Subconjunctival conbercept for the treatment of corneal neovascularization
Cun Sun, Fang Ruan, Shang Li, Jian-Qiang Zhang, Ying Jie
1Department of Ophthalmology, Beijing Huimin Hospital,Beijing 100053, China
2Department of Ophthalmology, Beijing You’an Hospital,Capital Medical University, Beijing 100054, China
3Beijing Institute of Ophthalmology, Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University,Beijing Ophthalmology and Visual Sciences Key Lab, Beijing 100730, China
Abstract● AIM: To investigate the effectiveness and safety of subconjunctival injection of conbercept in the treatment of corneal neovascularization (CNV).
● KEYWORDS: corneal neovascularization; conbercept;anti-vascular endothelial growth factor; subconjunctival injection
INTRODUCTION
Corneal neovascularization (CNV) is the in-growth of new immature vasculature from the limbus into the avascular corneal tissue, which could lead to the opacification of the transparent cornea.As a highly prevalent cause of blindness in the world, CNV affects over 1.4 million people annually,12% of whom suffer subsequent vision loss[1-2].To date,the treatment of CNV includes local use of glucocorticoids and non-steroid anti-inflammatory medications, laser photocoagulation, fine needle diathermy, photodynamic therapy, and conjunctival, limbal, and amniotic membrane transplantation, all with however suboptimal effectiveness[1,3].One potential explanation for the suboptimal effectiveness might be that none of these treatments targets the molecular mediators of angiogenesis.
Recently, inhibitors of vascular endothelial growth factor(VEGF), which is the most important regulatory factor for angiogenesis, have shown good clinical application prospects and been approved for the treatment of neovascular fundus abnormalities[4].Existing pre-clinical and clinical research has shown the effectiveness of subconjunctival or corneal stromal injection of different anti-VEGF agents (e.g., bevacizumab,ranibizumab, and aflibercept) in the treatment of CNV[5-6].Conbercept (KH902; Chengdu Kanghong Biotech Co., China)is a recombinant fusion protein with key domains 2, 3, and 4 from VEGF receptors 1 and 2.Ⅰt has a high affinity for all VEGF isoforms as well as placental growth factors[7].In 2013,it was approved in China for the treatment of neovascular(wet) age-related macular degeneration and choroidal neovascularization secondary to pathological myopia[7].Whether conbercept could be used in the treatment of CNV is however largely unknown.The present study aimed thereforeto understand the effectiveness and safety of conberceptviasubconjunctival injection in modulating the characteristics of CNV.
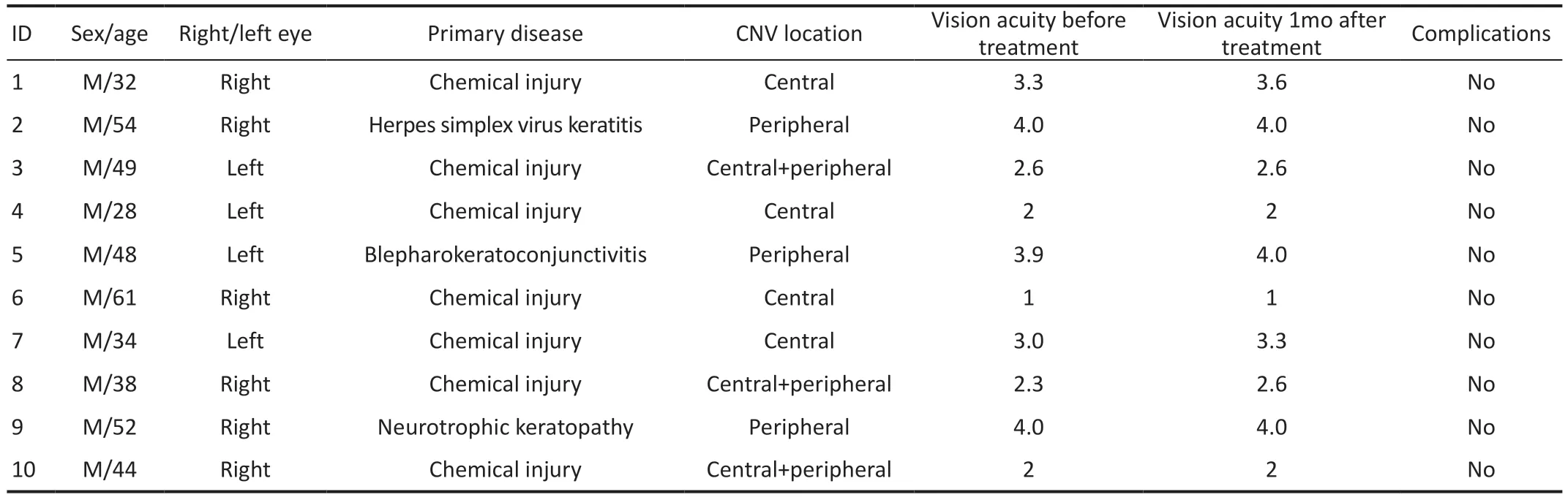
Table 1 Clinical characteristics of the 10 patients with corneal neovascularization
SUBJECTS AND METHODS
Ethical ApprovalThis study was additionally approved by the biomedical research ethics committee of Beijing Huimin Hospital (No.2018.1012).All recruited patients gave their written informed consent before their inclusion as participants in this study.
