Herbicidal activity and biochemical characteristics of the botanical drupacine against Amaranthus retroflexus L.
YU Hua-long,TIAN Ci,SHEN Rong-yan,ZHAO Han,YANG Juan,DONG Jin-gao,,ZHANG Li-hui,#,MA Shu-jie,#
1 College of Plant Protection,Hebei Agricultural University,Baoding 071001,P.R.China
2 State Key Laboratory of North China Crop Improvement and Regulation,Baoding 071001,P.R.China
3 College of Agronomy and Biotechnology,Hebei Normal University of Science &Technology,Qinhuangdao 066004,P.R.China
Abstract Botanical herbicide has been a hot topic in the research and development of novel pesticides. The herbicidal activity and biochemical characteristics of the botanical compound drupacine were studied by evaluating its effects on seed germination,seedling growth,morphological and physiological characteristics of Amaranthus retroflexus. Drupacine inhibited seed germination and seedling growth,and had a median inhibition concentration (IC50) value of 38.99 mg L?1 against A.retroflexus root. The α-amylase activity and soluble sugar content in treated plants were significantly lower than that of the control. The expression of α-amylase gene was dosage-dependently inhibited compared to the untreated control. This suggested that inhibition of α-amylase activity was a mode of action on seed germination. The root hairs were significantly decreased and part of the root cap fell off after treatment with drupacine. The ultrastructure observation showed that cell damage of root tips increased with the treatment time. Drupacine also increased the relative conductivity and malondialdehyde (MDA) content. Peroxidase (POD),catalase (CAT),and superoxide dismutase (SOD) activities were significantly enhanced in the treatment compared to the control. These findings indicated that the physiological and biochemical reaction changes leading to morphological and membrane injuries were the main effects of drupacine on the inhibition of seedling growth. Drupacine can be developed as a botanical herbicide.
Keywords: drupacine,herbicidal activity,physiological characteristic,defense capacity,Amaranthus retroflexus
1.Introduction
AmaranthusretroflexusL.is one of the most prevailing agricultural weeds in maize and other autumn-crop fields in China (Buhleret al.2010;Duet al.2021) that causes substantial enormous economic losses in cereal crops (Knezevicet al.2010).Amaranthusretroflexuscompetitively reduces soybean yields especially at the commencement stage after sown (Benschet al.2010).In addition,A.retroflexusextract has allelopathic effects on cucumber and wheat,and this may be the main reason for crop yield loss (Bakhshayeshan-Agdamet al.2019). Chemical herbicides have been the major tools for controlling ofA.retroflexus(Huanget al.2019). However,excessive use of chemical herbicides has evoked weed resistance,environmental pollution and risks to human health (Wanget al.2017;Agostiniet al.2019;Westonet al.2019). High selective pressure caused by extensive applications of the protoporphyrinogen oxidase (PPO)-inhibiting herbicide fomesafen has causedA.retroflexusto evolve resistance to this herbicide (Duet al.2021). It has been also reported that the resistant population ofA.retroflexusexhibited 41.8-fold resistance to fomesafen compared to a susceptible population in Heilongjiang Province,China (Huanget al.2020).
Herbicidal compounds from plant extracts and their derivatives are possible alternatives to conventional chemical control of weeds because they are biodegradable,effective,ecologically acceptable,and may be less toxic to humans and other non-target organisms (Dukeet al.2019). The natural amine alkaloid sarmentosine is the main herbicidal compound isolated from the medicinal plantPipersarmentosumthat is toxic toA.retroflexus(Fenget al.2019). The herbicidal alkaloid berberine has been isolated fromCoptischinensisFranch,and has an inhibition ratio exceeding 90% againstBidenspilosa(Wuet al.2017).Herbicides based on natural products can also provide new target resources (Zhanget al.2017;Diaztielaset al.2019). The natural product sarmentine can inhibit enoyl-ACP reductase and photosynthesis by competing for the binding of plastoquinone on photosystem II (Dayanet al.2015). Pelargonic acid can cause rapid desiccation of plant foliage by removing the cuticular waxes that cover photosynthetic organs (Ciriminnaet al.2019). Thus,herbicidal compounds from botanical sources can play a role in the development of new agrochemicals and the exploitation of new targets (Liuet al.2016).
Drupacine has been extracted from the leaves and branches of the endemic Chinese speciesCephalotaxussinensis(Taxaceae) (Fig.1) (Zhaoet al.2019). Drupacine is highly toxic to the plant-parasitic nematodesMeloidogyneincognitaandBursaphelenchusxylophilus(Wenet al.2013). We previously confirmed that drupacine was the main herbicide-active compound ofC.sinensis,and demonstrated its inhibitory effect on young bud growth ofA.retroflexus,Trifoliumpretense,Loliumperenne,andSorghumsudanense(Maet al.2016a). In a pot experiment,drupacine inhibited fresh weights ofT.pretenseandA.retroflexusat levels greater than 70% (Maet al.2016b). However,systematic research on the herbicidal activity of drupacine has not been reported and the biochemical effects and mode of action of drupacine against weeds are unknown.

Fig.1 Chemical structure of drupacine.
