Rabdosia serra alleviates dextran sulfate sodium salt-induced colitis in mice through anti-inflammation,regulating Th17/Treg balance,maintaining intestinal barrier integrity,and modulating gut microbiota
Hongyi Li ,Yi Wng ,Shumin Sho ,Hui Yu ,Dqin Wng ,Chuyun Li ,Qin Yun ,Wn Liu ,Jiling Co ,Xiojun Wng ,Hibio Guo ,Xu Wu ,Shngpng Wng ,f,**
a State Key Laboratory of Quality Research in Chinese Medicine,Institute of Chinese Medical Sciences,University of Macau,Macao,999078,China
b Laboratory of Molecular Pharmacology,Department of Pharmacology,School of Pharmacy,Southwest Medical University,Luzhou,Sichuan,646000,China c Hutchison Whampoa Guangzhou Baiyunshan Chinese Medicine Co.,Ltd.,Guangzhou,510000,China
d College of Pharmacy,Shenzhen Technology University,Shenzhen,Guangdong,518118,China
e School of Pharmacy,Lanzhou University,Lanzhou,730000,China
f Macau Centre for Research and Development in Chinese Medicine,University of Macau,Macao,999078,China
Keywords:Rabdosia serra Chemical profile Colitis Inflammation Gut microbiota
ABSTRACT Rabdosia serra(R.serra),an important component of Chinese herbal tea,has traditionally been used to treat hepatitis,jaundice,cholecystitis,and colitis.However,the chemical composition of R.serra and its effect against colitis remain unclear.In this study,the chemical composition of the water extract of R.serra was analyzed using ultra performance liquid chromatography coupled with a hybrid linear ion trap quadrupole-orbitrap mass spectrometer(UPLC-LTQ-Orbitrap-MS).A total of 46 compounds,comprising ent-kaurane diterpenoids,flavonoids,phenolic acids,and steroids,were identified in the water extract of R.serra,and the extract could significantly alleviate dextran sulfate sodium salt-induced colitis by improving colon length,upregulating anti-inflammatory factors,downregulating proinflammatory factors,and restoring the balance of T helper 17/T regulatory cells.R.serra also preserved intestinal barrier function by increasing the level of tight junction proteins(zonula occludens 1 and occludin)in mouse colonic tissue.In addition,R.serra modulated the gut microbiota composition by increasing bacterial richness and diversity,increasing the abundance of beneficial bacteria(Muribaculaceae,Bacteroides,Lactobacillus,and Prevotellaceae_UCG-001),and decreasing the abundance of pathogenic bacteria(Turicibacter,Eubacterium_fissicatena_group,and Eubacterium_xylanophilum_group).Gut microbiota depletion by antibiotics further confirmed that R.serra alleviated colitis in a microbiota-dependent manner.Overall,our findings provide chemical and biological evidence for the potential application of R.serra in the management of colitis.
1.Introduction
Inflammatory bowel disease(IBD),which mainly includes Crohn's disease(CD)and ulcerative colitis(UC),is a chronic and multifactorial inflammatory disease of the intestinal tract[1].Over the last few decades,the incidence of IBD increased by more than two times in several East Asian countries and will continue to increase in next decade[2].Recent studies have indicated that IBD is closely related to disrupted intestinal barrier function and immune function imbalance[3-5].Moreover,changes in the diversity,structural composition,and function of the intestinal microbiome caused by abnormal inflammatory response can lead to the development of IBD[6-8].The intestinal microbiome plays a critical role in nutrition uptake,immune response,metabolism,and pathogen defense[9].
Current treatments for IBD include aminosalicylates(e.g.,5-aminosalicylic acid(5-ASA)and mesalazine),corticosteroids(e.g.,methylprednisolone and dexamethasone),antibiotics(e.g.,metronidazole and ciprofloxacin),immunomodulators(e.g.,methotrexate and 6-mercaptopurine),biological agents,and surgery in severe IBD cases[10,11].However,owing to the limited therapeutic efficacy,high economic cost,and side effects of long-term treatments,there is an urgent need to discover novel effective drugs for IBD treatment.Previous studies have reported phytotherapy as one of the most valuable therapies for the management of intestinal disorders[7,12].Certain medicinal herbs,such asAbelmoschus Manihot[7],Rhodiola crenulate[12],Curcuma longa[13],andGinkgo biloba[14],and their derived natural compounds have received considerable attention for their marked alleviation of IBD symptoms.
