Serum anti-blood type IgG/IgM binding and cytotoxicity to pig PBMC and RBC
CHEN Liang-jie, FENG Hao, DU Jia-xiang, LI Tao, XIA Qiang-bing, JIANG Hong-tao,PAN Deng-ke, CHEN Gang, WANG Yi
1.Department of Organ Transplantation, The Second Affiliated Hospital of Hainan Medical University; The Transplantation Institute of Hainan Medical University, Haikou 570105, China
2.Key Laboratory of Organ Transplantation, Ministry of Education; NHC Key Laboratory of Organ Transplantation; Key Laboratory of Organ Transplantation, Chinese Academy of Medical Sciences, Wuhan 430030, China
3.Chengdu Clonorgan Biotechnology Co., Ltd., Chengdu 610072, China
4.Institute of Organ Transplantation, Sichuan Academy of Medical Sciences, Sichuan Provincial People’s Hospital, Chengdu 610072, China
Keywords:
ABSTRACT
1.Introduction
The shortage of donor organs seriously affects the lives of patients with terminal renal organ diseases, and xenotransplantation is one of the effective ways to solve this problem.Pigs are considered to be the best donors for xenotransplantation at present.[1,2]The size and physiological characteristics of pig organs are similar to those of human beings.[3] With the development of special genetic engineering technology, genetically modified pigs can reduce the combination of human IgM and IgG with pig cells.[4].The transplantation of genetically modified pig organs into non-human primates, NHPs) has achieved exciting results.At present, the longest survival time of pig heart and pig kidney after xenotransplantation into monkeys exceeds 945 days respectively.[5]And 500 days.[6] Further improve the feasibility of clinical transformation of pig organs transplanted into human body.[7,8]In 2021, the American transplantation research team successfully transplanted pig kidney to brain dead people, and the experiment was terminated 54 hours later, and there was no obvious evidence of rejection of the transplanted kidney.[9] In 2022, the transplant team of the University of Maryland transplanted the pig heart to a patient with heart failure[10], and died after surviving for 2 months.The cause of death is still unclear.All these results indicate that xenotransplantation is about to shift from preclinical research to clinical application.
However, before the clinical application of xenotransplantation,there are still some problems that need to be clarified, such as whether the blood type of the recipient is required.Human organ transplantation requires the blood type of donor and recipient, and of course, there are also organ transplants with ABO incompatible blood types.[11] However, this kind of recipient needs to deal with the pre-stored anti-blood group antibody before operation.Does xenotransplantation have the same problem? There are 16 blood group systems in pigs, among which AO blood group system (pigs have no B gene, so they lack B blood group and AB blood group possessed by humans) is the most extensive, important and clinically significant antigen system.[12] Therefore, for the safety of clinical xenotransplantation, it is theoretically best to choose O blood group for donor pigs.However, for the receptor, whether there are differences in antibody binding and CDC of different blood types to O-type pig cells is the focus of this study.
2.Materials and methods
2.1 Materials
2.1.1 Experimental materials
Serum, pig whole blood PBMC and RBC (Chengdu Zhongke Og Company), lymphocyte separation solution (article number LTS1077, Tianjin Haoyang Biological Company), fetal bovine serum (article number WS500T, Ausbian Company, Australia), FITC anti-human IgM antibody (article number AB_2339692, Jackson Company, USA), FITC anti-human IgG antibody (article number AB _ Jackson Company, USA), Propidium iodide PI (No.S19136,Shanghai Yuanye Bio), PBS (No.PB180327, Wuhan Pusino Life Science and Technology), isolectin BSI-B4 (No.L2140, Sigma Company, Germany), Dolichos biflorus agglutinin DBA (No.FL-1031-5, Vector Labs, USA), Anti-Neu5Gc Antibody (No.146901,BioLegend, USA), APC Anti-Human β 2-Microglobulin Antibody(No.316312, American BioLegend Company), APC Anti-CD55 Antibody (No.ab275667, British Abcam Company), Rabbit Anti-Chicken Igy/Alexa Fluor 555 AntibodyNo.bs-0432R-AF555,Beijing Boao Biotechnology Co., Ltd.).
2.1.2 Instruments
Flow cytometer (model BD FACSCanto II, Becton Dickinson Company, USA), high-speed centrifuge (model TGL-25M, Shanghai Luxiangyi Company), water bath pot (model BL-3K, Shanghai Binglin Company) and inverted microscope (model wt031425,Shenzhen Weite Optoelectronics Company).