In this study,we evaluated the herbicidal activity and biochemical characteristics of drupacine. We studied the inhibitory effect of drupacine on seed germination and seedling growth ofA.retroflexus,and its effect on the morphological and physiological characteristics ofA.retroflexusseedlings. The results of this research could provide new information for further investigation of the mode of action of drupacine againstA.retroflexusand other weeds.
2.Materials and methods
2.1.Weed seeds and chemicals
Mature seeds ofA.retroflexusL.were collected in October from the experimental farm of Hebei Agricultural University,Baoding City,Hebei Province,China. The seeds were rubbed gently with a cotton cloth to remove the peel and impurities and then stored in a refrigerator at 4°C.
Drupacine and the atrazine standard (>98% purity) were purchased from Aladdin Industrial Corporation (Shanghai,China).
2.2.Petri plate assay
Germination bioassayDrupacine was dissolved in dimethylsulfoxide (DMSO),and then diluted with 0.1% Tween 80 (Polysorbate 80,a hydrophilic nonionic surfactant) water solution to obtain 200,100,50,25 and 12.5 mg L?1concentrations. The maximum final concentration of DMSO was no greater than 1% to ensure that the DMSO did not interfere with the bioassay. Two sheets of filter paper (Whatman No.1)were placed in each Petri dish (9 cm diam.) and moistened with 5 mL of drupacine solutions. A Tween 80 aqueous solution treatment (0.1%) served as the control.Amaranthusretroflexusseeds were treated with 0.8% gibberellic acid to break dormancy,sterilized with 2% (w/v)sodium hypochlorite solution for 15 min,and rinsed 4 times in sterile distilled water. A completely randomized experimental design was used for the germination bioassay. Each Petri dish contained 20 seeds,and each treatment was replicated 4 times. There were 24 Petri dishes representing 6 treatments (5 drupacine concentrations+untreated control)×4 replicates. A total of 480 seeds were used including 20 seeds for each Petri dish×6 treatments (5 drupacine concentrations+untreated control)×4 replicates. The Petri dishes were placed in a growth chamber with controlled temperature ((25±2)°C),relative humidity (80%),and a 12 h:12 h (L:D) photoperiod. All the germinated and non-germinated seeds were counted daily for 7 d.
Seedling growth bioassayAfter-ripened seeds were incubated in deionized water in darkness at (25±2)°C for 24–48 h. The seeds were rinsed with deionized water and the germinated seeds were transferred onto 2 sheets of filter paper moistened with 5 mL of Tween 80 water solution containing 200,100,50,25,and 12.5 mg L–1drupacine. Tween 80 water solution treatments were used as the control. A completely randomized experimental design was used for the seedling growth bioassay. Each Petri dish contained 20 seeds,and each treatment was replicated four times for a total of 480 seeds. There were 20 seeds for each Petri dish×6 treatments (5 drupacine concentrations+untreated control)×4 replicates. The seeds were incubated in a growth chamber. After 3,5,and 7 d,seedling root and shoot lengths were measured. The inhibition rate and 50% inhibitory concentration (IC50) were calculated.
2.3.Pot culture assay
Pre-emergence and post-emergence herbicidal activities were tested using pot a culture assay (Maet al.2018). Drupacine was dissolved in DMSO,and then diluted with distilled water to obtain 4,2,and 1 g L–1concentration. After-ripened seeds were incubated in deionized water in darkness at (25±2)°C for 24–48 h.The germinated seeds were chosen for the pot culture assay. The sand was sterilized by dry heat and was then placed in plastic pots (10 cm depth). Then,20 germinatedA.retroflexusseeds were placed on top of the sand and they were then covered with 1 cm of additional sand. For pre-emergence herbicidal activity,drupacine solution (5 mL) was applied directly to the soil surface,using a laboratory spray bottle. To study post-emergence herbicidal activity,germinatedA.retroflexusseeds with 2–3 true leaves were sprayed with the drupacine solution (5 mL of each treatment).Atrazine (1 g L–1) was applied as the positive control.A completely randomized experimental design was used for this experiment. Each plastic pot contained 20 seeds,and each treatment was replicated 4 times for a total of 40 plastic pots. There were 2 methods (pre-emergence and post-emergence)×5 treatments (3 drupacine concentrations+untreated control+positive control)×4 replicates/treatment. A total of 800 seeds were needed: 20 seeds/pot×40 plastic pots. After treatment,the pots were placed in a greenhouse (20°C) where they received regular overhead watering.Amaranthusretroflexusplants were harvested 14 and 21 d after sowing and their fresh weights were recorded.
2.4.α-Amylase activity and gene expression,soluble sugar content,and total starch content assays
The α-amylase activity was tested following the method of Poonpaiboonpipatet al.(2013). TheA.retroflexusseeds (0.5 g) treated with drupacine (48 h) were put into a mortar and ground to a fine powder. Distilled water (3 mL) was added,extracted for 20 min,and then centrifuged at 8 000 r min?1for 20 min. The supernatant was used as the enzyme extract. The control tube (4 mL) contained 1 mL enzyme extract,1 mL 1% starch,and 2 mL DNS reagent (40 mmol L–13,5-dinitrosalicylic acid,1 mol L–1K–Na tartrate and 0.4 mol L–1NaOH),and incubated at 37°C for 5 min. The sample tube (2 mL) including 1 mL enzyme extract and 1 mL 1% starch was incubated at 37°C for 5 min and then terminated by adding 2 mL DNS reagent.The control and sample tubes were heated for 5 min,and immediately cooled. They were diluted with distilled water up to 20 mL. The absorbance values were measured with a spectrophotometer set at 540 nm wavelength. The amount of α-amylase present in the control and samples was calculated from the standard curve,which was prepared using maltose (Carlaet al.2012). The activity of α-amylase was expressed as mg min?1g?1. The concentration inhibition of α-amylase during the early growth of seedlings was also tested as described above.
