microRNA-627-5p inhibits colorectal cancer cell proliferation,migration and invasion by targeting Wnt2
Dong-Yan Zhao, Teng-Fei Yin, Xi-Zhen Sun, Yuan-Chen Zhou, Qian-Qian Wang, Ge-Yujia Zhou, Shu-Kun Yao
Dong-Yan Zhao, School of Biology & Basic Medical Sciences, Soochow University, Soochow 215213, Jiangsu Province, China
Teng-Fei Yin, Department of Gastroenterology, Qilu Hospital of Shandong University, Jinan 250012, Shandong Province, China
Xi-Zhen Sun, Department of Gastroenterology, Beijing Jishuitan Hospital, Beijing 100035, China
Yuan-Chen Zhou, Qian-Qian Wang, Ge-Yujia Zhou, Shu-Kun Yao, Department of Gastroenterology, China-Japan Friendship Hospital, Beijing 100029, China
Yuan-Chen Zhou, Qian-Qian Wang, Graduate School, Peking University China-Japan Friendship School of Clinical Medicine, Beijing 100029, China
Ge-Yujia Zhou, Graduate School, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100029, China
Abstract BACKGROUND microRNA-627-5p (miR-627-5p) dysregulation has been observed in several cancer types, such as hepatocellular carcinoma, oral squamous cell carcinoma, glioblastoma multiforme, and gastric cancer. The biological function of miR-627-5p in colorectal cancer (CRC) growth and metastasis is yet unclear.AIM To investigate the effects of miR-627-5p on the malignant biological properties of colorectal malignant tumour cells by targeting Wnt2.METHODS The levels of miR-627-5p in colorectal tumour tissues were assessed in Gene Expression Omnibus datasets. In order to identify Wnt2 transcript expression in CRC tissues, quantitative real-time polymerase chain reaction (qRT-PCR) analysis was used. Luciferase reporter tests were used to explore whether miR-627-5p might potentially target Wnt2. Wnt2 transcript and protein levels were detected in CRC cells with high miR-627-5p expression. To learn more about how miR-627-5p affects CRC development, migration, apoptosis, and invasion, functional experiments were conducted. Cotransfection with the overexpression vector of Wnt2 and miR-627-5p mimics was utilized to verify whether overexpression of Wnt2 could cancel the impact of miR-627-5p in CRC. Western blot and qRT-PCR were conducted to investigate the effects of miR-627-5p on the Wnt/β-catenin signalling pathway.RESULTS miR-627-5p was notably decreased in colorectal tumour tissues, while the gene level of Wnt2 was notably upregulated. A dual luciferase reporter assay revealed that miR-627-5p specifically targets the 3’-untranslated regions of Wnt2 and miR-627-5p upregulation markedly reduced the protein and gene expression of Wnt2 in CRC cells. In vitro gain-of-function assays displayed that miR-627-5p overexpression decreased CRC cells’ capabilities to invade, move, and remain viable while increasing apoptosis. Wnt2 overexpression could reverse the suppressive functions of miR-627-5p. Moreover, upregulation of miR-627-5p suppressed the transcript and protein levels of the downstream target factors in the canonical Wnt/β-catenin signalling, such as c-myc, CD44, βcatenin, and cyclinD1.CONCLUSION miR-627-5p acts as a critical inhibitory factor in CRC, possibly by directly targeting Wnt2 and negatively modulating the Wnt/β-catenin signalling, revealing that miR-627-5p could be a possible treatment target for CRC.
Key Words: miR-627-5p; Wnt2; Colorectal cancer; β-catenin; Progression
INTRODUCTION
Colorectal cancer (CRC) is one of the most prevalent malignant tumours and has high incidence and mortality rates globally, posing a great threat to the health of human beings[1]. Despite great advances in the diagnosis and treatment of CRC over the span of a few decades, CRC is still incurable and often has a poor prognosis, especially in patients with advanced tumours, which necessities clarification of the underlying mechanisms of CRC[2,3]. Generally, a vast number of genetic and molecular changes, such as epigenetic aberrations, genetic alterations, microsatellite instability, and chromosomal instability, have been shown to be correlated with colorectal carcinogenesis and tumour metastasis[4,5]. All of these genomic events can contribute to the activation of important signalling pathways (Wnt/βcatenin, MAPK/PI3K, and SMAD/TGF-β) and the initiation of colorectal tumorigenesis[6]. Since the human genome has been extensively characterized, miRNAs have become a hot spot and have offered fresh understanding of the molecular mechanisms underpinning CRC progression[7].
microRNA (miRNA) is an endogenously derived non-coding RNA sequence composed of 19-25 nucleotides that can modulate the levels of downstream genes through direct binding to their 3’-untranslated regions (3’-UTRs)viacleavage or translational arrest[8]. Many published papers have implicated the role of microRNA-627-5p (miR-627-5p) in controlling the emergence and development of a number of tumours[9-12]. For instance, Wanget al[9] reported that miR-627-5p acted as a inhibitory factor by reducing the expression of Bcl3 in hepatocellular carcinoma. miR-627-5p was also identified to be involved in suppressing the malignant behaviors of glioma cells by binding to the 3’-UTR of NR2C2 and downregulating its expression in glioblastoma multiforme[10]. These studies indicated the tumour inhibitory function of miR-627-5p. Furthermore, Shinet al[11] found miR-627-5p was observably upregulated in gastric cancer tissues than in normal controls, suggesting that miR-627-5p might stimulate gastric cancer progression. In other words, miR-627-5p may play different roles in different cancer types. A growing body of evidence has emphasized that abnormally expressed miRNAs play vital roles in promoting or suppressing the progression from normal to hyperproliferative epithelium, then to precancerous advanced adenoma (AA), and later invasive adenocarcinoma[13-15]. Nevertheless, the functions of miR-627-5p are largely unknown in colorectal carcinogen.
In the present study, TargetScan website speculated that miR-627-5p could be complementary with the 3’-UTR of Wnt2. Wnt2, an evolutionarily conserved secreted-type glycoprotein secreted by the Wnt signalling, performs a crucial role in promoting the malignant progression of gastrointestinal cancers through activation of the Wnt/β-catenin signalling[16-18]. Consequently, the current study’s goal was to investigate how miR-627-5p and Wnt2 contribute to the emergence of CRC, and to assess the relationship between them.
MATERIALS AND METHODS
Bioinformation analysis
From the Gene Expression Omnibus (GEO) database, the original series matrix files of the miRNAs of patients with colorectal tumours and healthy controls (HCs) were gathered. GSE41655 and GSE18392 were employed to analyze the differential expression of miR-627 between CRC tissues and control tissues. GSE41655 contained 33 tumour tissues and 15 normal control tissues while GSE18392 included 116 tumour tissues and 29 control tissues.