2.2 Methods
2.2.1 Collection and experimental grouping of human serum
Serum of healthy people (n=20), serum of patients with end-stage renal disease (n=20) and serum of brain-dead organ donors (n=20)were collected, centrifuged and stored at -80 ℃.According to ABO blood type, they were divided into 4 groups (blood type A: n=20; B blood type: n=17; AB blood type: n=7; O blood type: n=16).The serum of healthy people comes from healthy volunteers, and the blood of patients and the serum of brain-dead organ donors come from residual/discarded clinical laboratory samples.Human blood extraction was approved by the hospital ethics committee.
2.2.2 Extraction of pig PBMC and RBC
Five pigs of O blood group were collected by heparin anticoagulant blood collection, and their genotypes were WT, GTKO, GTKO/β 4 Galnt2KO, GTKO/CMAHKO and TKO/hCD55, respectively.To extract PBMC and RBC from pig whole blood, the simple process is as follows: after pig whole blood is added into a centrifuge tube containing lymphocyte separation solution, density gradient centrifugation is performed.Take out the centrifuge tube.The tube is divided into four layers, the upper layer is plasma, the lower layer is red blood cells and separation solution, and the second layer is white cloud-like PBMC.Suck the second layer of PBMC and the bottom layer of red blood cells at room temperature for later use.
2.2.3 Identification of pig PBMC and RBC xenoantigens
100 μL PBMC was added into the flow tube, and the expressions of Gal, Sda, Neu5Gc, SLA-I and hCD55 were detected by adding isolectin BSI-B4, DBA, Anti-Neu5Gc Antibody, APC anti-human β2-microglobulin antibody and APC Anti-CD55 Antibody,respectively.BSI-B4, DBA and CD55 were incubated in the dark for 30 d.The anti-Neu5Gc mAb was incubated for 30 min, then the second antibody Rabbit Anti-ChickenIgY/Alexa Fluor 555 Antibody was added to incubate in the dark for 30 min, and the results were detected by flow cytometry.
2.2.4 antibody binding experiment
The blank group and antibody group were added with FACS buffer (PBS containing 1% fetal bovine serum) and PBMC (RBC)respectively, while the experimental group was added with FACS buffer, PBMC (RBC) and human serum (the ratio of serum is 1:10),which were mixed evenly and incubated at 4 ℃.After centrifugation,FITC anti-human IgM antibody or FITC anti-human IgG antibody were added to all groups except the blank group, mixed evenly, and incubated at 4 ℃ in the dark.Add FACS buffer to each flow tube,centrifuge, control drying, and finally add FACS buffer, and detect the results by flow cytometry.
2.2.5 CDC experiment
The blank group and the simple rabbit complement group were added with FACS buffer and PBMC, while the experimental group was added with FACS buffer, PBMC and human serum (the ratio of serum is 1:6), which were mixed evenly and incubated at 4 ℃.After centrifugation, all groups except the blank group were added with standard rabbit complement, mixed evenly, and incubated in a 37 ℃water bath.After centrifugation, add propidium iodide, incubate at 4 ℃ in the dark, and then add FACS buffer.The cell death rate was detected by flow cytometry.
Cytotoxicity is calculated as follows[13]:
Among them, A represents the percentage of dead cells, B represents the percentage of the highest dead cells (killing PBMC),C with 70% ethanol), and C represents the percentage of the lowest dead cells (only adding rabbit complement).
2.3 Statistical processing
The experimental data were analyzed by GraphPad Prisn8 mapping software, and the comparison among groups was analyzed by oneway ANOVA test (tukey test) or nonparametric test (Dunn's test).The measurement data is expressed by the mean standard deviation(), and the difference is statistically significant with P<0.05.
3.Results
3.1 The expression of Gal, Sda, Neu5Gc and SLA-I on PBMC and RBC surface of WT, GTKO, GTKO/β4GalNT2KO,GTKO/CMAHKO and TKO/hCD55 pigs
WT pig PBMC expressed Gal, Sda and Neu5Gc.PBMC of GTKO pig did not express Gal antigen, but expressed Sda and Neu5Gc.GTKO/β4GalNT2KO pig PBMC did not express Gal or Sda, but expressed Neu5Gc.GTKO/CMAHKO pig PBMC did not express Gal or Neu5Gc, but expressed Sda.However, TKO/hCD55 pigs did not express these three xenoantigens, but expressed hCD55.SLA-I was expressed in PBMC of all genotype pigs.The expression of RBC xenoantigens of different pig genotypes is consistent with PBMC, except that SLA-I is not expressed.