In order to clarify whether drupacine inhibits α-amylase activity itself or its gene expression,resulting in the reduction of the enzyme formation,the relative expression of α-amylase mRNA ofA.retroflexusseeds and seedlings exposed to different concentrations of drupacine were assayed. Specific primer pairs were designed using Primer 5 and were synthesized by AuGCT Tech Co.in China,and GAPDH was selected for normalization. Total RNA was extracted by RNAprep Pure Plant Kit (TIANGEN,China). cDNA synthesis was performed using FastKing RT Kit with gDNase (TIANGEN,China) according to the manufacturer’s protocol. The relative expression of α-amylase mRNA was examined by Real-Time PCR Detection System (BIORAD,USA). The 2–ΔΔCtmethod (Livak and Schmittgen 2001) was used for data analysis.
After treatment with drupacine for 48 h,theA.retroflexusseeds were used for soluble sugar content and total starch content assays. Soluble sugar and total starch contents were determined using commercial kits (E-nitiate Biopharmaceuticals (Hangzhou) Co.,Ltd.,Hangzhou) according to manufacturer instructions. The experiment was repeated 3 times,and Tween 80 water solution treatments were used as controls.
2.5.Light microscopy
After treatment with drupacine for 3,5,and 7 d at concentrations of 0,50,and 100 mg L?1,the roots ofA.retroflexusseedlings were rinsed with distilled water and cut into 0.3–0.5 cm strips. The roots were dyed with hydrochloric acid-phenylene trihydroxybenzene solution for 15 s (Carmenet al.2002),and photographed under an Olympus CX21FS1 Microscope (Tokyo,Japan).
2.6.Relative conductivity assay
GerminatedA.retroflexusseeds were incubated in deionized water in darkness at (25±2)°C for 3–5 d untilA.retroflexusseedlings grew to about 2 cm. Then the seedlings were treated with 0,25,50 and 100 mg L?1drupacine for 3,6,12,and 24 h,respectively. Seedling samples (0.5 g) were suspended in 20 mL of distilled water and the initial conductivity was measured after 12 h. Then the seedling samples were boiled for 10 min to completely kill the tissues and the final conductivity was also determined. The experiment was repeated 3 times and the controls were treated with a Tween 80 water solution. The relative conductivity was calculated using the formula: Relative conductivity (%)=(Initial conductivity/Final conductivity)×100.
2.7.Assay of defense enzymes activities and malondialdehyde (MDA) content
GerminatedA.retroflexusseeds were incubated in drupacine solutions at 25,50 and 100 mg L?1for 72 h.Samples (0.2 g) were homogenized and suspended in 2 mL of 0.1 mol L–1PBS buffer,pH 7.0,and centrifuged at 12 000 r min?1for 20 min. The crude enzyme was used for the assay. Determination of peroxidase (POD) and superoxide dismutase (SOD) activities followed methods used by Carmenet al.(2002). Catalase (CAT) activity was determined using commercial kits (Shanghai) according to manufacturer instructions. MDA content was determined using the methods described by Liet al.(2013). The experiment was repeated 3 times and each treatment had 3 replicates. According to the above method,the activities of POD,CAT,SOD and MDA content of theA.retroflexusroot tip induced by 50 mg L–1drupacine for 0,3,6,12,and 24 h were also tested.
2.8.Scanning electron microscopy (SEM)
In order to clarify the physiological and biochemical reaction or the stability of the membrane occurs firstly,the cellular structural change ofA.retroflexusroot tip induced by drupacine. After treatment with drupacine for 3,6,12,24,72,and 120 h at concentrations of 50,and 100 mg L?1,the root tips were rinsed with distilled water and cut into 0.5 cm strips. The root tips were dried with filter paper and then dehydrated with an ethanol series (30,40,50,60,70,80,90,and 100%). The samples were processed with freeze drying technique by Bench Lyophilizer (SP Scientific,USA) for 4–6 h. The sputter coater (CRESSIN-GTON,UK) was also used for coating spraying gold 20–30 s,and observed under a Scanning Electron Microscopy (Thermo Fisher,USA).
2.9.Data analysis
The measurement data were expressed as mean± standard error. Data were transformedviathe deltamethod (Weisberg 2005). One-way analysis of variance (ANOVA) was performed using SPSS 18.0.Mean comparisons were performed using the Tukey test,and effects were considered significant forP<0.05. The dose-response model used was the method described by Onofriet al.(2010,2011),and the IC25,IC50,and IC75values were calculated.