Human tissue collection
A total of 30 patients with colorectal tumours, 33 AA and 20 HCs aged between 18 and 80 years were employed to explore the tissue levels of Wnt2 in the study. All patients diagnosed by histology as AA and colorectal adenocarcinoma were enrolled as case group. All healthy participants were recruited through advertisements and screened by careful history taking, physical examinations, essential laboratory examinations and colonoscopy. Individuals with negative colonoscopy results were selected as HC group. During the endoscopies, biopsy samples were taken from the rectosigmoid colon in the HCs. The excluding protocol for all subjects were indicated below: (1) Subjects with a history of other major organic diseases, malignant tumours in other organs, and psychiatric disorders; (2) pregnant or lactating female subjects; (3) patients with hereditary CRC or hereditary intestinal polyposis syndromes; (4) participants with a history of radiotherapy, chemotherapy, and major abdominal surgery; and (5) patients who presented with inflammatory or infective diseases. The clinicopathological characteristics of HCs and colorectal neoplasm patients are described in Supplementary Table 1. China-Japan Friendship Hospital’s ethics committee gave the study’s protocol approval under the number 2018-116-K85-1, and all participants gave their written informed permission.
Cell culture
The American Type Culture Collection was used to obtain a colonic epithelial cell line (FHC), human colonic malignant tumor cell lines (HCT116, RKO, and SW480), and a human embryonic kidney cell line (HEK-293T). For the purpose of investigating Wnt2 mRNA expression, FHC and three CRC cell lines were used. Gain of function tests were carried out using HCT116 and SW480 cells. Dual luciferase reporter tests were performed using HEK-293T cells because of their high transfection efficiency[19]. Incubation conditions included 5% CO2in the air and a minimum relative humidity of 95%, for the RPMI 1640 medium or Dulbecco’s Modified Eagle’s Medium in which cells were placed.
Cell transfection
miR-627-5p mimics, mimics negative control oligonucleotides (NC mimics), Wnt2 overexpressing plasmids (pcDNA-Wnt2) and matched negative controls (pcDNA-NC) were designed by GenePharma (Shanghai Province, China). In 6-well culture plates, SW480 and HCT116 cells were plated for 24 h. Following the manufacturer’s instructions, LipofectamineTM 3000 Transfection Reagent (Invitrogen, Carlsbad, CA, United States) was used to transfect a mixture of miR-627-5p mimics or NC mimics (150pmol) with pcDNA-Wnt2 or pcDNA-NC (3 g). In Supplementary Table 2, the oligonucleotide sequences are displayed.
Quantitative real-time polymerase chain reaction
Total RNA that included miRNA from tissues or cells was isolated by the RNAprep Pure Cell Kit (Solarbio, Beijing, China). cDNA was obtained using the Hifair?II 1st Strand cDNA Synthesis Kit (YESEN, Shanghai, China). PCR amplification was conducted in a LineGene 9600 Plus Real-Time PCR system (Bioer Technology, Hangzhou, China) by using Hieff?qPCR SYBR? Green Master Mix (No Rox) (YESEN, Shanghai, China). The comparative threshold method was used to quantify the relative expression of miRNAs and mRNAs. In Supplementary Table 3, the primer sequences used in the study are displayed.
Western blotting
RIPA lysis buffer (Beyotime, Shanghai, China) was used for protein extraction, and the BCA Protein Assay Kit was used to measure the amount of protein (Solarbio, Beijing, China). Total proteins were then subjected to sodium dodecyl sulfate/polyacrylamide gel electrophoresis and electrotransferred to PVDF membranes (Bio-Rad, Hercules, CA, United States). Nonspecific binding sites were blocked using 5% nonfat milk for 2 h and the membranes were incubated at 4 °C overnight with rabbit anti-Wnt2 antibody (1:1000; Affinity, Melbourne, United States), rabbit anti-c-myc antibody (1:1000; Bioss, Beijing, China), rabbit anti-CD44 antibody (1:1000; Affinity, Melbourne, United States), rabbit anti-cyclin D1 antibody (1:1000; Abcam, Cambridge, United Kingdom), and rabbit anti-β-tubulin antibody (1:4000; Proteintech, Chicago, United States). The PVDF membranes were then treated for 1 hour with a goat anti-rabbit secondary antibody that was HRP conjugated (1:5000; Bioss, Beijing, China). Enhanced chemiluminescence reagents (Beyotime, Shanghai, China) were used to visualize the bands. Image J was utilized to quantify the chemiluminescent signals of protein bands using β-tubulin as an internal control.
Cholecystokinin octapeptide assay
Cell viability was monitored using cholecystokinin octapeptide (CCK-8) reagents (Solarbio, Beijing, China). After transfection, CRC cells plated in 96-well plates were added to CCK-8 solution and incubated for 1 h. The number of viable cells was determined at a 24 h interval for four consecutive days following the manufacturer’s instructions.
Matrigel invasion assay
Corning Transwell insert chambers (Corning Incorporated, New York, NY, United States) with a 6.5-μm pore size were used to assess invasive capability. Cancer cells were planted in the upper chamber, cultured with foetal bovine serum (FBS) free medium, and allowed to invade for 72 h. The lower chamber was added to culture medium comprising 10% FBS to attract the invaded cells. The invading cells that broke through the Matrigel were then fixed in paraformaldehyde, stained in crystal violet, and counted in five randomly selected high-power fields.
Scratch assay
Homogeneous single cell suspensions were plated in 6-well plates until a single layer formed before being wounded by scraping a straight line with a yellow micropipette tip. The plates were incubated with complete medium after 3 PBS solution washes. All lengthy wounds were captured on camera at 0 and 24 h after the wound.
Flow cytometry analysis
An Annexin V-fluorescein isothiocyanate/propidium iodide (PI) apoptosis kit (7seabiotech, Shanghai, China) was utilized to detect the proportion of apoptotic cells after transfection. All processes were performed following the manufacturer’s protocols. Flow cytometry (BeckMan, United States) and FlowJoTM(Becton, New York, NY, United States) software were used to determine the cell apoptosis rates. Detailed experimental procedures are described in our previous study[20].
Dual luciferase reporter assay
The human Wnt2 3’-UTR comprising the expected complementary site of miR-627-5p (wild type), and its identical sequence with the mutant sequences of specific complementary sites of miR-627-5p (mutant) were inserted into the pmirGLO luciferase vector. HEK-293T cells were cotransfected with pmirGlo-Wnt2 3’-UTR wild type or pmirGLO-Wnt2 3’-UTR mutant and miR-627-5p mimics. The luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, United States).
Statistical analysis
All data are shown as mean ± SD or median (interquartile range). The Shapiro-wilk test was used to verify the normal distribution. Student’sttest or Wilcoxon rank-sum test were employed to decide significant differences between two groups where appropriate. Spearman correlation analysis was used to calculate the relationship between Wnt2 gene expression and miR-627-5p expression in colorectal neoplasm tissues. All calculations were conducted with IBM SPSS (Chicago, IL, United States) and diagrams were described using GraphPad Prism (La Jolla, CA, United States). APvalue of 0.05 or lower was considered significant.