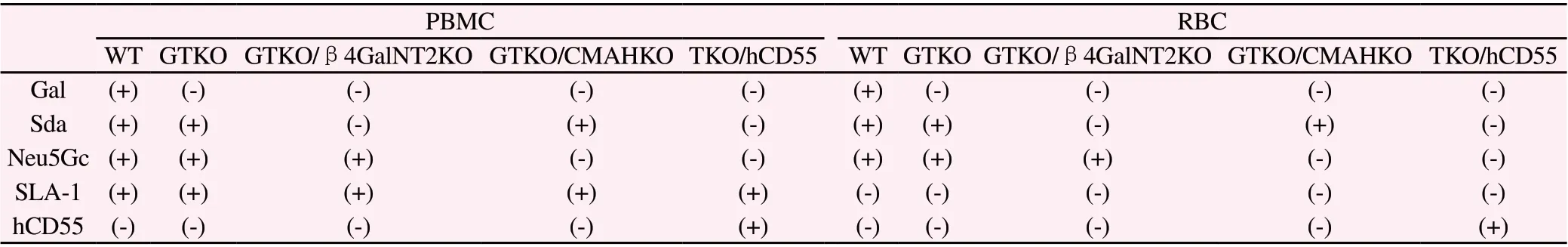
Tab1 Expression of PBCM and RBC xeno-antigens and hCD55 in WT and various genotypes of pigs
3.2 There was no significant difference in antibody binding and CDC between human serum of different blood types and WT and PBMC of various pig genotypes
(1) The binding data of four groups of serum IgM (type A, type B, type AB and type O) with different genetically modified pig PBMC are listed in Table 2.Overall comparison shows that there is no significant difference in these indexes among the four groups(P>0.05), and there is no statistical significance between the two groups (P>0.05).
(2) The binding data of four groups of serum IgG (type A, type B, type AB and type O) with different genetically modified pig PBMC are listed in Table 3.Overall comparison shows that there is no significant difference in these indexes among the four groups(P>0.05), and there is no statistical significance between the two groups (P>0.05).
(3)The killing data of four groups of serum (type A, type B,type AB and type O) on PBMC of different genetically modified pigs are listed in Table 4.Overall comparison shows that there is no significant difference in these indexes among the four groups(P>0.05), and there is no statistical significance between the two groups (P>0.05).