Study ParticipantsThis is a case series study, including 10 patients with CNV who were consecutively recruited during December 2018-November 2019 in Beijing Huimin Hospital,Beijing, China.
Ⅰnclusion criteria: age ≥18y, clinical diagnosis of CNV without active eye diseases, and neovascularization more than 2 mm into the corneal limbus.
Exclusion criteria: corneal trauma, infection, or ophthalmic surgery during the past month; use of contact lenses during the past month; or severe systemic diseases.
TreatmentLevofloxacin eye drops were applied four times a day for three days before the injection of conbercept.On the day of injection, we used proparacaine hydrochloride eye drops as anesthesia, iodophor solution to sterilize the skin around the eyes, and povidone-iodine solution to sterilize the conjunctival sac.We injected 0.1 mL of conbercept(10 mg/mL) into the corneoscleral limbus near the root of the new blood vessels.All injections were administered by the same ophthalmologist (Sun C).
Study OutcomesVisual acuity and area, length, and diameter of neovascularization at five different time points (before treatment,1d, 1, 2wk, and 1mo after treatment), as well as systemic and ocular complications after treatment, were documented.Corneal images were taken at all five time points, using an anterior segment slit-lamp microscope at the magnification required to delineate the fine details of the vessels.
Data AnalysisWe used Image pro plus 6.0 to measure the area, length, and diameter of neovascularization.Area was calculated by using the following formula: area(mm2)=CN/12×3.1416×[R2?(R?VL)2], where CN is the clock hours of neovascularization, R is the radius of the cornea, and VL is the longest vessel length, extending from the limbal vasculature[8].In terms of length, we identified three blood vessels with the greatest length in the densest quadrant of CNV and measured the greatest distance between the starting point of each vessel at the corneal limbus and anywhere along the vessel.We then calculated the mean value of the length of these three vessels as the measured length.In terms of diameter, we measured the diameters of all new blood vessels at the corneal limbus and calculated the average value as the diameter.
Statistical AnalysisStatistical analysis was performed using SPSS 19.0.Shapiro-Wilk method was used to test the normality of data, and one-way repeated measure analysis of variance was used to compare data across the five time points.We also performed within-individual analysis to study the temporal trend of different parameters across the time points.Data are reported as mean±standard deviation (SD).AP<0.05 was considered statistically significant.
RESULTS
Clinical CharacteristicsAmong the 10 patients with CNV,all were male, with a mean age of 44±10.15y (Table 1).
The mean±SD area of neovascularization was 42.46±12.80 mm2before treatment, and there was a statistically significant difference between all the time points after treatment and the time point before treatment (allP<0.05, Table 2, Figure 1).There was also a clearly declining area across the four different time points after treatment (F=31.373,P<0.001).
The mean±SD length of neovascularization was 4.64±1.77 mm before treatment and there was a statistically significant difference between the length measured at 1, 2wk, as well as 1mo after treatment and the length measured before treatment(allP<0.01; Table 2, Figure 1).There was also a declining trend of length across the four time points after treatment(F=19.652,P<0.001).

Figure 1 Outcomes of corneal neovascularization (CNV) parameters at five different time points A: CNV area; B: CNV length; C: CNV diameter.Compared with before treatment, aP<0.05, bP<0.01, cP<0.001.The significant therapeutic response which was evidenced as early as 1d, then peaked at 2wk, and reduced at 1mo.
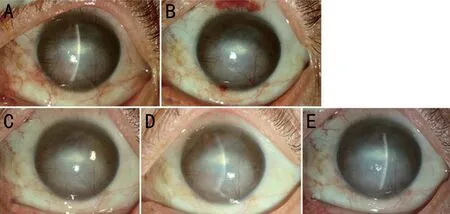
Figure 2 The effect of subconjunctival conbercept in patient 3, a 49-year-old man with chemical injury in the left eye complicated by corneal scarring, and neovascularization A: Baseline picture shows two main vessel branches emerging from the 12-o’clock and 5-o’clock position at the limbus and passing into the depressed scar in the corneal mid-periphery where it branched several times into smallercaliber vessels.B, C, D, E: 1d, 1, 2wk, and 1mo after subconjunctival conbercept.