RESULTS
The expression levels of miR-627 in CRC tissues
The miR-627 levels were contrasted between the CRC subgroup and the normal subgroup in two GEO datasets, GSE41655 (33 CRCsvs15 HCs) and GSE18392 (116 CRCsvs29 HCs), and the findings showed that CRC tissues had noticeably lower levels of miR-627 (Figure 1A and B). The AUCs of miR-627 in the GSE41655 and GSE18392 datasets were 0.90 (P< 0.001) and 0.67 (P= 0.006), respectively, according to receiver operating characteristic analysis (Figure 1C and D).
Relationship between miR-627-5p and Wnt2 expression in colorectal neoplasm tissues
The mRNA levels of Wnt2 in clinical tissues or in the colonic epithelial cells and cancer cells were determined by quantitative real-time polymerase chain reaction (qRT-PCR). The findings indicated Wnt2 expression were sequentially upregulated from HC tissues and AA tissues to CRC tissues (Figure 2A). This observation was supported by the fact that Wnt2 expression in cancer cells was observably higher than it was in epithelial cell line (FHC) cells (Figure 2B). Our previous study examined miR-627-5p expression in the same clinical tissues[20] and we next analyzed the relationship among the expressions of miR-627-5p and Wnt2 in colorectal neoplasm tissues and found an inverse relationship between miR-627-5p and Wnt2 gene levels (r= -0.61,P< 0.001, Figure 2C).
Direct binding relationship between miR-627-5p and Wnt2
First, the possible miR-627-5p downstream genes were speculatively identified using the TargetScan website. As a result, a sequence located at bases 1287-1293 of the Wnt2 3’-UTR was binding to the seed sequence of miR-627-5p (Figure 3A). To verify that miR-627-5p and Wnt2 have a direct complimentary interaction, we conducted dual luciferase reporter assays. Then, the Wnt2 pmirGLO vector comprising wild type or mutant miR-627-5p target sites was constructed (Figure 3A). Then, the constructed vectors were transfected into 293T cells with the cotransfection of miR-627-5p mimics or scrambled controls. miR-627-5p overexpression reduced the luciferase levels of the reporter vector carrying the wild type sequence of Wnt2 3’-UTR (0.58 ± 0.04vs1.00 ± 0.05,P< 0.001), but not those of the reporter vector comprising the mutant target sequence in 293T cells (1.12 ± 0.09vs1.03 ± 0.11,P= 0.35, Figure 3B). Moreover, gain-of-function experiments were performed by transfection of miR-627-5p mimics in SW480 and HCT116 cells to verify whether miR-627-5p could influence the expression levels of Wnt2 in CRC cells. RT-PCR analysis demonstrated that miR-627-5p mimics markedly increased miR-627-5p level in SW480 (35.90 ± 3.09vs1.02 ± 0.20,P< 0.001) and HCT116 cells (31.30 ± 5.14vs1.02 ± 0.23,P< 0.001, Figure 3C). As depicted in Figure 3D-E, upregulation of miR-627-5p directly reduced the transcript expression (SW480, 0.09 ± 0.03vs1.01 ± 0.16,P< 0.001; HCT116, 0.02 (0.01-0.31)vs0.93 (0.91-1.16),P< 0.001) and protein expression (SW480, 0.81 ± 0.01vs1.17 ± 0.10,P= 0.004; HCT116, 1.03 ± 0.01vs1.26 ± 0.03,P< 0.001) of Wnt2 in SW480 and HCT116 cells. In summary, miR-627-5p functions as a specific complement to Wnt2.
Role of miR-627-5p in CRC cells
Then, we investigated the biological function of miR-627-5p through gain-of-function tests in SW480 and HCT116 cells. As depicted in Figure 4A and D, wound healing assays showed that miR-627-5p overexpression contributed to a weakened ability of migrating cells (SW480, 23.63% ± 9.62%vs139.11% ± 29.36%,P< 0.001; HCT116, 36.03% ± 15.15%vs168.69% ± 31.75%,P< 0.001). Matrigel invasion assays demonstrated that exogenetic upregulation of miR-627-5p markedly blocked cancer cells’ ability to invade (SW480, 112.00 ± 39.77vs236.20 ± 33.10,P= 0.001; HCT116, 144.60 ± 35.78vs335.20 ± 14.02,P< 0.001; Figure 4B and E). Next, we used flow cytometry analysis to verify whether miR-627-5p overexpression could influence cell apoptosis and the findings showed that miR-627-5p upregulation accelerated cell apoptosis (SW480, 33.91% ± 5.61%vs17.08% ± 1.40%,P= 0.007; HCT116, 42.15% ± 1.00%vs21.35% ± 0.61%,P< 0.001, Figure 4C and F). Furthermore, according to CCK-8 experiments, miR-627-5p overexpression attenuated cell growth (Figure 4G). Collectively, miR-627-5p inhibits CRC cells migration, invasion, and proliferation but promotes cell apoptosis.
Impact of the miR-627-5p/Wnt2 axis on the malignant behaviours of CRC cells
To explore the functional effects of the miR-627-5p/Wnt2 axis in CRC cells, we generated a Wnt2 overexpression vector (pcDNA-Wnt2) and designed rescue assays. As presented in Figure 5, pcDNAWnt2 effectively increased the gene [SW480, 44.84 ± 5.98vs1.00 ± 0.08,P< 0.001; HCT116, 51.39 (45.06-56.67)vs1.15 (0.63-1.39),P< 0.001] and protein (SW480, 3.73 ± 0.16vs2.70 ± 0.10,P= 0.001; HCT116, 1.32 ± 0.01vs1.04 ± 0.05,P= 0.001) expression of Wnt2 in SW480 and HCT116 cells, proving that the construction of this vector was successful. Then, we used it to transfect CRC cells overexpression miR-627-5p. The results of wound healing and Matrigel invasion assays revealed that upregulation of Wnt partially canceled the suppressive functions of miR-627-5p on cell migration [SW480, 102.95% (96.05%-132.64%)vs22.22% (15.48%-32.49%),P= 0.008; HCT116, 116.23% ± 20.46%vs36.03% ± 15.15%,P< 0.001] and invasion (SW480, 265.80 ± 36.89vs112.00 ± 39.77,P< 0.001; HCT116, 322.00 ± 28.61vs144.60 ± 35.78,P< 0.001, Figure 4A, B, D, and E). Besides, flow cytometry analysis demonstrated that cell apoptosis induced by miR-627-5p could be attenuated by Wnt2 overexpression (SW480, 17.69% ± 1.35%vs33.91% ± 5.61%,P= 0.008; HCT116, 24.17% ± 1.00%vs42.15% ± 1.00%,P< 0.001, Figure 4C and F). CCK-8 assays demonstrated that the decreased in cell viability caused by miR-627-5p could be attenuated by Wnt2 overexpression (Figure 4G).