Tab2 Binding of serum IgM of four groups to pig PBCM of various genotypes (G.M, )
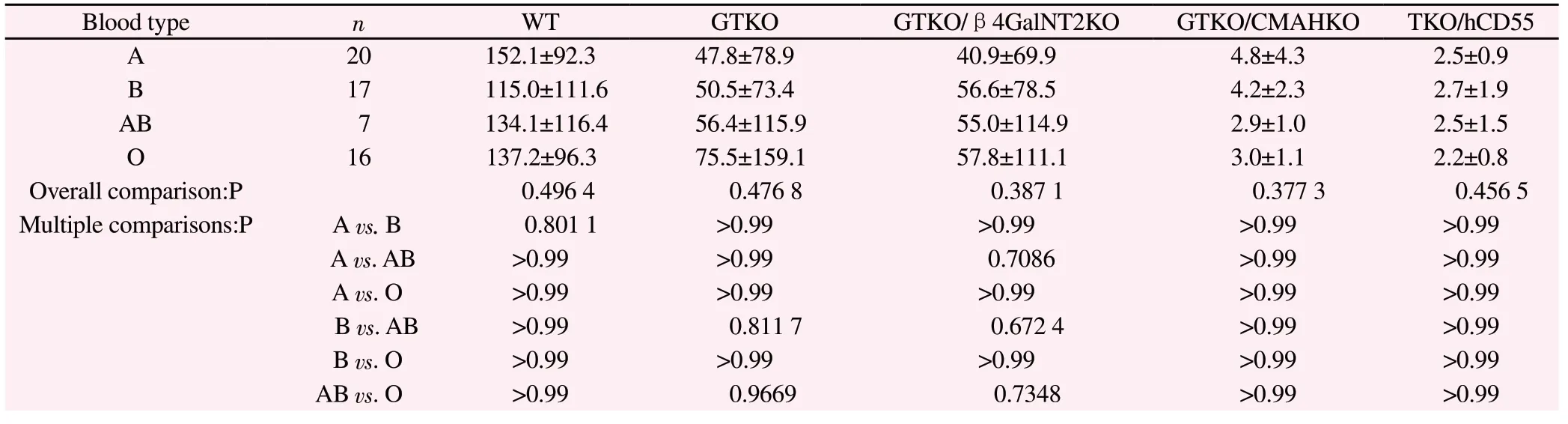
Tab2 Binding of serum IgM of four groups to pig PBCM of various genotypes (G.M, )
Blood type n WT GTKO GTKO/β4GalNT2KO GTKO/CMAHKO TKO/hCD55 A 20 152.1±92.3 47.8±78.9 40.9±69.9 4.8±4.3 2.5±0.9 B 17 115.0±111.6 50.5±73.4 56.6±78.5 4.2±2.3 2.7±1.9 AB 7 134.1±116.4 56.4±115.9 55.0±114.9 2.9±1.0 2.5±1.5 O 16 137.2±96.3 75.5±159.1 57.8±111.1 3.0±1.1 2.2±0.8 Overall comparison:P 0.496 4 0.476 8 0.387 1 0.377 3 0.456 5 Multiple comparisons:P A vs.B 0.801 1 >0.99 >0.99 >0.99 >0.99 A vs.AB >0.99 >0.99 0.7086 >0.99 >0.99 A vs.O >0.99 >0.99 >0.99 >0.99 >0.99 B vs.AB >0.99 0.811 7 0.672 4 >0.99 >0.99 B vs.O >0.99 >0.99 >0.99 >0.99 >0.99 AB vs.O >0.99 0.9669 0.7348 >0.99 >0.99
Tab3 Binding of serum IgG of four groups to pig PBCM of various genotypes(G.M, )
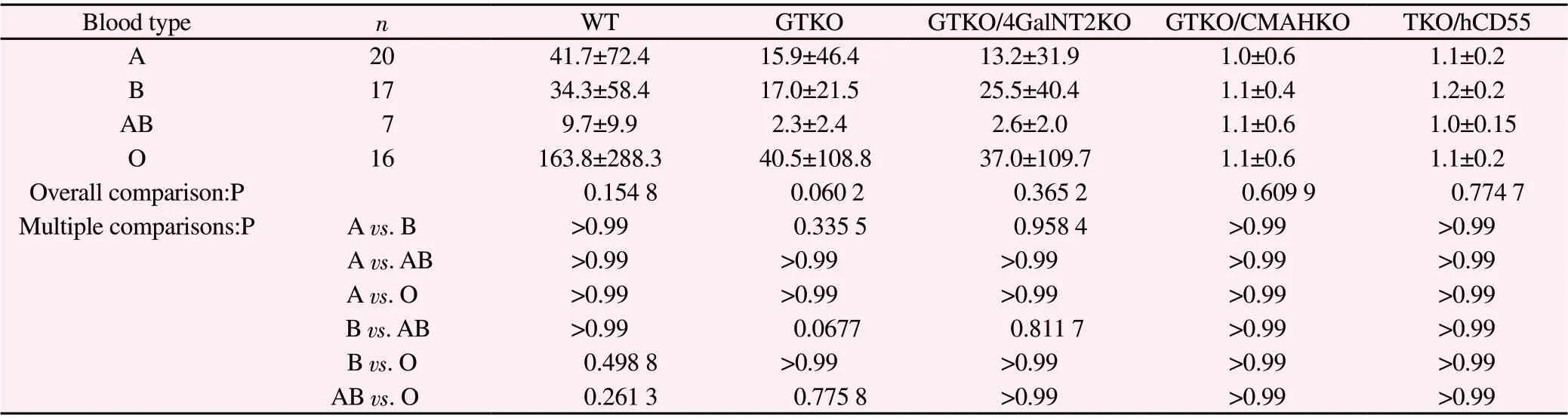
Tab3 Binding of serum IgG of four groups to pig PBCM of various genotypes(G.M, )
Blood type n WT GTKO GTKO/4GalNT2KO GTKO/CMAHKO TKO/hCD55 A 20 41.7±72.4 15.9±46.4 13.2±31.9 1.0±0.6 1.1±0.2 B 17 34.3±58.4 17.0±21.5 25.5±40.4 1.1±0.4 1.2±0.2 AB 7 9.7±9.9 2.3±2.4 2.6±2.0 1.1±0.6 1.0±0.15 O 16 163.8±288.3 40.5±108.8 37.0±109.7 1.1±0.6 1.1±0.2 Overall comparison:P 0.154 8 0.060 