3.Results
3.1.Effect of drupacine on seed germination and seedling growth
The effects of different concentrations of drupacine on seed germination are shown in Fig.2. The seed germination rate ofA.retroflexuswas delayed as exposure time increased and was reduced with increased drupacine concentration. The seed germination rate of the control group reached a maximum of 91.67% at 4 d. However,the seed germination was delayed at concentrations of 12.5 and 25 mg L?1,and reached its maximum of 83.33 and 80.00% at 5 and 6 d,respectively.At 4 d,germination rates were 91.67,70.00,58.33,25.00,16.67,and 5.00% at concentrations of 0 (control),12.5,25,50,100,and 200 mg L?1,respectively.

Fig.2 Germination of Amaranthus retroflexus in presence of different concentrations of drupacine. Data are mean±standard error (n=3).
Drupacine inhibited the growth ofA.retroflexusroots and shoots (Fig.3). The IC25,IC50,and IC75values were (25.89±0.93),(38.99±1.25),and (61.58±2.06) mg L?1againstA.retroflexusroots after treatment with drupacine for 3 d,respectively (Fig.3-A). Fig.3-B shows that the root and shoot lengths ofA.retroflexusseedlings became shorter with an increase of drupacine concentration.
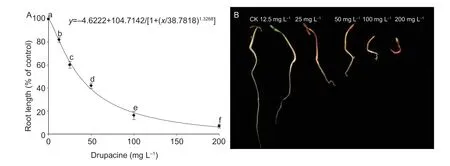
Fig.3 The herbicidal activity of drupacine against Amaranthus retroflexus. A,dose–response curve in A.retroflexus root length after treatment with drupacine for 3 d. Root length of A.retroflexus: observed proportions of seeds germinated relative to the total number of seeds at the beginning shown together with the fitted germination curves based on the inappropriate nonlinear regression model (solid line). For this approach,the four-parameter logistic model was used. B,effect of drupacine on root and shoot length of A.retroflexus seedlings. Data are mean±standard error (n=3). Values followed by different letters are significantly different at P<0.05 according to Tukey’s test.
3.2.Fresh weight inhibition effect in pot experiment
At 14 and 21 d after treatment,the 4 g L–1drupacine concentration showed 87.45 and 85.26% pre-emergence inhibition againstA.retroflexuswhich was equivalent to the effects of atrazine at 1 g L–1(Table 1). Postemergence herbicidal activity of drupacine (4 g L–1) was also potent,with inhibition rates of 47.50 and 51.35% againstA.retroflexus,respectively. This inhibition was lower than the inhibition provided by atrazine (1 g L–1).
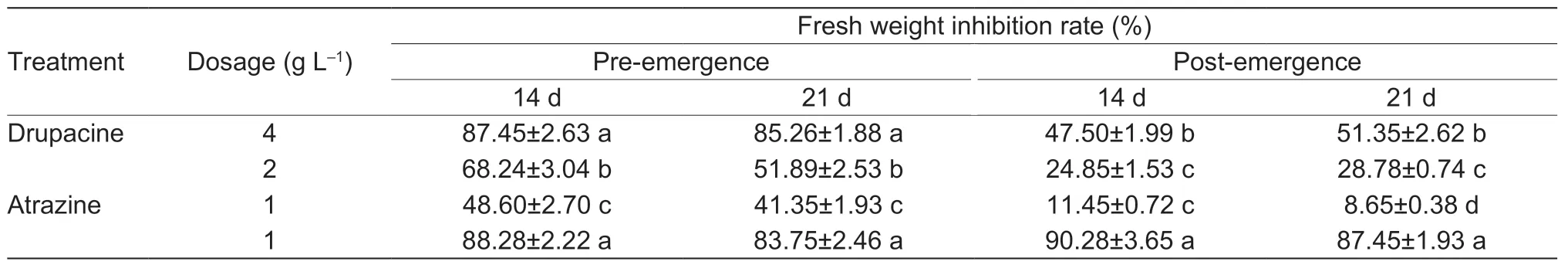
Table 1 Fresh weight inhibition effect of drupacine against Amaranthus retroflexus in pot experiment
3.3.Effects on α-amylase activity and gene expression,soluble sugar content and total starch content
Fig.4-A shows the effect of different concentrations of drupacine on the α-amylase activity ofA.retroflexusseeds. The activity of α-amylase was reduced as the concentration of drupacine increased. The α-amylase activity ofA.retroflexusseeds was 1.09 mg min?1g?1after treatment with 200 mg L?1drupacine;this value was much lower than that of the control. The relative expression of α-amylase mRNA ofA.retroflexusseeds was also reduced as the concentration of drupacine increased (Fig.4-B),which is consistent with enzyme activity. As for the growth inhibition of roots during the early growth of seedlings,α-amylase activity was is reduced to 0.06 mg min?1g?1and the gene expression was downregulated after treatment with 200 mg L?1drupacine (Fig.4-C and D).
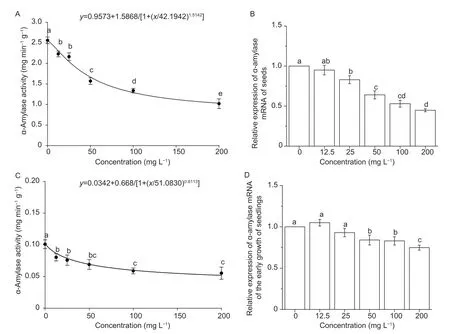
Fig.4 Effect of different concentrations of drupacine on α-amylase activity and relative expression of α-amylase mRNA of Amaranthus retroflexus seeds (A and B) and seedlings (C and D). Data are mean±standard error (n=3). Values followed by different letters are significantly different at P<0.05 according to Tukey’s test.