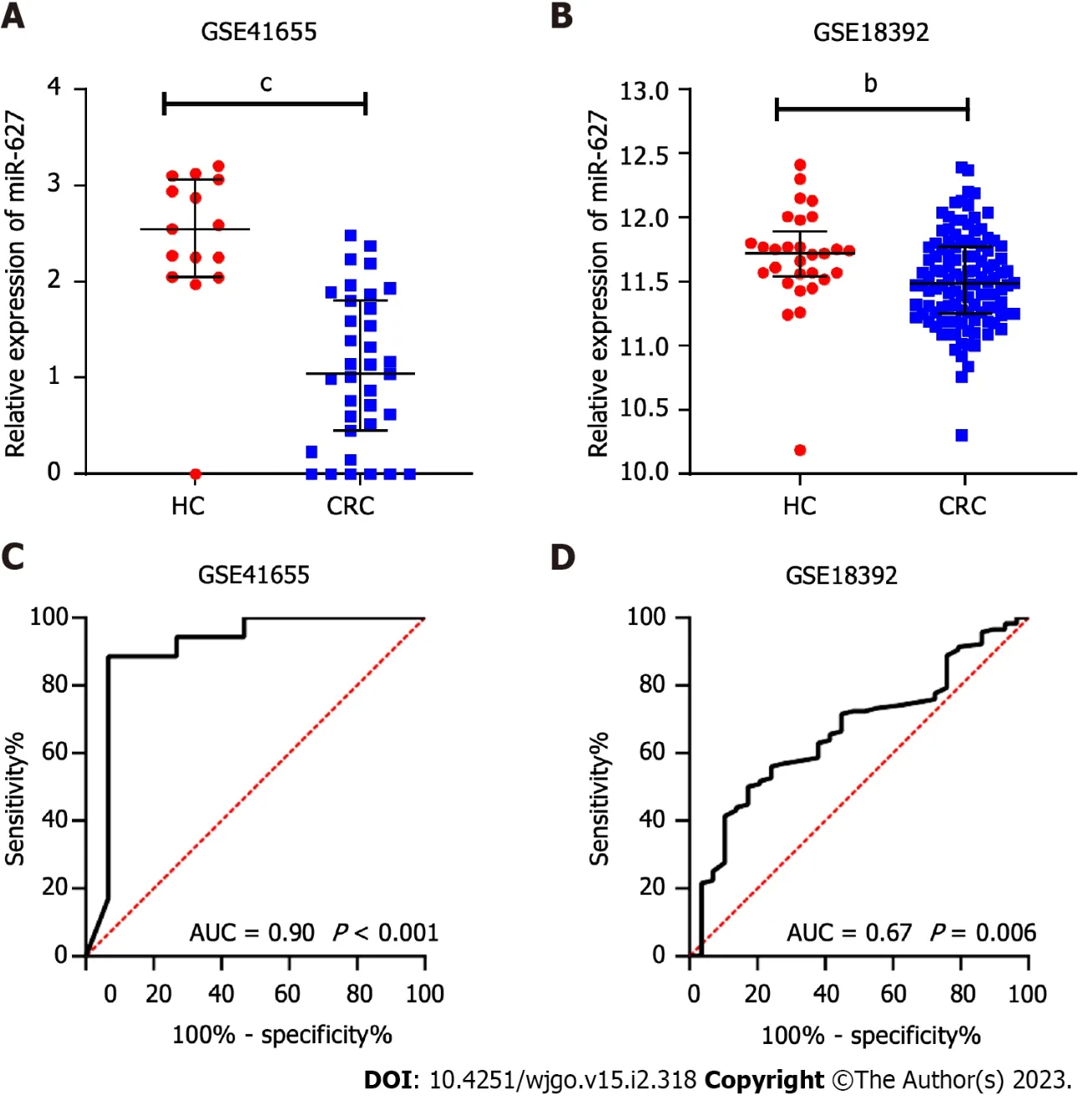
Figure 1 Evaluation of the tissue expression and diagnostic utility of microRNA-627 in the GSE41655 and GSE18392 datasets. A: Tissue expression of microRNA-627 (miR-627) in the healthy controls (HCs) and colorectal cancer (CRC) patients in the GSE41655 dataset; B: Tissue expression of miR-627 in the HCs and CRC patients in the GSE18392 dataset; C: Receiver operating characteristic analysis of miR-627 to distinguish CRC patients from HCs in the GSE41655 dataset; D: Receiver operating characteristic analysis of miR-627 to distinguish CRC patients from HCs in the GSE18392 dataset. HCs: Healthy controls; CRC: Colorectal cancer. bP < 0.01; cP < 0.001.
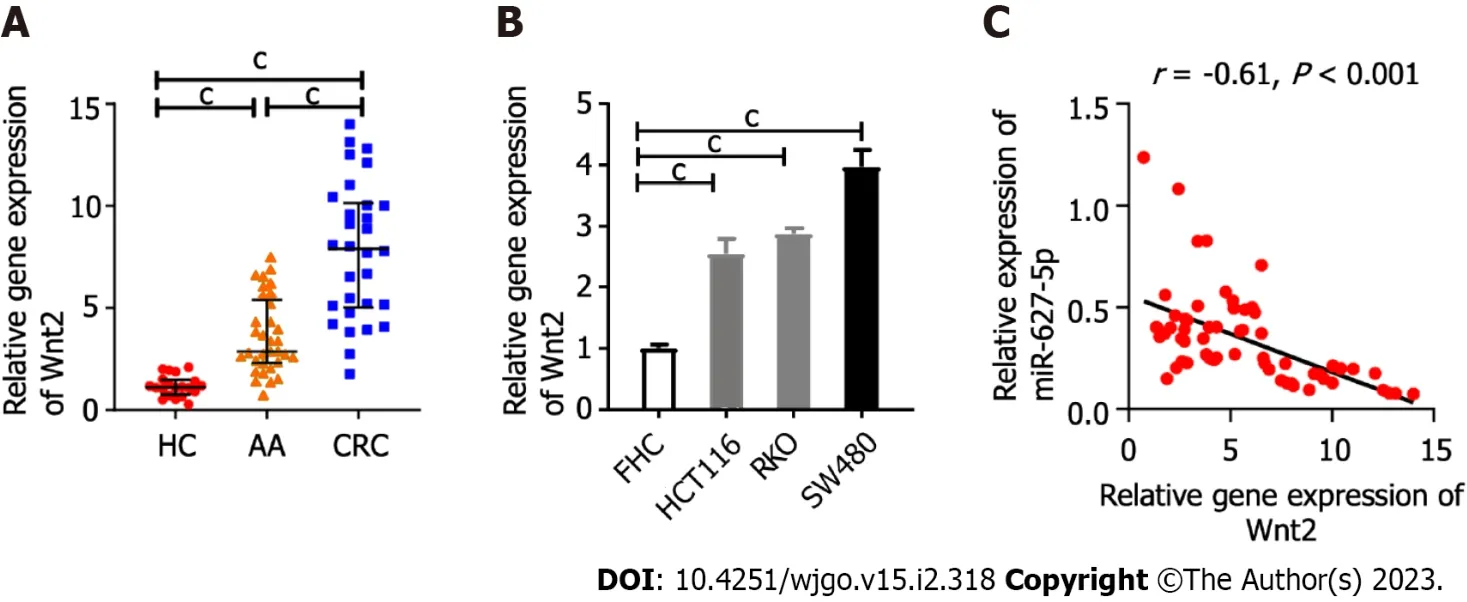
Figure 2 Inverse correlation between Wnt2 and microRNA-627-5p expression in colorectal neoplasm tissues. A: The mRNA expression levels of Wnt2 in healthy control tissues, advanced adenoma tissues and colorectal cancer (CRC) tissues; B: The mRNA expression levels of Wnt2 in CRC cell lines (SW480, HCT116, and RKO cells) and epithelial cell line cell line; C: The relationship between miR-627-5p and Wnt2 mRNA expression in colorectal neoplasm tissues. HCs: Healthy controls; CRC: Colorectal cancer; AA: Advanced adenoma; FHC: Colonic epithelial cell line. cP < 0.001.