2 0.365 2 0.609 9 0.774 7 Multiple comparisons:P A vs.B >0.99 0.335 5 0.958 4 >0.99 >0.99 A vs.AB >0.99 >0.99 >0.99 >0.99 >0.99 A vs.O >0.99 >0.99 >0.99 >0.99 >0.99 B vs.AB >0.99 0.0677 0.811 7 >0.99 >0.99 B vs.O 0.498 8 >0.99 >0.99 >0.99 >0.99 AB vs.O 0.261 3 0.775 8 >0.99 >0.99 >0.99
Tab4 The killing effect of serum of four groups to pig PBCM of various genotypes(%,)
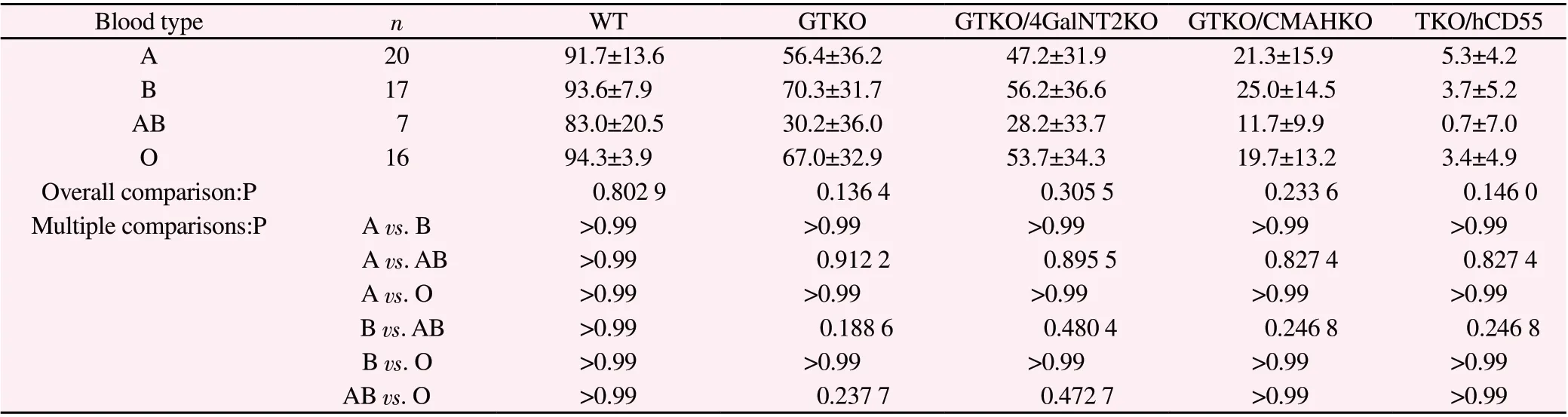
Tab4 The killing effect of serum of four groups to pig PBCM of various genotypes(%,)
Blood type n WT GTKO GTKO/4GalNT2KO GTKO/CMAHKO TKO/hCD55 A 20 91.7±13.6 56.4±36.2 47.2±31.9 21.3±15.9 5.3±4.2 B 17 93.6±7.9 70.3±31.7 56.2±36.6 25.0±14.5 3.7±5.2 AB 7 83.0±20.5 30.2±36.0 28.2±33.7 11.7±9.9 0.7±7.0 O 16 94.3±3.9 67.0±32.9 53.7±34.3 19.7±13.2 3.4±4.9 Overall comparison:P 0.802 9 0.136 4 0.305 5 0.233 6 0.146 0 Multiple comparisons:P A vs.B >0.99 >0.99 >0.99 >0.99 >0.99 A vs.AB >0.99 0.912 2 0.895 5 0.827 4 0.827 4 A vs.O >0.99 >0.99 >0.99 >0.99 >0.99 B vs.AB >0.99 0.188 6 0.480 4 0.246 8 0.246 8 B vs.O >0.99 >0.99 >0.99 >0.99 >0.99 AB vs.O >0.99 0.237 7 0.472 7 >0.99 >0.99
Table 2, Table 3 and Table 4 show that there is no significant difference in binding of human ABO blood group serum antibody to WT, GTKO, GTKO/β 4 galnt2KO, GTKO/CMAHKO and TKO/hCD55 pig PBMC antigens.