Rabdosia serra(R.serra)(Maxim.)Hara(genus ofIsodon),also known as“Xihuangcao”,is mainly distributed in the southern regions of China,including Guangdong,Fujian,and Guangxi,as well as other subtropical and tropical parts of Asia[15-17].It is one of the most important herbs in Chinese herbal tea.In China,it has long been used as a local indigenous medicine for the treatment of enteritis,jaundice,hepatitis,and acute cholecystitis[17].Although the chemical profile ofR.serrais unclear,previous phytochemical studies have shown thatent-kaurane diterpenoids,flavonoids,phenolic acid,and steroids are its main bioactive constituents[18-20].Some of these compounds exhibit promising antioxidative,antiinflammatory,antihypotonic,antibacterial,and anticancer activities[16,19,21-23].Furthermore,oridonin and its derivatives from plants belonging to the genusIsodonhave been shown to inhibit the proliferation of splenic Th1/Th17 cells and affect memory CD4+T cells in trinitrobenzene sulfonic acidinduced colitis.Because of their abundant diterpenes,Isodonplants have recently been shown to have a significant effect on colorectal cancer treatment[22,23].Furthermore,the dietary supplementation of oridonin,the mainent-kaurane diterpenoid inIsodonplants,can significantly improve intestinal health[24].Therefore,investigating the effects ofR.serraagainst IBD and the underlying mechanisms is clinically relevant.
This study aimed to investigate the effects of the water extract ofR.serraas a dietary supplement on dextran sulfate sodium(DSS)salt-induced colitis as well as its interaction with the gut microbiota,inflammatory factors,tight junction proteins,and T helper 17(Th17)/regulatory T(Treg)cells and its regulation of the gut microbiota.In addition,the chemical components of the water extract ofR.serrawere analyzed using ultra-performance liquid chromatography coupled with linear trap quadrupole-Orbitrap mass spectrometry(UPLC-LTQ-Orbitrap-MS).
2.Materials and methods
2.1.Chemicals and materials
Reference standards,including caffeic acid,quercetin,isoquercitrin,ferulic acid,vitexin,vicenin II,schaftoside,isoschaftoside,rutin,hyperoside,salicylic acid,rosmarinic acid,daidzein,oleanolic acid,stigmasterol,epinodosin,methyl rosmarinate,kamebakaurin,shikokianin,lasiokaurin,oridonin,lasiodonin,ponicidin,and nodosin were purchased from Refmedic Biotechnology Co.,Ltd.(Chengdu,China).Additionally,5-ASA was purchased from Aladdin Biochemical Technology Co.,Ltd.(Shanghai,China),while DSS(molecular weight:36-50 kDa)was obtained from International Laboratory USA(San Francisco,CA,USA).The antibiotics,namely,vancomycin,neomycin sulfate,ampicillin,and metronidazole,were purchased from Shanghai Macklin Biochemical Co.,Ltd.(Shanghai,China).Mouse serum interleukin(IL)-17,IL-4,transforming growth factor(TGF)-β1,and interferon(IFN)-γ enzyme-linked immunosorbent assay(ELISA)kits were obtained from Novus Biologicals(Littleton,CO,USA).A lipopolysaccharide(LPS)ELISA kit was provided by CUSABIO(Wuhan,China).A fixation/permeabilization solution kit(BD GolgiPlug)and CD25,Foxp3,CD4,and IL-17A antibodies used for Th17 and Treg cell phenotyping were purchased from BD Biosciences(Franklin Lakes,NJ,USA).
2.2.Preparation of the water extract of R.serra
The aerial parts of air-driedR.serra(100 g)were boiled(100°C)in ultrapure water(600 mL)for 1 h.This step was repeated twice under the same extraction conditions;then both the samples were centrifuged(5,000 rpm for 10 min at 4°C)and the supernatants were pooled.This supernatant was concentrated and dried in vacuo to yield a 10.3 g of the water extract ofR.serra.