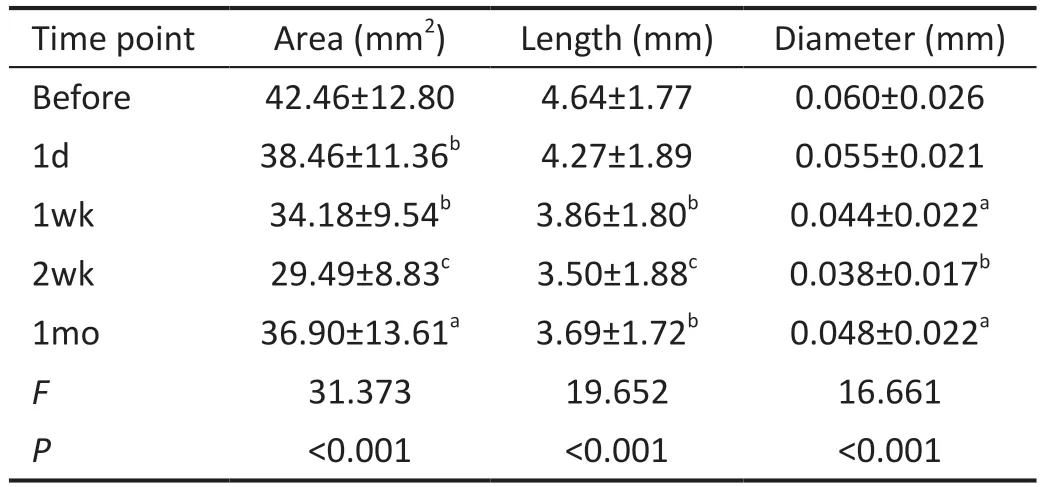
Table 2 Area, length, and diameter of neovascularization before and after subconjunctival conbercept
The mean±SD diameter of neovascularization was 0.060±0.026 mm before treatment and there was a statistically significant difference between the diameter measured at 1wk,2wk, as well as 1mo after treatment and the diameter measured before treatment (allP<0.05; Table 2, Figure 1).There was also a declining trend of diameter across the four time points after treatment (F=16.661,P<0.001).
The slit-lamp photographs of patient 3 were shown in Figure 2.
DISCUSSION
The formation of CNV is a complex process, involving vascular endothelial cells, keratinocytes, and inflammatory cells[9].Neovascular patterns can be separated into three groups, namely deep neovascularization overlying Descemet’s membrane (herpetic and luetic interstitial keratitis), stromal neovascularization (as a result of stromal keratitis), and vascular pannus (ocular surface disorders)[10].Multiple factors might contribute to the development of CNV.For instance,VEGF is the most important pro-angiogenic factor[2]and demonstrates a significantly increased concentration in the vascularized cornea[9].On the other hand, VEGF antagonists have been shown to reduce CNV in animal models[10], whereas anti-VEGF drugs have been shown to improve the survival rate of transplanted corneal grafts by restoring the “immune privilege”of the cornea[11].Subconjunctival bevacizumab,ranibizumab, and aflibercept crossed the blood and seemed to be effective in inhibiting CNV without causing epitheliopathy in an experimental rat model compared to the controls.No significant results were noted between these three anti-VEGF molecules, however, multiple-dose bevacizumab treatment seems to be more effective[11-12].
Several anti-VEGF agents are currently used in clinical offlabel for the treatment of CNV, and most of them appear to be a safe, effective, and practical method[5,13-16].In the first report of human use of topical bevacizumab for CNV, two patients showed significant reductions in superficial and deep stromal neovascularization without impairment in the corneal endothelium[17].Others have also shown that perilimbal and intrastromal injection of bevacizumab before penetrating keratoplasty can improve surgical outcomes of high-risk patients[18].Ranibizumab, another monoclonal antibody against VEGF-A, has also been used to reduce CNV in both animal models and clinical studies[19-20].Aflibercept (VEGF TrapR1R2) has strong anti-VEGF activity and is a soluble fusion protein with binding domains for both VEGF receptors 1 and 2.Subconjunctival injection of aflibercept can reduce donor vascularization and improve graft survival in a high-risk graft murine model[21].However, Sellaet al[22]showed that a single subconjunctival aflibercept injection was ineffective to regress formed CNV.
So far, the only topical antiangiogenic agent tested in phase II and III trials is aganirsen.Administration of topical aganirsen eye drops (86 μg/d per eye) in patients with keratitis related progressive CNV has been shown to lead to significant amelioration of CNV and reduced need for corneal transplantation[23].Although these agents are effective in the treatment of actively growing vessels, they have limited efficacy in well-established CNV[24].