3.3 There was no significant difference in antibody binding between serum of different human blood groups and WT and RBC of various pig genotypes
(1) The binding data of four groups of serum IgM (type A, type B, type AB and type O) with different genetically modified pig RBC are listed in Table 5.Overall comparison shows that there is no significant difference in these indexes among the four groups(P>0.05), and there is no statistical significance between the two groups (P>0.05).
(2) The binding data of four groups of serum IgG (type A, type B, type AB and type O) with different genetically modified pig RBC are listed in Table 6.Overall comparison shows that there is no significant difference in these indexes among the four groups(P>0.05), and there is no statistical significance between the two groups (P>0.05).
Tables 5 and 6 show that there is no significant difference between the binding of human ABO blood group serum antibody and WT,GTKO, GTKO/β 4 galnt2ko, GTKO/CMAHKO and TKO/hCD55 pig RBC antigens.
Tab5 Binding of serum IgM of four groups to pig RBC of various genotypes (G.M,))
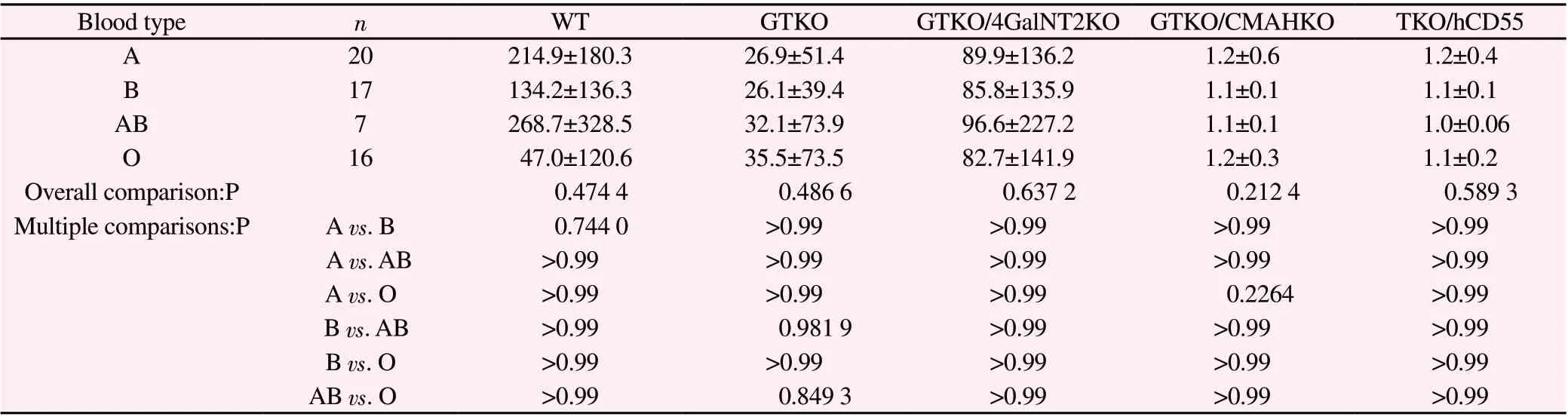
Tab5 Binding of serum IgM of four groups to pig RBC of various genotypes (G.M,))
Blood type n WT GTKO GTKO/4GalNT2KO GTKO/CMAHKO TKO/hCD55 A 20 214.9±180.3 26.9±51.4 89.9±136.2 1.2±0.6 1.2±0.4 B 17 134.2±136.3 26.1±39.4 85.8±135.9 1.1±0.1 1.1±0.1 AB 7 268.7±328.5 32.1±73.9 96.6±227.2 1.1±0.1 1.0±0.06 O 16 47.0±120.6 35.5±73.5 82.7±141.9 1.2±0.3 1.1±0.2 Overall comparison:P 0.474 4 0.486 6 0.637 2 0.212 4 0.589 3 Multiple comparisons:P A vs.B 0.744 0 >0.99 >0.99 >0.99 >0.99 A vs.AB >0.99 >0.99 >0.99 >0.99 >0.99 A vs.O >0.99 >0.99 >0.99 0.2264 >0.99 B vs.AB >0.99 0.981 9 >0.99 >0.99 >0.99 B vs.O >0.99 >0.99 >0.99 >0.99 >0.99 AB vs.O >0.99 0.849 3 >0.99 >0.99 >0.99
Tab6 Binding of serum IgG of four groups to pig RBC of various genotypes (G.M, ))
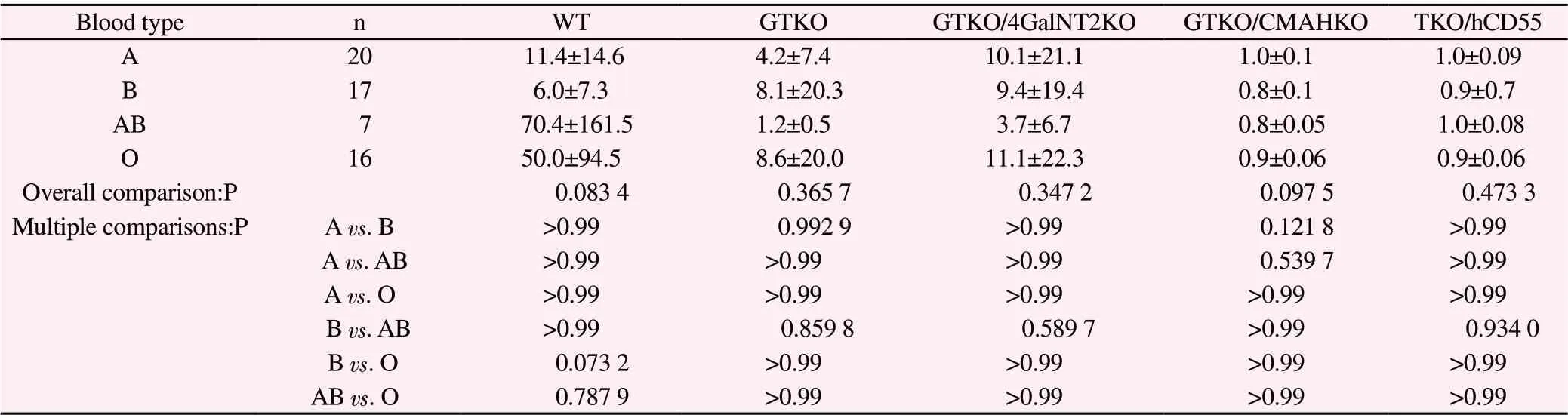
Tab6 Binding of serum IgG of four groups to pig RBC of various genotypes (G.M, ))
Blood type n WT GTKO GTKO/4GalNT2KO GTKO/CMAHKO TKO/hCD55 A 20 11.4±14.6 4.2±7.4 10.1±21.1 1.0±0.1 1.0±0.09 B 17 6.0±7.3 8.1±20.3 9.4±19.4 0.8±0.1 0.9±0.7 AB 7 70.4±161.5 1.2±0.5 3.7±6.7 0.8±0.05 1.0±0.08 O 16 50.0±94.5 8.6±20.0 11.1±22.3 0.9±0.06 0.9±0.06 Overall comparison:P 0.083 4 0.365 7 0.347 2 0.097 5 0.473 3 Multiple comparisons:P A vs.B >0.99 0.992 9 >0.99 0.121 8 >0.99 A vs.AB >0.99 >0.99 >0.99 0.539 7 >0.99 A vs.O >0.99 >0.99 >0.99 >0.99 >0.99 B vs.AB >0.99 0.859 8 0.589 7 >0.99 0.934 0 B vs.O 0.073 2 >0.99 >0.99 >0.99 >0.99 AB vs.O 0.787 9 >0.99 >0.99 >0.99 >0.99