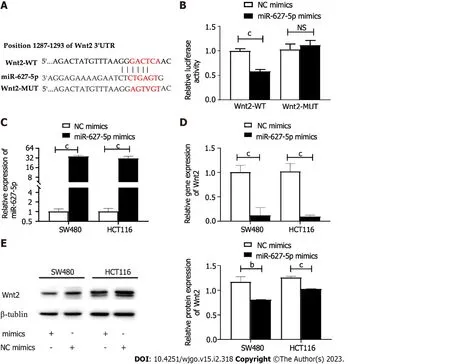
Figure 3 The direct binding relationship between microRNA-627-5p and Wnt2. A: Schematic illustration of the predicted binding sites between microRNA-627-5p (miR-627-5p) and Wnt2 mRNA; B: Dual luciferase reporter assays in HEK-293T cells. Experimental group: NC mimics + pcDNA-Wnt2-WT, miR-627-5p mimics + pcDNA-Wnt2-WT, NC mimics + pcDNA-Wnt2-MUT, miR-627-5p mimics + pcDNA-Wnt2-MUT; C: The transfection efficiency of miR-627-5p mimics in SW480 and HCT116 cells; D: The effects of miR-627-5p overexpression on the transcript expression levels of Wnt2 in SW480 and HCT116 cells; E: The effects of miR-627-5p overexpression on the protein expression levels of Wnt2 in SW480 and HCT116 cells. 3’ UTR: 3’-untranslated region; NS: Not significant; bP < 0.01; cP < 0.001.
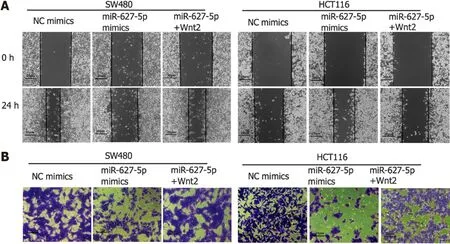
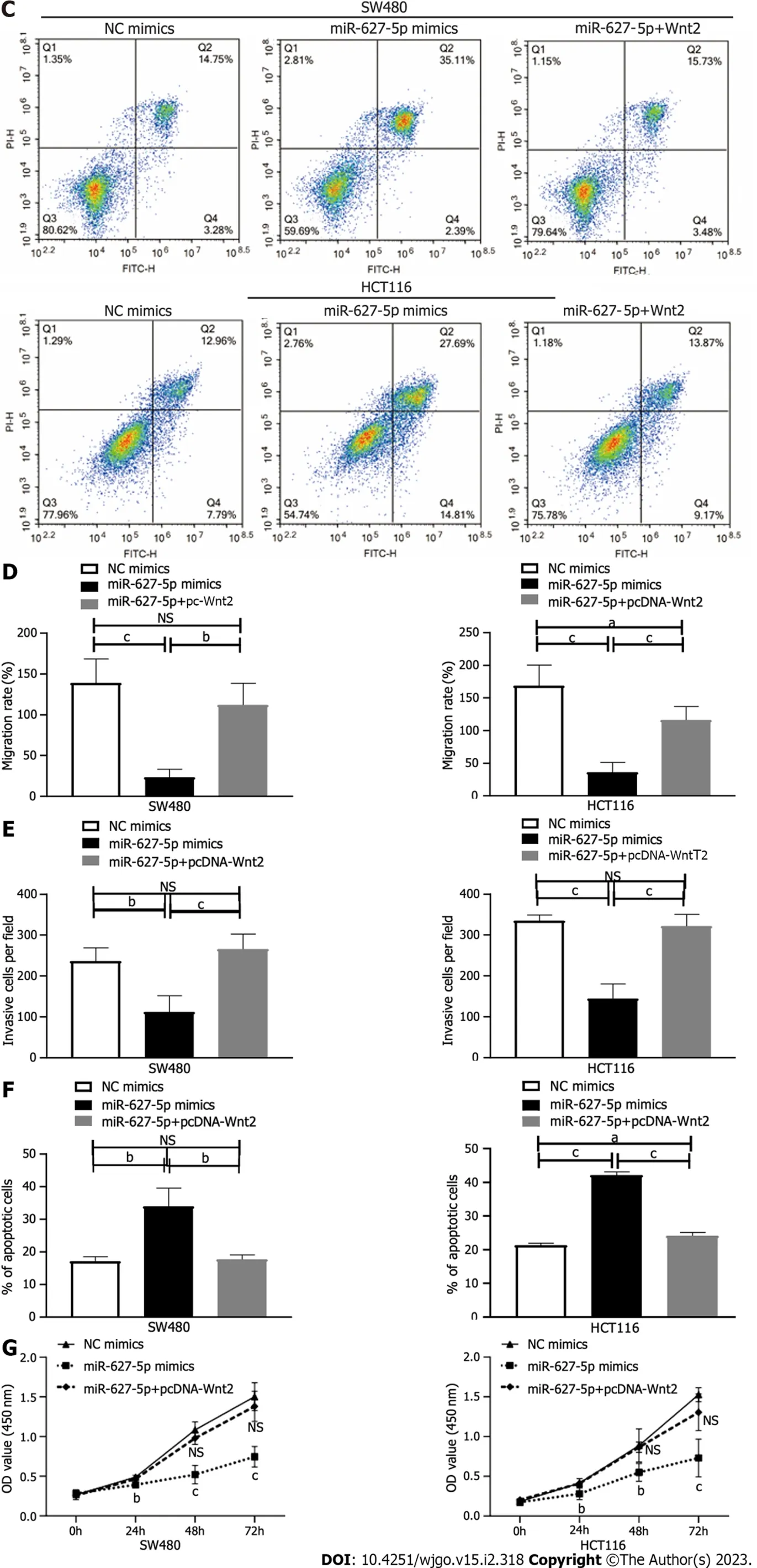
Figure 4 Cellular behaviours induced by microRNA-627-5p mimics and Wnt2 overexpression plasmids in SW480 and HCT116 cells. Experimental group: NC mimics, microRNA-627-5p (miR-627-5p) mimics, miR-627-5p mimics + pcDNA-Wnt2. A and D: Scratch assay was used to detect the migration of colorectal cancer (CRC) cells in each group; B and E: Matrigel invasion assay was used to detect the invasive capability of CRC cells in each group; C and F: Flow cytometry analysis was used to evaluate the apoptosis of CRC cells in each group; G: Cholecystokinin octapeptide (CCK-8) assay was used to detect the viability of CRC cells in each group. NS: Not significant; CRC: Colorectal cancer. aP < 0.05; bP < 0.01; cP < 0.001.