2.3.UPLC-LTQ-Orbitrap-MS for chemical characterization
The chemical analysis of the water extract ofR.serrawas performed based on our previously reported methods with slight modification[25,26].UPLC-LTQ-Orbitrap-MS analysis was performed using a DionexUltiMate 3000 rapid separation binary UPLC system(Thermo Fisher Scientific Inc.,Bremen,Germany)equipped with an LTQ-Orbitrap XL mass spectrometer and electrospray ionization(ESI)source.Hypersil gold C18column(150 mm×2.1 mm,1.9μm)was used for the chromatographic separation at 35°C.Formic acid(0.1%,V/V)solution(A)and acetonitrile(B)were used as mobile phases.The gradient elution program was performed as follows:0-2 min,5% B;2-25 min,5%-95% B;25-28 min,95% B;28-29 min,95%-5% B;29-30 min,5% B.The flow rate was set at 0.4 mL/min,and 4μL of the water extract ofR.serra(final concentration of 0.5 mg/mL)was injected for analysis.
Detection using LTQ-Orbitrap-MS was performed in both negative and positive ESI modes.The MS parameters of the positive/negative ion modes were as follows:capillary temperature,380°C/380°C;capillary voltage,+35 V/-35 V;ion spray voltage,+4.5 kV/-4.5 kV;and tube lens voltage,+110 V/-100 V.Reference masses were recorded at anm/zrange of 100-1,500.
2.4.Animal experiments
All animal experiments and animal care procedures were performed in accordance with the guidelines of Committee on Use and Care of Animals of Southwest Medical University(reference no.:2020226).SPF-grade C57BL/6 male mice aged 6-8 weeks were obtained from Spelford Biotechnology Co.,Ltd.(Beijing,China)and housed under pathogen-free conditions in a regular 12 h light/dark cycle at 22°C±2°C and relative humidity of 55%-60%.The mice were givenad libitumaccess to food and sterilized water.All mice were acclimatized for 7 days before the experiment.
Mice were randomly divided into five groups(n=8)as follows:control,DSS,5-ASA(positive control),R.serra(RS)-75,and RS-150.Mice in the DSS,5-ASA,RS-75,and RS-150 groups were administered with DSS(2.8%,m/V)in sterilized drinking water for 6 days(from day 0 to day 6)to induce acute colitis.From day 5 to day 10,mice in the DSS,5-ASA,RS-75,and RS-150 groups were orally administered with water,5-ASA(100 mg/kg),R.serra(75 mg/kg),andR.serra(150 mg/kg),respectively,daily.The control group was orally administered with water daily.Body weight change and disease activity index(DAI)score were assessed daily.The DAI score criteria are outlined in Table S1.On days 0 and 10,mouse feces were collected and stored at-80°C within 2 h for further microbial analysis.On day 11,mice were euthanatized and their entire colons were excised.The colons were measured and then washed gently with phosphate-buffered saline(PBS,pH=7.4).Blood was collected and centrifuged to obtain serum samples for ELISA.
2.5.Histological analysis
Hematoxylin and eosin(H&E)staining of colonic tissue was performed for histological analysis as previously described[12].Briefly,distal colon segments(approximately 1 cm)were fixed in formalin(4%,m/V)and embedded in paraffin after dehydration.Subsequently,images of the H&E stained sections were acquired using a Nikon Eclipse Ts2R+FL microscope(Nikon Instruments Inc.,Tokyo,Japan).The histological score was recorded according to the previously reported criteria[12].
2.6.Evaluation of inflammatory factors
The serum and colonic levels of pro-inflammatory(IL-17 and IFN-γ)and anti-inflammatory(TGF-β1 and IL-4)factors were evaluated using ELISA kits according to the manufacturer's instructions.
