR5),with or without IAA treatments (Fig.4-A and B).
To further determine whetherGhHB12attenuates the sensitivity to auxin,auxin-mediated inhibition of root growth was compared betweenGhHB12transgenic and WT cotton lines. The results showed that overexpression ofGhHB12significantly promoted root growth under 10 μmol L-1IAA treatment conditions compared with WT plants (Fig.4-C and D). All the results indicated that the auxin signaling response was repressed byGhHB12in cotton.
3.5.GhHB12 attenuates the distribution of auxin
To further verify the auxin concentrations difference between WT andGhHB12transgenic cotton lines,we first determined the content of free IAA in the top first leaf on the main stem and axillary bud.GhHB12-overexpressing cotton lines showed lower IAA content compared with WT lines,whileGhHB12-RNAi lines showed higher IAA content than the WT plants in the top first leaf on the main stem (Fig.5-A).On the contrary,GhHB12-overexpressing cotton lines showed higher IAA content compared with WT lines in the axillary bud (Fig.5-B). It indicated thatGhHB12attenuates the spatial-temporal distribution of auxin in cotton.
3.6.GhHB12 attenuates the polar transport of auxin
To investigate whether the polar transport of auxin was affected byGhHB12,we positioned the hypocotyl segments of WT-DR5 and OE37-DR5 horizontally,vertically (the basal side of segment insert in the medium) and inversely(the apical side of segment insert in the medium) on the 1/2 MS medium with IBA for 24 h. GUS staining was only observed in the basal side of the hypocotyl segment when positioned on 1/2 MS medium horizontally and inversely,while it is lesser in the hypocotyl segments of OE37-DR5 than in WT-DR5 (Fig.6-A and B). Nevertheless,little or no GUS staining was observed in both the basal and apical sides of the hypocotyl segment when positioned on 1/2 MS medium vertically (Fig.6-C). It indicated thatGhHB12inhibited the polar transport of auxin.
4.Discussion
4.1.GhHB12 negatively regulates cotton plant height by repressing the auxin signal transduction
Plant height (PH) is an important agronomic trait affectingcrop yield and mechanical harvesting (Maet al.2019;Yanet al.2019). Therefore,developing compact-type cultivars with semi-dwarf plant height is critical in cotton breeding.To date,a good few QTLs controlling plant height have been identified in cotton (Song and Zhang 2009;Shanget al.2015;Maet al.2019),but only a few genes were identified and verified their function in cotton plant height(Yanget al.2014;Qanmberet al.2019;Jiet al.2021).In previous studies,an HD-Zip gene (GhHB12),which regulates the abiotic and biotic stress responses,growth and development processes,was isolated and identified in cotton (Zhuet al.2011;Heet al.2018a,b;He Xet al.2020). In this study,we confirmed the role ofGhHB12in regulating cotton plant height,which could be achieved by
affecting the auxin signaling and cell wall expansion.
Plant height is controlled by multiple phytohormones(such as auxin,BRs,GAs,and SLs) and associated complex regulatory networks (Silverstone and Sun 2000;Breweret al.2013;Gallavotti 2013;Wanget al.2018;Castorina and Consonni 2020). A large number of studies have shown that auxin promotes cell elongation by increasing cell wall extensibility (Perrot-Rechenmann 2010;Majda and Robert 2018) and the defect of auxin biosynthesis,perception,transport,and signaling transduction usually leads to dwarf and semi-dwarf phenotype in plants (Nagpalet al.2000;Dharmasiriet al.2005;Chaeet al.2012). In the present study,overexpression ofGhHB12in cotton resulted in a shorter hypocotyl and main stem than the WT (Fig.1);in contrast,down-regulation ofGhHB12by RNAi resulted in opposite effects (Fig.1),indicating thatGhHB12had a negative effect on the elongation of hypocotyl and main stem in cotton. RNA sequencing analysis showed that the key genes involved in auxin signaling and cell wall expansion were altered inGhHB12-overexpression cotton lines,includingARF6,IAA4/16,PIN7,SAUR42,EXPA8/10/15,PLY5,PGLR4/5,CSLE1,andXTH6/22/23(Fig.3;Appendix B). Additionally,the reduction of auxin signaling response (Fig.4) and polar transport (Fig.6)inGhHB12-overexpression cotton lines were visualized by a DR5::GUS reporter. Therefore,it is reasonable to hypothesize thatGhHB12negatively regulates cotton cell expansion and internode elongation by repressing the auxin signal transduction.