The effects of different concentrations of drupacine on soluble sugar and total starch contents ofA.retroflexusseeds are shown in Fig.5. The soluble sugar contents were 31.45,30.47,28.38,26.88,and 24.47 mg g?1after treatment with concentrations of 12.5,25,50,100,and 200 mg L?1drupacine,respectively. The total starch content was increased as the concentration increased when compared with the change of soluble sugar content (Fig.5-A). The total starch content was 42.50 mg g?1at a drupacine concentration of 200 mg L?1,which was higher than that of the control (Fig.5-B).
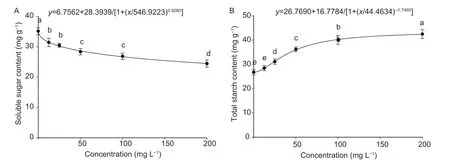
Fig.5 Effect of different concentrations of drupacine on soluble sugar content (A) and total starch content (B) of Amaranthus retroflexus seeds. Data are mean±standard error (n=3). Values followed by different small letters are significantly different at P<0.05 according to Tukey’s test.
3.4.Effect on seedling morphology
The roots ofA.retroflexusseedlings treated with drupacine were observed under a light microscope (Fig.6). In the control group,well-characterized differentiation,elongation,and maturation zones were observed and the root hairs were regularly arranged (Fig.6-A,D and G). In contrast,the root hairs were reduced and shortened after drupacine treatment.These changes were more obvious as the concentration increased (Fig.6-B and C),and increasing exposure time (Fig.6-B,E and H). After 7 d of treatment with 100 mg L?1drupacine,there was no specific division pattern and obvious boundary in differentiation,elongation,and maturation zones. The root hairs were not observed and part of the root cap had fallen off (Fig.6-I).
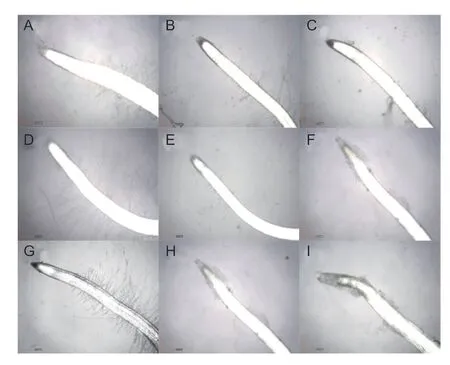
Fig.6 Effects of drupacine on the root morphological characteristics of Amaranthus retroflexus seedlings. A,D and G,control pictures taken at 3,5 and 7 d post treatment,respectively. B,E and H,the root morphological changes after the treatment of drupacine (50 mg L–1) at 3,5,and 7 d,respectively. C,F and I,the root morphological changes after the treatment of drupacine (100 mg L–1) at 3,5,and 7 d,respectively. Scale bar: 5.0 mm.
3.5.Effect on cell membrane permeability
The relative conductivity changed with increased concentration and increased exposure time was also tested (Fig.7). At 50 and 100 mg L?1concentrations of drupacine the relative conductivity was increased as exposure time increased. However,the relative conductivity was significantly changed at a concentration of 25 mg L?1. The relative conductivity was 49.92% after treatment with 100 mg L?1drupacine for 24 h,which was much higher than that of the control. These results suggest that the cell membrane can be damaged by drupacine and lead to the release of intracellular electrolytes inA.retroflexusseedlings.
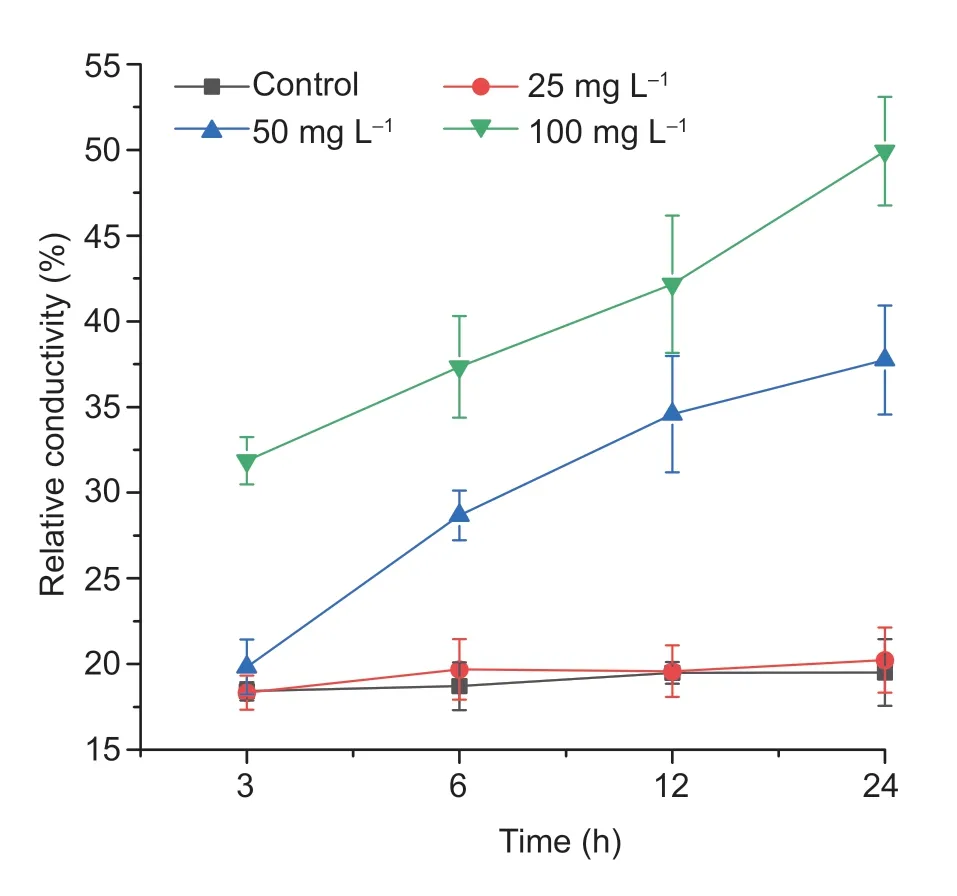
Fig.7 The relative conductivity of Amaranthus retroflexus seedlings with drupacine treatments at concentrations of 25,50 and 100 mg L–1. Data are mean±standard error (n=3).