2.7.Flow cytometry analysis
Mouse mesenteric lymph nodes(MLNs)were harvested and gently pressed through a cell strainer in precooled PBS to obtain a single cell suspension(1×106cells/mL).For Th17 cell intracellular staining,single cells were prestimulated using GolgiPlug(purchased from BD Biosciences,Franklin Lakes,NJ,USA)for 6 h at 37°C at a concentration of 1μL/mL.For the staining of cell surface markers,cells were incubated with anti-CD4 antibody at 4°C for 30 min in the dark and then fixated and permeabilized for 20 min under the same conditions.Subsequently,the cells were incubated with anti-IL-17A antibody for 1 h at 4°C for intracellular staining.For the staining of Treg cells,single cells were incubated with anti-CD25 antibody for 0.5 h.After fixation and permeabilization,the cells were stained with anti-Foxp3 antibody at 4°C for 1 h in the dark.The flow cytometric experiment was performed using FACSVerse(BD Biosciences),and the data were analyzed using FlowJo 10 software(TreeStar,San Carlos,CA,USA).
2.8.Immunohistochemical(IHC)analysis
To determine the colonic levels of tight junction proteins(such as zonula occludens 1(ZO-1)and occludin),IHC analysis was performed according to our previously reported method[12].Briefly,after deparaffinization,rehydration,and antigen retrieval,the colonic sections were treated with H2O2(3%,V/V)to block endogenous peroxidase and then incubated with bovine serum albumin(3%,m/V).Colonic sections were then incubated with primary antibodies against ZO-1 and occludin at 4°C overnight in the dark.After washing with PBS,the slides were immersed in corresponding horseradish peroxidase-linked secondary antibodies.Antibody signals were visualized with diaminobenzidine(DAB)and hematoxylin under an optical microscope.Positive DAB-stained areas were quantified using the ImageJ software(Version 1.48v,National Institute of Health,Bethesda,MD,USA).
2.9.16S rRNA analysis
Microbiome DNA extraction and 16S rRNA gene sequencing were performed according to our previously reported method[12].16S rRNA gene sequencing was performed on an Illumina MiSeq platform according to the manufacturer's instruction(Majorbio Bio-Pharm Technology Co.,Ltd.,Shanghai,China).
The diversity of the gut microbiota was analyzed using Majorbio Cloud Platform.16S rRNA raw fastq files were demultiplexed and quality-filtered using Trimmomatic[12].Operational taxonomic units(OTUs)were clustered with 97% sequence similarity using UPARSE.UCHIME and Silva(SSU123)were used for chimeric sequences and taxonomy,respectively.R tools accompanied with the package mixOmics were used for principal coordinate analysis(PCoA)and partial least squares discriminant(PLS-DA)analysis.Distance-based redundancy analysis(db-RDA)and Spearman correlation were performed to analyze the correlation of microbial structure change with colitis abnormalities.
2.10.Statistical analysis
Statistical analysis was performed using the GraphPad Prism 8.0 software(GraphPad Software,La Jolla,CA,USA),and the results are expressed as means±standard deviation.One-way analysis of variance followed by a post hoc Tukey's test was used for multiple group comparisons.Pvalue less than 0.05 was considered statistically significant.
3.Results and discussion
3.1.Chemical analysis of the water extract of R.serra
Previous studies reported thatR.serraextracts contain diterpenoids,triterpenoids,flavonoids,phenolic acids,and other chemical compounds[15,16,19,27-29].As shown in Fig.1 and Table S2,a total of 46 compounds,includingent-kaurane diterpenoids,flavonoids,phenolic acid,steroids,and other compounds,were rapidly identified in the water extract ofR.serrathrough UPLC-LTQ-Orbitrap-MS analysis by comparison with reference standards and/or a previous report[18].The representative chromatograms are shown in Figs.1A and B.
As shown in Fig.1C,a total of 19ent-kaurane diterpenoids,including 3 C-20-nonoxygenatedent-kaurane diterpenoids and 16 C-20-oxygenatedent-kaurane diterpenoids(nine 7,20-epoxyent-kaurane,four enmein type,one 7,20-diepoxy-ent-kaurane,one 3,20-epoxy-ent-kaurane,and one spiro-lactone type)were tentatively identified.The C-20-nonoxygenated type,including compounds 26(amethystonoic acid),34(umbrosin B),and 44(inflexarabdonin J),were tentatively identified in the extract.The continuous loss of neutral H2O(-18 Da)at C-7 and C-11 was observed through cleavage of a hydroxyl group.Taking amethystonoic acid as an example,them/zvalues 345([M-H-H2O]-)and 327([M-H-2H2O]-)were obtained through a successive loss of H2O.Moreover,the characteristic ion fragments atm/z301([M-H-H2O-CO2]-)andm/z283([M-H-2H2O-CO2]-)were obtained through the cleavage of the hydroxyl group at C-7 and loss of CO2of the carbonyl group at C-15.