Conbercept can both bind to placental growth factor and all isoforms of VEGF-A and VEGF-B[25].As it has multiple targets, strong affinity, and a long half-life cycle, conbercept has proved to be effective and safe in the treatment of diabetic macular edema[26].In cases of refractory macular edema secondary to central retinal vein occlusion (CRVO), after switching from bevacizumab/ranibizumab, conbercept can improve macular thickness and extend interval of injection[27].Duet al[28]confirmed that conbercept, especially early administration, can effectively inhibit CNV in rabbits, intrastromal administrations has the better effect than subconjunctival injection.
The present study used conbercept in patients with CNV and showed that the area of CNV reduced significantly already one day after treatment whereas the length and diameter of CNV reduced significantly one week after treatment.As the reduction in all three parameters peaked at two weeks after treatment, it might be beneficial to administer new injections to maintain or enhance effectiveness.For instance, although there were still significant reductions in all three indicators at one month after treatment, compared to the pre-treatment period,the magnitude of reductions diminished compared to two weeks after treatment.The observation time of this study was only 1mo, and it is speculated that with the extension of time(such as 3mo), the CNV may recur, suggesting the potential need of repeated treatments[15].Alternatively, for patients with stable CNV, transplantation of corneal graft might be considered at this time point, with the aim to achieve the best treatment effect for reducing neovascularization.
Although all patients in the present study demonstrated a reduction in CNV after treatment, the reduction varied in magnitude and none of the patients had a complete resolution.There are multiple potential explanations for the partial effectiveness.First, the dosage administered in the present treatment is not sufficient to completely antagonize the corneal VEGF burden.Second, we only injected conbercept once and this might have led to a low cumulative concentration of the medication.Third, cytokines other than VEGF, such as transforming growth factor-α, transforming growth factor-β1,and fibroblast growth factor, can also induce CNV[29]and are not targeted by conbercept.Because neovascularization likely involves multiple processes, targeting VEGF alone might prevent the pro-angiogenic cascade but not others.Finally,conbercept works mostly on newly formed blood vessels,whereas our case series included patients with CNV due to different reasons and patients with both newly formed and long-standing neovascularization.As a result, some of the patients included in this study had more severe CNV with more mature vessels.As discussed above, the limited number of treatments might have also affected the treatment effectiveness.Previous evidence suggests that VEGF inhibitors can influence corneal healing by interfering with macrophage infiltration[30].Soleimaniet al[31]reported two cases with Descemet membrane detachment after intrastromal bevacizumab injection in patients with previous lamellar keratoplasty.The hemorrhagic Descemet membrane detachment was not resolved, the donor had to exchange with a new one because of staining in one of the patients.We should emphasize that the complications were not related to bevacizumab, instead the injection method was involved in these situations.In this study, we injected conbercept in the corneoscleral limbus near the root of the CNV to avoid damaging the corneal tissue.The subconjunctival injection may be a safer method, and the medication can however diffuse in a short distance through the conjunctival tissue to the central blood vessels.There was no thinning of the corneal epithelium or stroma, injury in corneal epithelium, conjunctivitis, keratitis, endophthalmitis,cataract, or other complications, demonstrating the safety of subconjunctival conbercept.
In conclusion, subconjunctival conbercept may be an effective and safe option for the treatment of CNV, significantly reducing the area, length, and diameter of CNV without severe complications.Conbercept may also be considered an adjunct to other medical or surgical treatments of CNV.The use of conbercept for CNV is however still off-label.Further studies with a larger number of patients and longer followup are needed to validate our findings and to optimize the treatment regimen (e.g., number and timing of injections as well as injections versus other routes of administration).Comparison studies with other anti-VEGF agents are also helpful.
ACKNOWLEDGEMENTS
Foundation:Supported by the National Natural Science Foundation of China (No.81970764); the Youth Beijing Scholar (No.2020-022).
Conflicts of Interest:Sun C, None; Ruan F, None; Li S,None; Zhang JQ, None; Jie Y, None.
 International Journal of Ophthalmology2023年6期
International Journal of Ophthalmology2023年6期
- International Journal of Ophthalmology的其它文章
- Role of 7-methylxanthine in myopia prevention and control: a mini-review
- How internal limiting membrane peeling revolutionized macular surgery in the last three decades
- Photoreceptor changes in Leber hereditary optic neuropathy with m.G11778A mutation
- Efficacy and safety of subthreshold micropulse laser in the treatment of acute central serous chorioretinopathy
- Efficacy of ripasudil in reducing intraocular pressure and medication score for ocular hypertension with inflammation and corticosteroid
- Different serum levels of lgG and complements and recurrence rates in IgG4-positive and negative lacrimal gland benign lymphoepithelial lesion