4.2.The auxin-mediated dwarf architecture in GhHB12-overexpression lines is independent of miR157-SPL signaling in cotton
A previous study found thatGhHB12directly interacted withGhSPL10 andGhSPL13(Heet al.2018a).MiR156/157-SPL is one of the most conserved regulating pathways and modulates auxin signaling in the plant kingdom. Over expression of miR156/157 usually causes bushy and dwarf architecture in many plants (Wanget al.2009;Jiaoet al.2010;Miuraet al.2010;Fuet al.2012;Wanget al.2015;Liu Jet al.2017;Daiet al.2018;Sunet al.2019). Like most miR156/157s of high plant species,ectopic expressionGhmiR157inArabidopsiscause a bushy and dwarf architecture,delaying flowering time and causing smaller floral organ size (Liu Net al.2017). Unexpectedly,the overexpression of GhmiR157 slightly delayed the flowering time and increased plant height in cotton,though it caused bushy architecture(only increased the number of vegetative branches)and smaller floral organ size (Liu Net al.2017;Heet al.2018a). This indicates that GhmiR157 promotes the outgrowth of vegetative branches independent of the reduction of apical dominance. Co-expression ofGhHB12and GhmiR157 dramatically increases the vegetative branches,decreases the fruit branches,and delays the flowering time (Heet al.2018a),indicating that the promotion of vegetative branches inGhHB12-overexpression lines is dependent on miR157-SPL signaling. But,the auxin-mediated dwarf architecture inGhHB12-overexpression lines is independent of miR157-SPL signaling in cotton.
4.3.The auxin-mediated dwarf architecture in Gh-HB12-overexpression lines maybe dependent of HY5,ATH1,and HAT4 in cotton
Among the DEGs between WT andGhHB12-overexpression cotton lines,the key transcriptional factors(TFs) involved light signaling and shade avoidance (HY5,ATH1,andHAT4/ATHB2) were induced inGhHB12-overexpression lines (Fig.3). HY5 is a basic leucine zipper (bZIP) transcription factor that inhibits hypocotyl growth and promotes photomorphogenesis in light,while COP1 (CONSTITUTIVELY PHOTOMORPHOGENIC1)mediates the degradation of HY5viathe 26S proteasome system to promote skotomorphogenesis in darkness (Anget al.1998).hy5mutant exhibits longer hypocotyl than WTArabidopsisseedlings under light conditions (Anget al.1998;Chattopadhyayet al.1998).ATH1is a lightregulated homeobox gene and is repressed by COP1 in etiolated seedlings,overexpressing ofATH1inhibits stem length inArabidopsis(Quaedvlieget al.1995;Gomez-Mena and Sablowski 2008).HAT4/ATHB2is an early auxin-responsive and light-regulated HD-Zip II subfamily gene that repressesArabidopsisplant height (He Get al.2020). We suspect thatGhHB12directly or indirectly inducesHY5,ATH1,andHAT4to affect auxin signaling,cell wall formation,or cell wall extension to repress the cotton height. Hence,further investigation of the possible relationships betweenGhHB12and HY5,ATH1,and HAT4 will expand our understanding of the biological functions ofGhHB12in cotton plant height.
5.Conclusion
This study demonstrated that the cotton HD-Zip geneGhHB12was induced by auxin,and overexpression ofGhHB12induces the expression ofHY5,ATH1,andHAT4,represses the spatial-temporal distribution,polar transport,and signaling of auxin,alters the expression of genes involved in cell wall expansion,and restrains the plant height in cotton. Taken together,our findings revealed thatGhHB12is an essential negative regulator in cotton plant height and provide novel insight into the regulatory network for plant architecture in cotton.
Acknowledgements
This work was supported by the Science and Technology Innovation Program of Hunan Province,China(2020RC2057). We thank Prof.Xu Jian (Department of Biological Science,National University of Singapore)for kindly providing the DR5 promoter. We also thank Liu Hongbo and Li Dongqin (National Key Laboratory of Crop Genetic Improvement,Huazhong Agricultural University) for their assistance with the determination of IAA content.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendicesassociated with this paper are available on https://doi.org/10.1016/j.jia.2022.09.022
 Journal of Integrative Agriculture2023年7期
Journal of Integrative Agriculture2023年7期
- Journal of Integrative Agriculture的其它文章
- Understanding changes in volatile compounds and fatty acids of Jincheng orange peel oil at different growth stages using GC-MS
- Untargeted UHPLC-Q-Exactive-MS-based metabolomics reveals associations between pre-and post-cooked metabolites and the taste quality of geographical indication rice and regular rice
- A double-layer model for improving the estimation of wheat canopy nitrogen content from unmanned aerial vehicle multispectral imagery
- The potential of green manure to increase soil carbon sequestration and reduce the yield-scaled carbon footprint of rice production in southern China
- lmprovement of soil fertility and rice yield after long-term application of cow manure combined with inorganic fertilizers
- A novel short transcript isoform of chicken lRF7 negatively regulates interferon-β production