The 7,20-epoxy-ent-kaurane diterpenoids included compounds 7(1-α-O-β-D-glucopyranosyl enmenol),13(shikokiaside A),16(rubescensin S),23(rabdosinate),25(lasiokaurin),27(lasiokaurinol),28(megathyrin A),37(effusanin A),and 38(rabdocoetsin).C ompound 28,a representative 7,20-epoxy-ent-kaurane diterpenoid,was characterized by its loss of 18 Da(-H2O)successively from a hydroxyl group at C-1 and C-14 to give a fragment atm/z329([M-H-H2O]-)and 311([M-H-2H2O]-).Simultaneously,the distinctive fragment ions atm/z301 and 271 were identified by their loss of CO([M-H-H2O-28 Da]-)from the D ring and CH2O([M-H-H2O-CO-30 Da]-)from the C-20 oxygenated B ring.Similar cleavage patterns were observed in the other 7,20-epoxyent-kaurane diterpenoids.
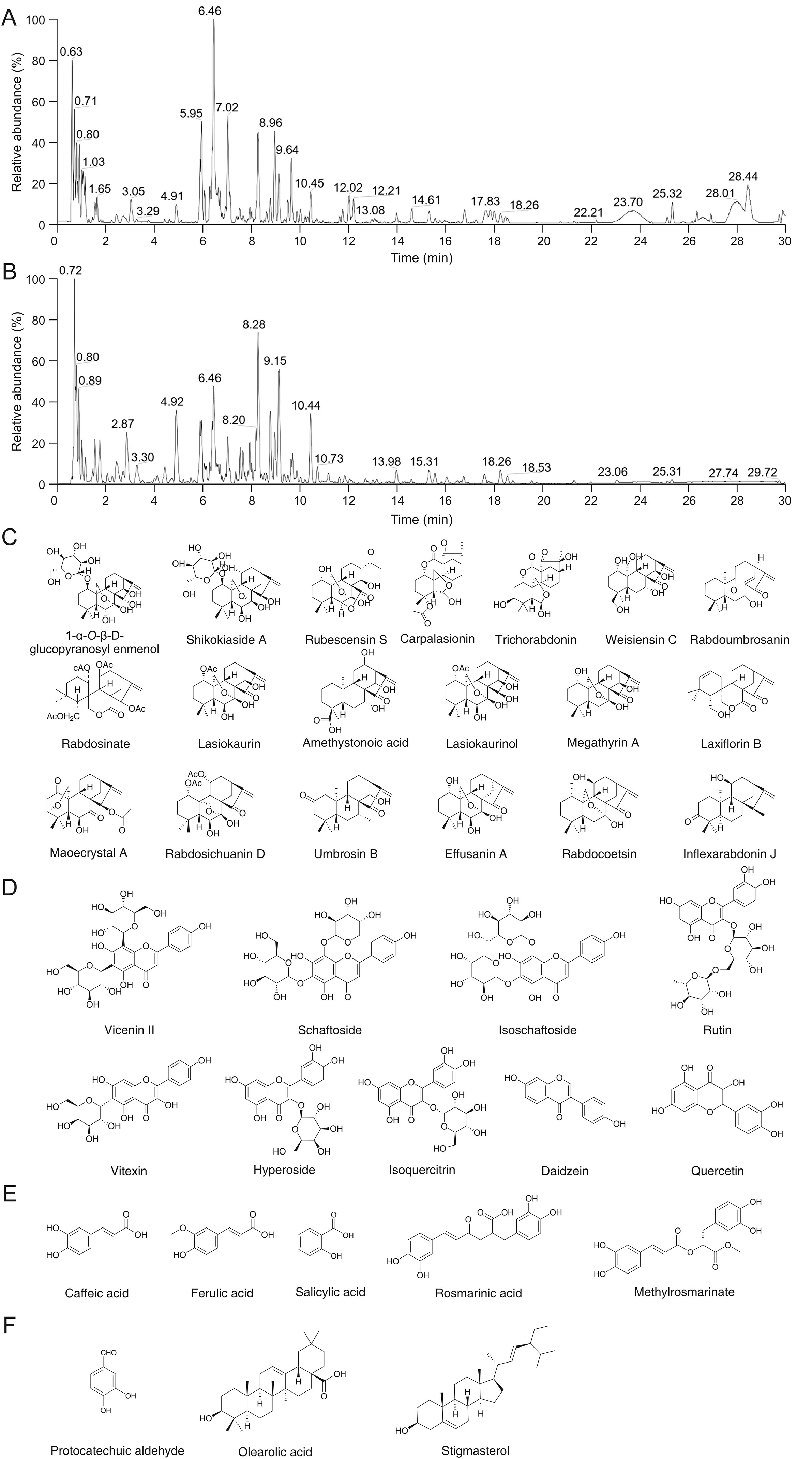
Fig.1.Chemical analysis of Rabdosia serra(R.serra).Ultra performance liquid chromatography total ion chromatogram of R.serra in(A)positive and(B)negative ion modes.(C)Entkaurane diterpenoids,(D)flavonoids,(E)phenolic acids,and(F)steroids and other compounds identified in R.serra.

Fig.2.R.serra showed strong efficacy in protecting against dextran sulfate sodium(DSS)salt-induced colitis in a mouse model.(A)Schematic illustration of the animal experimental procedure.(B)Body weight changes of each group were monitored daily and expressed as a percentage of initial weight.(C)Disease activity index(DAI)score.(D)Morphology of colons sampled from mice in each group.(E)Colon length.