Function of miR-627-5p in the Wnt/β-catenin signalling pathway
To clarify the signalling pathways influenced by the miR627-5p/Wnt2 axis in CRC cells, we conducted qRT-PCR and western blot analysis to evaluate dysregulated genes in the classical Wnt signalling. In Figures 6 and 7, miR-627-5p overexpression in SW480 and HCT116 cells led to a sharp decline in the transcript and protein levels of β-catenin, c-myc, CD44, and cyclin D1. Next, we monitored the expression of β-catenin, c-myc, CD44, and cyclin D1 by treating miR-627-5p overexpressing CRC cells with pcDNA-Wnt2 or scramble vector. The findings illustrated that the transcript and protein levels of β-catenin, c-myc, CD44, and cyclin D1 were rescued at least partly by the overexpression of Wnt2. Consequently, it was suggested miR-627-5p reduces the gene and protein levels of downstream Wnt/βcatenin signalling componentsviaWnt2.
DISCUSSION
In our study, we concentrated on whether miR-627-5p, a rarely reported miRNA in CRC, could exert a suppressive effect on CRC development. First, we selected two GEO datasets to compare the expression of miR-627 in colorectal tumour patients and HCs, and the findings showed the decreased levels of miR-627 in cancer tissues in both GEO datasets. Consistent with our results, a study published in 2013 found the expression of miR-627 were observably downregulated in CRC tissues when compared to those in control tissues[21]. Unfortunately, this study did not distinguish the 5p and 3p forms of miR-627. In our previous study, we collected CRC and AA tissues to assess the expression of miR-627-5p and showed significantly decreased expression in CRC and AA tissues compared to HC tissues. Besides, miR-627-5p was found to be deceased in CRC cell lines in comparison with those in FHC cells[20]. According to these results, miR-627-5p expression was reduced in CRC.
Next, we conducted functional experiments using SW480 and HCT116 cells by transfecting miR-627-5p mimics or NC mimics to clarify the biological function of miR-627-5p in colorectal tumour. According to the findings, miR-627-5p greatly reduced cancer cells’ ability to migrate, invade, and proliferate while also accelerating apoptosis, which was in accordance with past researches in other cancer types. For instance, miR-627-5p is markedly reduced in hepatocellular carcinoma and negatively correlated with the prognosis of cancer patients. miR-627-5p silencing promotes cell multiplication and cell cycle progression of hepatocellular carcinoma cells[9]. In oral squamous cell carcinoma, LINC00958 promotes tumour cell growth, delays apoptosis, and accelerates cell migration and epithelialmesenchymal transition by suppressing the expression of miR-627-5p[12]. Thus, miR-627-5p is regarded as a tumour suppressor and could serve as a target for the treatment of cancer in the future.
A variety of literature have elucidated that the loss or enhancement of miRNA function is mainly involved in cancer carcinogenesis and progression by targeting the expression of cancer-causing or cancer-suppressing genes[22]. To clarify the key mechanism of miR-627-5p in suppressing CRC growth, we used an online tool to excavate the possible downstream genes of miR-627-5p. We subsequently discovered that miR-627-5p might have a complementary site within the Wnt2 3′-UTR. A series of studies have claimed that Wnt2 contributes to the development of numerous malignant malignancies[17,23-26]. For example, the Wnt2 gene is almost undetectable in the normal gastrointestinal tract but is highly upregulated in precancerous adenomas, primary colorectal tumours and liver metastases[27]. High expression of Wnt2 is implicated as a critical factor in promoting the invasive and metastatic potential of CRC cells[26]. In concordance with past researches, the current study confirmed that Wnt2 mRNA levels were considerably elevated in colorectal neoplasm tissues and inversely related to miR-627-5p levels, suggesting that miR-627-5p might participate in intestinal carcinogenesis by regulating the expression of Wnt2.
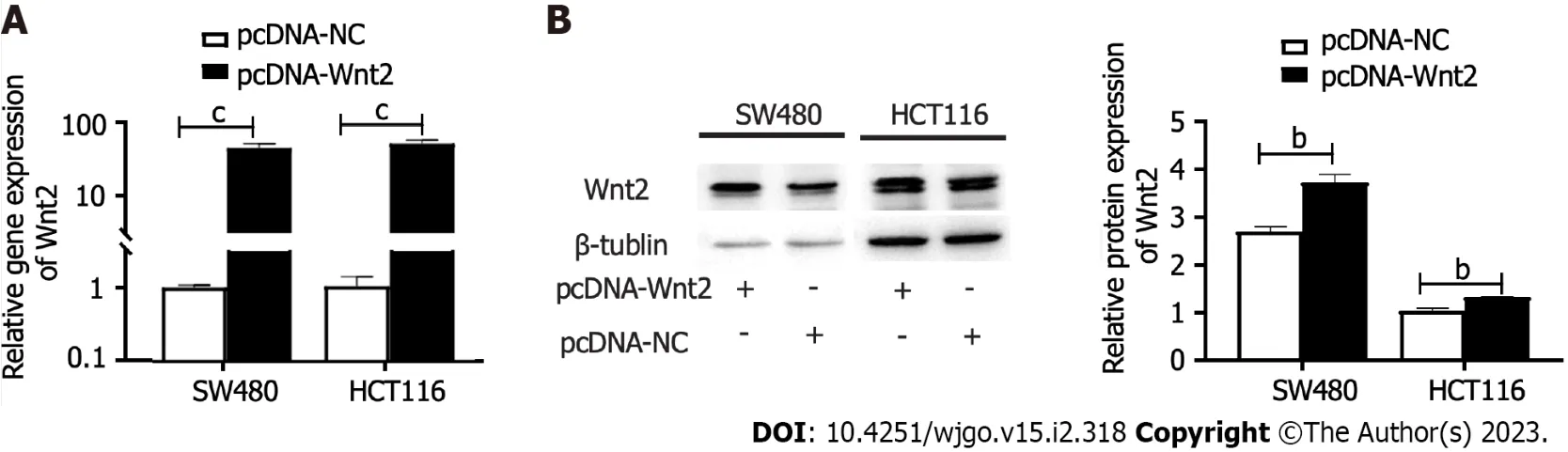
Figure 5 Identification of the transfected efficiency of Wnt2 overexpression plasmids. A: The mRNA expressions of Wnt2 in SW480 and HCT116 cells after the transfection of Wnt2 overexpression plasmids; B: The protein expressions of Wnt2 in SW480 and HCT116 cells after the transfection of Wnt2 overexpression plasmids. bP < 0.01; cP < 0.001.