3.6.Effect on defense enzymes activities and MDA content
The activities of POD,CAT,and SOD of theA.retroflexusseedlings treatment with different concentrations of drupacine are presented in Fig.8. All of the test indexes of treatment groups were enhanced in comparison with the control group. The activities of POD were increased to 1 679.11,2 577.60,and 1 849.39 U g?1min?1after treatment with 25,50 and 100 mg L?1,respectively. These were higher than the control value (1 143.48 U g?1min?1).The CAT activity was the highest at the IC50concentration and a 95.47% increase was calculated (Fig.8-B). For SOD activity,the highest enzyme activity occurred at 25 mg L?1which had a 102.63% increase compared to the control (Fig.8-C). The MDA content increased as the concentration of drupacine increased (Fig.8-D).
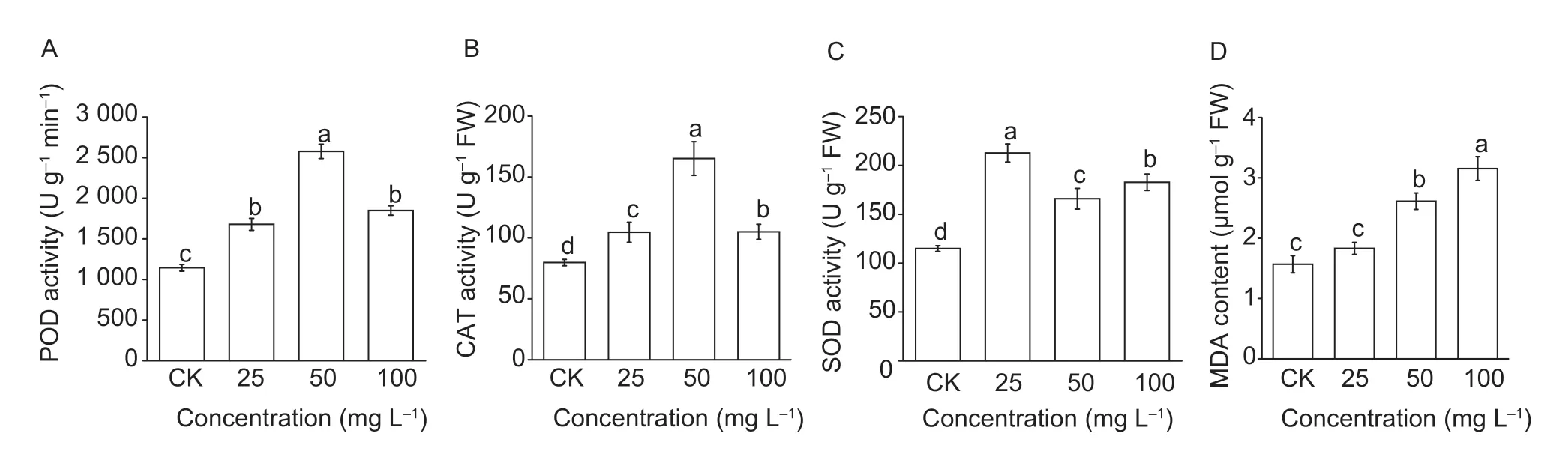
Fig.8 The activities of peroxidase (POD,A),catalase (CAT,B),suporoxide dismutase (SOD,C) and malondialdehyde (MDA) content (D) of the Amaranthus retroflexus seedlings with drupacine treatments at concentrations of 25,50 and 100 mg L–1. Data are mean±standard error (n=3) and the values followed by different small letters are significantly different at P<0.05 according to Tukey’s test.
The time ordered biochemical test ofA.retroflexusroot tip induced by drupacine are shown in Fig.9. After 3,6,12 and 24 h of treatment with drupacine,the activities of POD and SOD were significantly changed,and the maximum increases were observed after 12 h of treatment,which had 48.82 and 27.70% increases compared to the control,respectively (Fig.9-A and C).The activities of CAT were not significantly changed after 3 and 6 h treatment,and significantly increased after 12 and 24 h of treatment (Fig.9-B). The MDA content was not changed with elongated treated time within 12 h,and increased 33.76% after 24 h of treatment (Fig.9-D).