(F)Typical hematoxylin and eosin(H&E)-stained colonic sections.(G)Histopathological score.Ctrl:control group;DSS:DSS-induced colitis mice without other treatment;5-ASA:DSS-induced colitis mice treated with 5-aminosalicylic acid(5-ASA)as a positive control;RS-75:DSSinduced colitis mice treated with R.serra at a dose of 75 mg/kg;RS-150:DSS-induced colitis mice treated with R.serra at a dose of 150 mg/kg.Results are presented as means±SD,n=8 for each group.***P<0.001,compared to control;###P<0.001,compared to DSS.
Maoecrystal A was the only 3,20-epoxy-ent-kaurane diterpenoid found in the water extract of R.serra.In an ESI positive mode,it exhibited an ion peak[M-H]+at m/z 389.Distinctive fragment ions atm/z371 and 311 were obtained through a loss of H2O([M-H-18 Da]+)and AcOH([M-H-H2O-78 Da]+)from the deprotonated molecular ion[M-H]+.Moreover,a typical fragment ion atm/z269 was identified through the loss of 42 Da from the ion atm/z311([M-H-H2O-AcOH-CH2=CO]+).
Weisiensin C(22),the only 7,20-diepoxy-ent-kaurane found inR.serra,belongs to the C-20-oxygenated type.It was characterized from the tested ion([C20H30O6-H]-)atm/z365,and the characteristic fragment ions atm/z347([M-H-H2O]-),329([M-H-2H2O]-),and 321([M-H-3H2O]-)corresponded to the successive loss of neutral H2O.Simultaneously,the typical fragment ion([M-H-H2O-30 Da]-)was formed,thereby producing a characteristic ion atm/z307.A similar cleavage was observed for the fragment ion atm/z273([M-H-H2O-CO2]-)based on the fragment ion atm/z303.