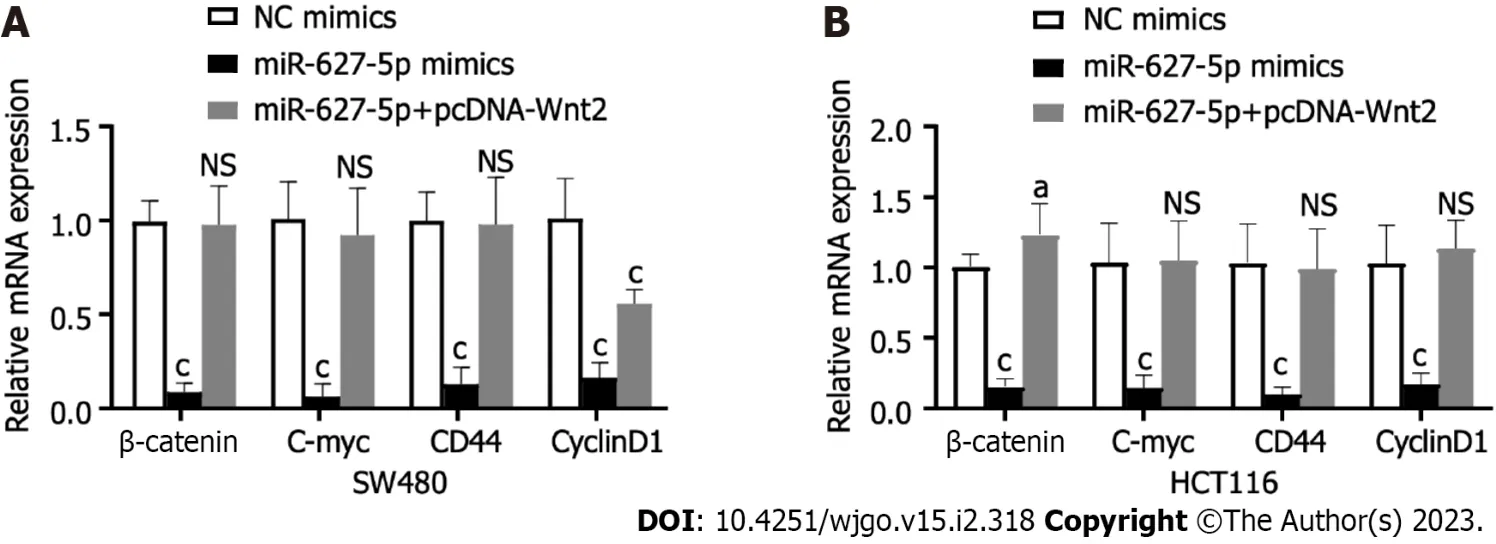
Figure 6 The mRNA expression alterations of downstream target genes in the Wnt/β-catenin signalling induced by miR-627-5p mimics and Wnt2 overexpression plasmids in SW480 and HCT116 cells. A: SW480 cells; B: HCT116 cells. Experimental group: NC mimics, miR-627-5p mimics, miR-627-5p mimics + pcDNA-Wnt2. NS: Not significant; bP < 0.01; cP < 0.001.
As is well-known, Wnt2 is one of the critical ligands that regulates the activity of the Wnt/β-catenin signalling, while aberrant activation of the classical Wnt signalling is a major driver of colorectal carcinogenesis[28,29]. Herein, we hypothesized that miR-627-5p might regulate Wnt2 expression, thereby modulating the Wnt/β-catenin signalling, and exerting its tumour suppressive function on CRCin vitro. Normally, β-catenin, the essential element of the canonical Wnt pathway, is continually eliminated by the destruction complex (AXIN, GSK3β, CK1, and APC) without canonical Wnt ligands. The constant degradation of β-catenin leads to low level of free β-catenin in the cytoplasm and the repression of Wnt target genes. Conversely, the Wnt pathway is activated when canonical Wnt ligands bind to their receptors on the cell surface and subsequently cause the aggregation of the degradation complex, resulting in the accumulation of β-catenin in the cytoplasm. Then, β-catenin gradually migrates to the nucleus, where it serves as a co-activator for T-cell specific factor/lymphoid enhancerbinding factor to activate Wnt target genes such as cyclinD1[30], CD44[31], and c-myc[32], which are identified to be involved in the malignant tendency of cancer cells, including stemness, tumorigenicity, metastasis, and chemoresistance[33-35]. To verify this hypothesis, we investigated how miR-627-5p affected the expression of the Wnt/β-catenin signalling like β-catenin, cyclinD1, c-myc, and CD44. Our current study revealed that upregulation of miR-627-5p could effectively decrease the mRNA and protein expression of β-catenin, cyclinD1, c-myc and CD44, whereas the suppressive effects of miR-627-5p could be partially canceled by Wnt2 overexpression. These results suggested that miR-627-5p/Wnt2 regulates the canonical Wnt pathway in CRC cells.
There are some limitations in the study. First, we performed transient transfection to increase the levels of miRNAs in CRC cell lines. Since miRNAs do not integrate into the cellular genome, the typical effects can only last for several days, and we could not assess the long-term effects of miRNAs. Stable transfection of miRNAs is required to achieve the long-term effects of miR-627-5p on tumour progression. Second, the translocation of cytoplasmic β-catenin to the nucleus is a crucial step in the activation of Wnt signaling. However, the current study only detected alterations in β-catenin in whole cells, not nuclear alterations. Finally, to learn more about the impact of miR-627-5p on the development of tumors in vivo, nude mouse carcinogenesis tests are necessary.