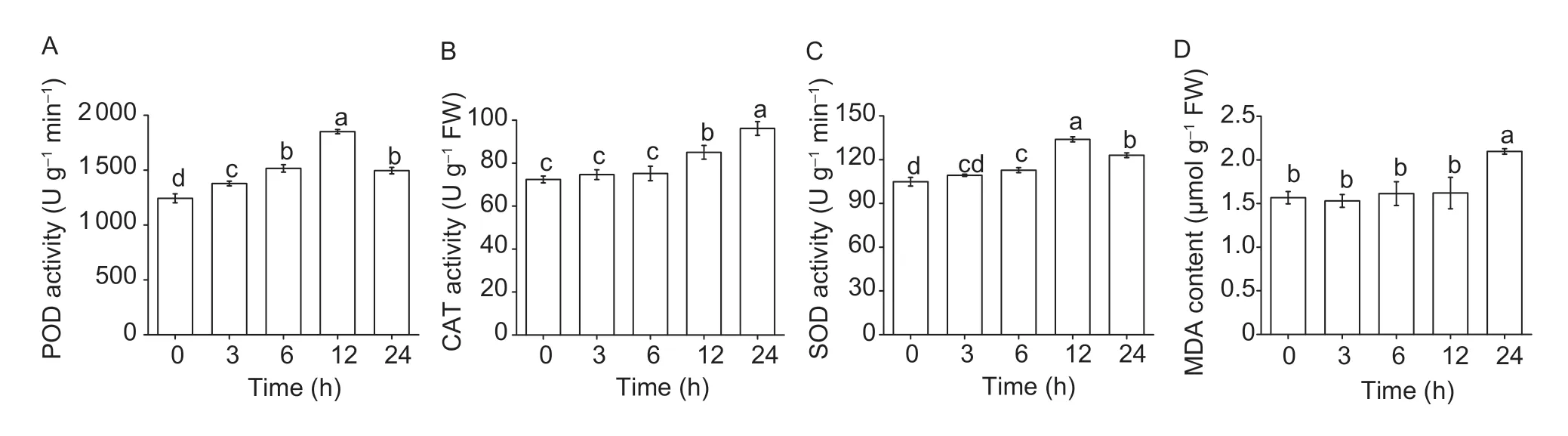
Fig.9 The activities of peroxidase (POD,A),catalase (CAT,B),suporoxide dismutase (SOD,C),and malondialdehyde (MDA) content (D) of the Amaranthus retroflexus root tip induced by 50 mg L–1 drupacine at different time points. Data are mean±standard error (n=3) and the values followed by different small letters are significantly different at P<0.05 according to Tukey’s test.
3.7.Effect on cellular structure of root tip
The cellular structural change ofA.retroflexusroot tip induced by 50 and 100 mg L–1drupacine at different times is shown in Fig.10. In the control group,cells arranged in regular and uniform and full cells were observed. In contrast,cellular structure of root tip was not significantly changed with elongated treated time within 24 h. After 72 h of treatment with 50 and 100 mg L?1drupacine,the cells were irregular and disorder arrangement. With the extension of treatment time,the cell damage became more serious. And the cellular structure of root tip was is severely damaged after 120 h of treatment with 100 mg L?1drupacine.
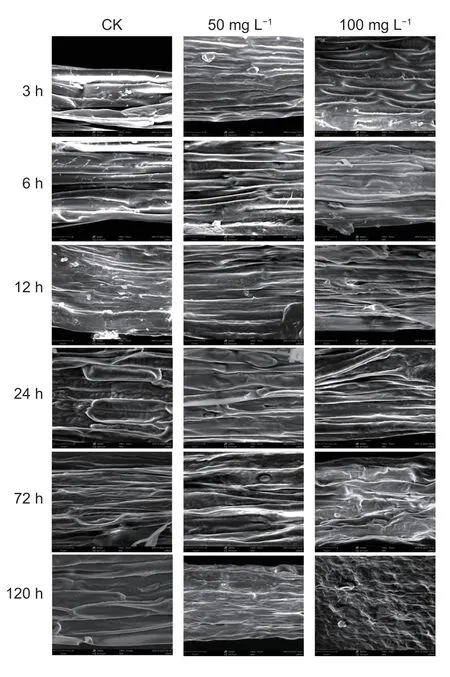
Fig.10 The cellular structural change of Amaranthus retroflexus root tip induced by 50 and 100 mg L–1 drupacine at different time points.