4.Discussion
Ultra-acute rejection in early research of xenotransplantation is the main cause of death after transplantation.At present, foreign antigens(GTKO,CMAHKO and β4GalNT2KO) have been knocked out by gene editing technology, resulting in a variety of genetically modified pigs.[14] In addition, the preclinical research of xenotransplantation has made great progress, and it is very likely to enter the clinic in the future.However, it is worth studying whether there are certain requirements for the selection of the recipient's blood type.Oostingh Gertie J et al.[15]The anti- -Gal IgM and IgG antibody titers in sera of renal dialysis patients and healthy people with different ABO blood types were analyzed.The results showed that there was no significant difference in anti- -Gal IgM antibody titers among people with different ABO blood types, but there was significant difference in anti- -Gal IgG antibody titers.The drop of anti- -Gal IgG antibody in serum of blood group O and A is higher than that of blood group B and AB, which suggests that blood group O may not be suitable for clinical trial of pig-human xenotransplantation.Other studies have also reported that the titer of anti- -Gal IgG antibody in blood group B and AB is low.[16-18].And Hurh S et al.[19]The binding degree of 100 human serum antibodies to swine antigen and complement-dependent cytotoxicity were analyzed.The results showed that among ABO blood groups, IgG antibody of blood group A had the highest binding degree to swine antigen.In our research,we found that the antibody binding or CDC of human serum with WT pig PBMC and RBC were significantly higher than those of other four genotypes of pig PBMC and RBC, indicating that anti-Gal antibody was the main antibody in human serum.However,there was no significant difference in the binding and killing effect between the sera of four groups of different blood types and WT pig PBMC and RBC, indicating that there was no significant difference in the anti-Nue5Gc antibody in the sera of people of any blood type.