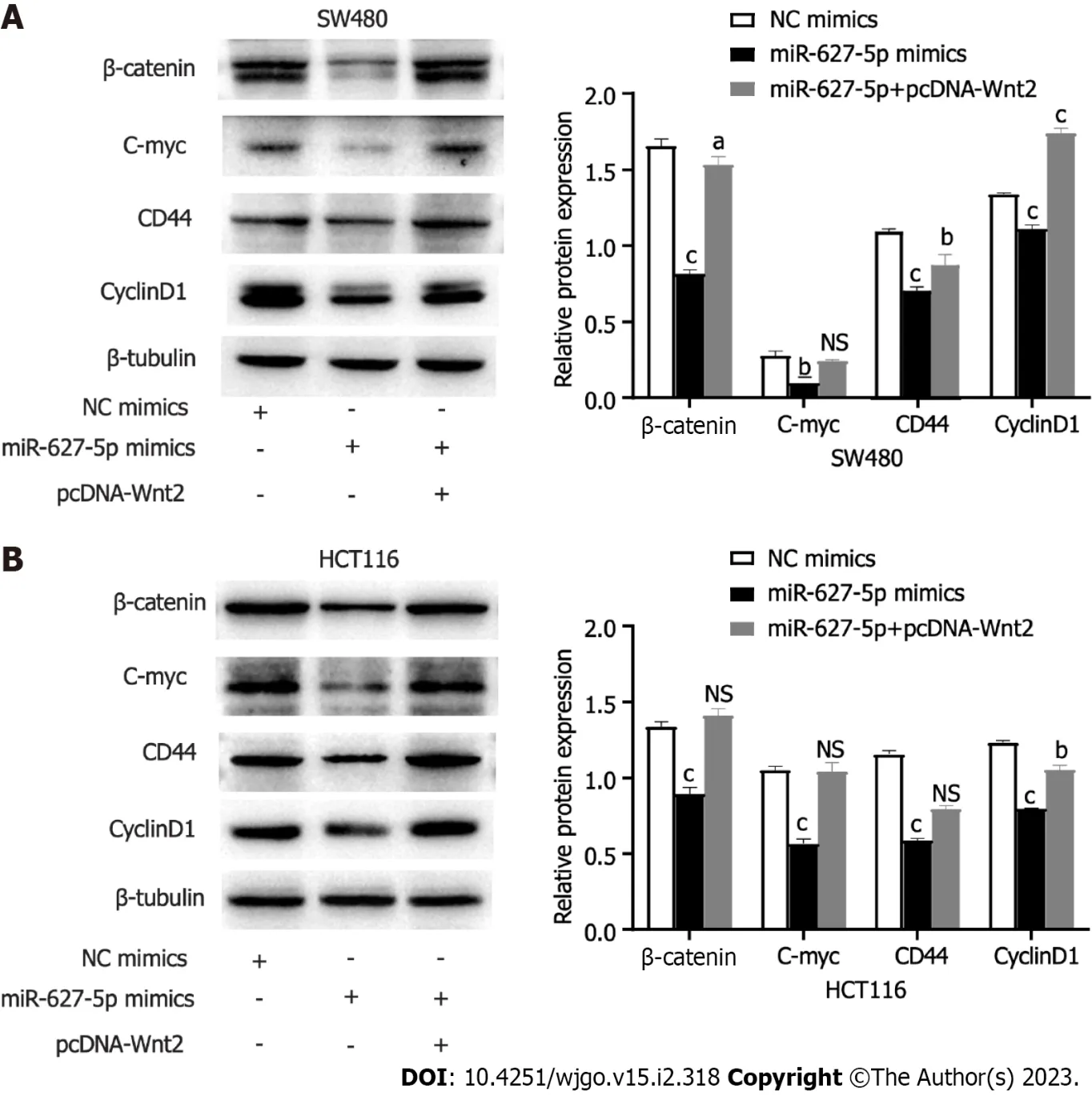
Figure 7 The protein expression alterations of downstream target genes in the Wnt/β-catenin signalling induced by microRNA-627-5p mimics and Wnt2 overexpression plasmids in SW480 and HCT116 cells. A: SW480 cells; B: HCT116 cells. Experimental group: NC mimics, miR-627-5p mimics, miR-627-5p mimics + pcDNA-Wnt2. NS: Not significant. aP < 0.05; bP < 0.01; cP < 0.001.
CONCLUSION
In summary, miR-627-5p could inhibit the malignant tendencies of CRC cells by directly inhibiting Wnt2 expression. The tumour suppressive effects were mainly achieved by inhibiting the activation of the classical Wnt/β-catenin signalling and the levels of its downstream target factors. These findings not only advance our understanding of the pathogenesis of CRC, but also provide evidence for an exploitable therapeutic target for CRC patients.
ARTICLE HIGHLIGHTS
Research background
Population aging has given rise to the incidence rate of colorectal cancer (CRC) worldwide. Better elucidation of the mechanisms underlying the formation and growth of CRC is very helpful for the development of new therapy.
Research motivation
Latest studies have shown that miRNAs generally regulate the expression of oncogenes or tumour suppressor genes and exert integral roles in modulating cancer-related pathways and mediating the formation and progression of CRC. However, whether miR-627-5p is involved in the tumorigenesis of colorectal tumours or not is largely unexplored.
Research objectives
This current study is designed to verify the function of miR-627-5p in colorectal tumorigenesis by targeting Wnt2/β-catenin signalling pathway.
Mickey Mantle7 drove the ball right over the center field wall, he said. Just a straight line climb in right out of the stadium. He looked out the window as if trying to pick the ball out of the cloud formations. I tried to imagine Mickey Mantle wearing an apron.
Research methods
The levels of miR-627-5p and Wnt2 were detected in CRC tissues. Functional experiments, including CCK-8, flow cytometry, Matrigel invasion, and scratch assays, were conducted to elucidate the function of miR-627-5p and wnt2 in colorectal tumour cells. Dual luciferase reporter tests were carried out to investigate how miR-627-5p and Wnt2 interact. The critical signalling pathway modulated by miR-627-5p was further identified.
Research results
Wnt2 transcript expression was markedly increased in colorectal tumour tissues and negatively correlated with miR-627-5p level. Upregualtion of miR-627-5p inhibited cancer cells’ abilities to invade growth and migrate by directly restraining Wnt2 expressions. Furthermore, miR-627-5p exerted the suppressive role in CRCviainactivating the Wnt2/β-catenin signalling.
Research conclusions
miR-627-5p restrained the malignant biological properties of CRC cellsviadirectly inhibiting Wnt2 expression to modulate the classical Wnt/β-catenin signalling.
Research perspectives
miRNA-627-5p/Wnt2/β-catenin may have potential therapeutic application for CRC.
FOOTNOTES
Author contributions:Zhao DY designed and performed the study, analyzed the data, and drafted the manuscript; Yin TF and Sun XZ collected colorectal samples from subjects, and provided guidance on experimental procedures; Zhou YC, Wang QQ, and Zhou GYJ collected the clinical data and colorectal samples from the subjects; Yao SK designed the study, supervised the study performance, revised the manuscript, and obtained the funding; all authors read and approved the final manuscript.
Supported bythe National Key Development Plan for Precision Medicine Research, No. 2017YFC0910002.
Institutional review board statement:This study was reviewed and approved by the Ethics Committee of China-Japan Friendship Hospital (Approval No. 2018-116-K85).
Informed consent statement:All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement:There are no conflicts of interest to report.
Data sharing statement:No additional data are available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Dong-Yan Zhao 0000-0002-7026-068X; Teng-Fei Yin 0000-0003-1140-8637; Xi-Zhen Sun 0000-0001-9967-5726; Yuan-Chen Zhou 0000-0001-6024-6246; Qian-Qian Wang 0000-0002-7709-2121; Ge-Yu-Jia Zhou 0000-0002-4196-1436; Shu-Kun Yao 0000-0002-8512-2589.
S-Editor:Chen YL
L-Editor:A
P-Editor:Zhang XD
 World Journal of Gastrointestinal Oncology2023年2期
World Journal of Gastrointestinal Oncology2023年2期
- World Journal of Gastrointestinal Oncology的其它文章
- Comment on “Crosstalk between gut microbiota and COVID-19 impacts pancreatic cancer progression”
- Prognostic value of claudin 18.2 expression in gastric adenocarcinoma
- Potent bromodomain and extraterminal domain inhibitor JAB-8263 suppresses MYC expression and exerts anti-tumor activity in colorectal cancer models
- Increased CD4/CD8 Lymphocyte ratio predicts favourable neoadjuvant treatment response in gastric cancer: A prospective pilot study
- Cancerous inhibitor of protein phosphatase 2A enhances chemoresistance of gastric cancer cells to oxaliplatin
- Evaluation of polygenic risk score for risk prediction of gastric cancer