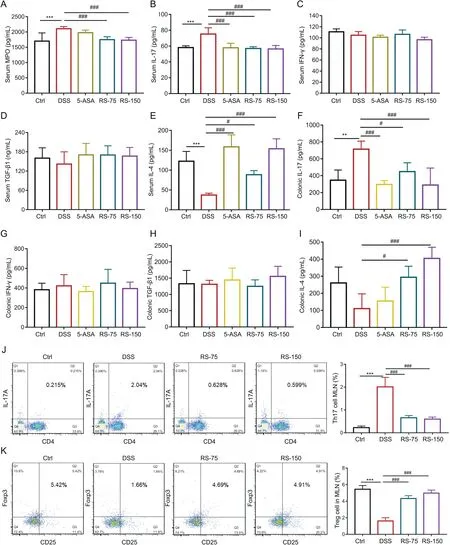
Fig.3.R.serra alleviated dextran sulfate sodium(DSS)-induced inflammation.Serum levels of(A)myeloperoxidase(MPO),(B)interleukin(IL)-17,(C)interferon(IFN)-γ,(D)transforming growth factor(TGF)-β1,and(E)IL-4.Colonic levels of(F)IL-17,(G)IFN-γ,(H)TGF-β1,and(I)IL-4.Flow cytometry analysis of(J)T helper 17(Th17)and(K)regulatory T(Treg)cells in mesenteric lymphoid nodes,with statistical data shown in the right panel.Th17 cells were assessed through CD4 and IL-17A.Treg cells were assessed through CD25 and Foxp3.Results are presented as means±SD,n=8 for each group.**P<0.01 and***P<0.001,compared to control;#P<0.05 and ###P<0.001,compared to DSS.Ctrl:control;DSS:DSS-induced colitis mice without other treatment;5-ASA:DSS-induced colitis mice treated with 5-aminosalicylic acid(5-ASA)as a positive control;RS-75:DSS-induced colitis mice treated with R.serra at a dose of 75 mg/kg;RS-150:DSS-induced colitis mice treated with R.serra at a dose of 150 mg/kg;MLN:mesenteric lymph nodes.
Four enmein typeent-kaurane diterpenoids were identified in the water extract ofR.serra:compounds 17(carpalasionin),19(trichorabdonin),31(rabdosichuanin D),and 42(rabdoumbrosanin).Carpalasionin,as an example,produced a deprotonated molecular ion[C22H28O7-H]-atm/z419.The characteristic fragment ions atm/z375([M-H-CO2]-)and 331(M-H-2CO2]-)were obtained through a successive loss of the CO2fragment.Moreover,the typical fragment ion atm/z263 occurred through the loss of CHOH based on the product ion atm/z290.
Laxiflorin B,a spiro-lactone typeent-kaurane diterpenoid,exhibited an ion peak[M-H]-atm/z343 with a distinctive deprotonated molecular ion[C20H24O5-H]-,which subsequently formed a typical ion atm/z299 through the loss of a neutral fragment CO2and obtaining a fragment ion atm/z281([M-H-CO2-H2O]-).The identified fragment ion atm/z255([M-H-CO-CH2O]-)was obtained through a subsequent loss of a CH2O fragment based on the product ion atm/z285.
Furthermore,nine flavonoids(Fig.1D),namely,compounds 4(vicenin II),5(schaftoside),6(isoschaftoside),8(rutin),9(vitexin),10(isoquercitrin),11(hyperoside),18(daidzein),and 21(quercetin),were characterized and identified through comparisons with standards and MS/MS data.Vicenin II produced abundant[C27H30O15-H]-ions atm/z593.The characteristic fragment ions[M-H-18 Da]-,[M-H-90 Da]-,and[M-H-C3H6O3-30 Da]-were formed through a loss of H2O,C3H6O3,and CH2O,respectively.Similar results were found for the fragment ions atm/z383[M-H-2C3H6O3-CH2O]-andm/z353[M-H-2C3H6O3-2CH2O]-based on the ion atm/z473.Schaftoside and isoschaftoside are isomers that formed a mass of[M+H]+ions atm/z565 in the ESI positive mode.A continual loss of a string of characteristic H2O fragment ions from the hydroxyl groups was observed atm/z547([M+H-H2O]+),529([M+H-2H2O]+),and 511([M+H-3H2O]+).Moreover,typical fragment ions atm/z499([M+H-2H2O-CH2O]+)and 469([M+H-2H2O-2CH2O]+)were formed through the cleavage of CH2O based on the product ionm/z529.
As shown in Fig.1E,five phenolic acids,namely,compounds 2(caffeic acid),3(ferulic acid),12(salicylic acid),15(rosmarinic acid),and 20(methyl rosmarinate)were identified in the extract and characterized further.All phenolic acids were identified by comparison with reference standards.