Gab et al[20]The differences of sex, ABO blood type, eating habits and vaccination history of healthy people were analyzed,and whether the expression levels of anti-Neu5Gc and anti-nonGal/nonNeu5Gc antibodies were different.They used healthy human serum to culture with arterial endothelial cells and red blood cells of GTKO/CD46/Neu5GcKO and GTKO/CD46/Neu5GcKO pigs.The results showed that there was no statistical difference between ABO blood group and antibody binding.In our other results, we found that after Neu5Gc was knocked out (CMAHKO) on PBMC and RBC of pigs, the binding of human serum antibodies to GTKO/CMAHKO and TKO/hCD55 was further lower than that of serum antibodies to GTKO and GTKO/4GalNT2KO pig cells, indicating that the amount of pre-existing antibodies in human serum was second to anti-Neu5Gc.Similarly, there was no significant difference in binding and killing between the sera of four groups of people with different blood types and GTKO/CMAHKO and TKO/hCD55 pig PBMC and RBC, indicating that there was no significant difference in anti-Nue5Gc antibody in the sera of people with different blood types.
In this study, it was also found that after the Gal antigen was knocked out, the binding and CDC of IgM and IgG antibodies of human AB blood group with GTKO, GTKO/β 4GALNT2KO,GTKO/CMAHKO and TKO/hCD55 pig PBMC and RBC antigens were generally low, and only a few individuals had a high binding level.If the number of samples was increased, there might be statistical differences.However, after Neu5Gc antigen was knocked out, the difference was reduced, and the binding water of serum antibodies of different blood groups with GTKO/CMAHKO and TKO/hCD55 pig PBMC and RBC antibodies was lower on average.When only Gal antigen is knocked out, AB blood group recipients may be more suitable for xenotransplantation than O blood group recipients and other blood group recipients.However, after further knockout of Neu5Gc antigen or simultaneous knockout of Neu5Gc and Sda antigen, the differences among recipients of different blood types gradually narrowed, and all recipients were suitable for xenotransplantation.
To sum up, there is no obvious difference in the binding or killing of PBMC and RBC of GTKO/CMAHKO and TKO/hCD55 pigs with O blood type by different ABO blood types, whether healthy people, patients with end-stage renal disease or brain dead organ donors, indicating that TKO pigs with O blood type may be the best genotype donor pigs at present, and the recipients may not need to consider blood type factors.
Author's contribution:
The experiment was designed by Chen Liang-jie, guided by Wang Yi, Chen Gang and Pan Deng-ke.Chen Liang-jie, Feng Hao and Du Jia-xiang collected data, and Li Tao was responsible for the flow index detection.Chen Liang-jie and Li Tao reviewed and statistically analyzed the experimental data.The paper was written by Chen Liang-jie and reviewed by Wang Yi and Jiang Hong-tao.
All authors declare no conflict of interest.
 Journal of Hainan Medical College2023年2期
Journal of Hainan Medical College2023年2期
- Journal of Hainan Medical College的其它文章
- Research progress of modern pharmacological effects of Pueraria lobata and its compound clinical application
- A systemaic review and meta-analysis of traditional Chinese medicine in the treatment of chronic cough
- Research progress on sensitive genes related to ferroptosis
- Identification of key genes underlying clinical features of hepatocellular carcinoma based on weighted gene co-expression network analysis and bioinformatics analysis
- METTL14 upregulates m6A modification of pri-miR-141 inhibiting ZEB1 to promote proliferation and inflammation of lung fibroblasts
- Effect of Cx32 over-expression on cell proliferation, migration, and invasion of hepatocellular carcinoma cell line Huh7 and its mechanism