4.Discussion
The natural compound drupacine exhibited significant inhibitory effects on both seed germination and seedling growth ofA.retroflexus. Many other herbicidal natural compounds have been reported previously. For example,lantadene A and B fromLantanacamarainhibited germination and seedling growth ofBidenspilosa(Gindriet al.2019). The botanical compound norharmane had an inhibitory effect on the germination ofAvenafatuaL.,and reduced the growth ofPlantagolanceolataL.andA.retroflexus(Davidet al.2020). Three flavonoids fromCynaracardunculushad a significant phytotoxic effect onTrifoliumincarnatum(Kaabet al.2019). In the current study,the inhibition rate of seed germination exceeded 80% after 4 d of drupacine-treatment and the IC50was only (38.99±1.25) mg L?1againstA.retroflexusroots after treatment with drupacine for 3 d. Meanwhile,the preemergence herbicidal activity of drupacine toA.retroflexuswas better than that of post-emergence in pot culture assay,which may be because drupacine mainly affect the process of seed germination (Maet al.2018). In consideration of the long-term use ofC.sinensisas a Chinese herbal medicine (Saeedet al.2008;Panet al.2010),the main herbicide-active compound drupacine is expected to be environmentally-friendly. Thus,drupacine has the potential to be developed into a natural herbicide to controlA.retroflexus,and potentially other weeds as well.
The α-amylase activity was analyzed to understand the characteristics of drupacine effects on inhibition ofA.retroflexusseed germination. The degradation of reserve carbohydrates to soluble sugars is mostly dependent on α-amylase,which is important for seed germination. In this study,the α-amylase activity was significantly decreased after treatment with drupacine,which was consistent with the change of gene expression. It indicates that drupacine inhibits its gene expression,resulting in the reduction of the enzyme formation. In addition,the soluble sugar and total starch contents were also changed,suggesting that α-amylase cannot normally convert starch into soluble sugar. Our study correlated herbicidal activity of natural herbicides with their α-amylase inhibitory effect. The essential oil fromCymbopogoncitratushad a significant inhibitory effect on α-amylase (Poonpaiboonpipatet al.2013).The inhibiting effect of fusilade on α-amylase activity of lentil seeds was reported (Hisashi and Francisco 2005).The compound 6-methoxy-2-benzox/azolinone also significantly decreased the α-amylase activity in lettuce seeds. These data indicate that α-amylase plays an important role in the inhibition of seed germination by drupacine.
The physiological and biochemical reaction changes leading to morphological and membrane injuries were the main effects of drupacine on the inhibition of the seedling growth ofA.retroflexus. Root hairs are tubular protrusions that can significantly increase the interaction between the plant and the soil (Jungket al.2001). Normal root morphology is important for the growth of plant seedlings (Stamp and Kiel 1992). However,there was no specific division pattern and obvious boundary in differentiation,elongation,and maturation zones after treatment with drupacine. However,the root hairs were decreased in number,and part of the root cap fell off. These results were consistent with the natural compoundtranschalcone,which can induce programmed cell death inArabidopsisthalianaroots (Carlaet al.2012). In addition,the relative conductivity was significantly increased,which suggested that the membrane integrity was disrupted by drupacine leading to solute leakage. The change of membrane permeability,in turn,affects lipid peroxidation (Jonaset al.2016). Interestingly,the MDA content was increased with increasing concentrations of drupacine,meaning that lipid peroxidation had occurred. Reactive oxygen species (ROS) can induce membrane injury and lipid peroxidation (Liuet al.2007). Antioxidant enzymes such as POD,CAT,and SOD are important for suppressing oxidative stress to remove excess ROS (Velikovaet al.2000). In the present study,POD,CAT,and SOD activities were significantly enhanced in comparison with the control,which is earlier occurs before the cellular structure damage,indicating that drupacine could interfere with the balance of antioxidant defensive enzymes leading to membrane injury. In consideration of the environmentally-friendly,high-activity,and unique structure of drupacine,it would be necessary to elucidate the molecular herbicidal mechanism in subsequent studies.
5.Conclusion
In summary,the natural compound drupacine had significant inhibitory effects on both seed germination and seedling growth ofA.retroflexus. The inhibition of α-amylase activity was one of the mechanisms by which drupacine inhibited seed germination. Morphological changes and membrane injury were the main effects of drupacine on the inhibition of seedling growth. Further studies are needed to understand the detailed mode of action of drupacine againstA.retroflexusbefore we can conclude that drupacine can be developed as a biological herbicide.
Acknowledgements
This research was funded by the Provincial Natural Science Foundation of Hebei for Excellent Young Scholar,China (C2021204071),the Science and Technology Project of Hebei Education Department (QN2021079),the Key Research and Development Project of Hebei Province (21326511D and 19226504D),and the China Agriculture Research System of MOF and MARA (CARS-02).
Declaration of competing interest
The authors declare that they have no conflict of interest.
 Journal of Integrative Agriculture2023年5期
Journal of Integrative Agriculture2023年5期
- Journal of Integrative Agriculture的其它文章
- Developing a duplex ARMS-qPCR method to differentiate genotype l and ll African swine fever viruses based on their B646L genes
- The effects of maltodextrin/starch in soy protein isolate–wheat gluten on the thermal stability of high-moisture extrudates
- Elucidation of the structure, antioxidant, and interfacial properties of flaxseed proteins tailored by microwave treatment
- Effects of planting patterns plastic film mulching on soil temperature, moisture, functional bacteria and yield of winter wheat in the Loess Plateau of China
- lnversion tillage with straw incorporation affects the patterns of soil microbial co-occurrence and multi-nutrient cycling in a Hapli-Udic Cambisol
- The effects of co-utilizing green manure and rice straw on soil aggregates and soil carbon stability in a paddy soil in southern China