Furthermore,three steroids and other compounds,including compounds 1(protocatechuic aldehyde),45(oleanolic acid),and 46(stigmasterol),were identified in the extract(Fig.1F),among which oleanolic acid and stigmasterol were identified by comparison with the standards.
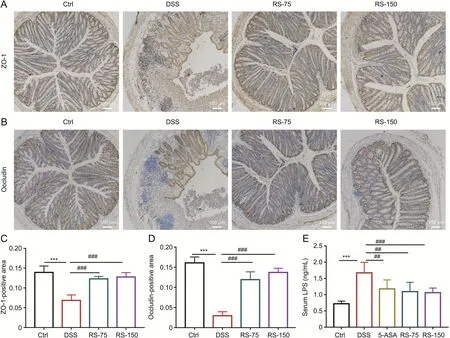
Fig.4.R.serra preserved intestinal barrier function.Immunohistochemical(IHC)analysis to determine the expressions of(A)zonula occludens 1(ZO-1)and(B)occluding proteins.The brown color between cells suggests positive expression of ZO-1 or occludin,and the blue staining represents the nucleus.(C)ZO-1 and(D)occludin quantified using ImageJ.(E)Serum lipopolysaccharide(LPS)levels in mice.Results are presented as means±SD,n=8 for each group.***P<0.001,compared to control;#P<0.05,##P<0.01 and ###P<0.001,compared to dextran sulfate sodium(DSS).Ctrl:control;DSS:DSS-induced colitis mice without other treatment;5-ASA:DSS-induced colitis mice treated with 5-aminosalicylic acid(5-ASA)as a positive control;RS-75:DSS-induced colitis mice treated with R.serra at a dose of 75 mg/kg;RS-150:DSS-induced colitis mice treated with R.serra at a dose of 150 mg/kg.
3.2.Antioxidant capacities of R.serra in vitro
Inflammation is closely linked to excessive oxidative stress.Medicinal herbs containing polyphenols can help increase antioxidant capacityand decrease oxidative stress[30].Antioxidant enzymes such as superoxide dismutase(SOD)play a key role in protecting cells against oxidative stress.Glutathione(GSH),an antioxidant substance,also plays a critical role in balancing oxidative stress[31].Therefore,in this study,the levels of SOD(Fig.S1A)and GSH(Fig.S1B)in H2O2-induced Caco-2 cells were determined to evaluate the antioxidant capacities ofR.serraaccording to the methods described in the Supplementary material.Compared to untreated cells,SOD and GSH levels in Caco-2 cells were significantly decreased after H2O2treatment,andR.serratreatment markedly alleviated the decrease in SOD and GSH levels in a concentration-dependent manner,indicating thatR.serracan balance oxidative stress in vitro.A previous study reported that the antioxidant activities of Chinese herbs are related to theirentkaurane diterpenoids,flavonoids,phenolic acids,and other phenolic compounds[30].Thus,the abovementioned findings indicate thatR.serra,owing to its strong antioxidant activities,can be used as a potential antioxidant for the management of inflammatory diseases.
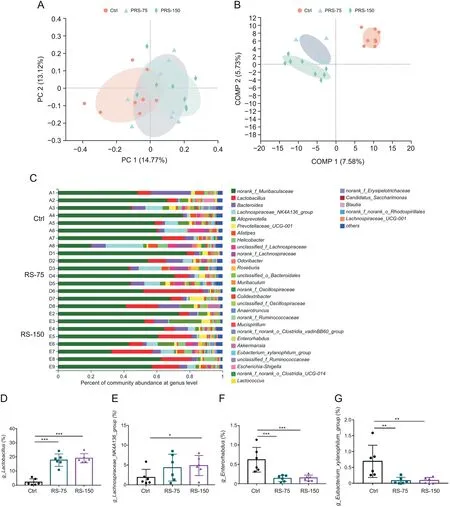
Fig.5.Gut microbial diversity and structural changes in mice after pre-adminisatration with R.serra(PRS)before dextran sulfate sodium(DSS)treatment.(A)Principal coordinate analysis(PCoA)at the operational taxonomic unit(OTU)level;(B)partial least squares discriminant analysis(PLS-DA)at the OTU level;(C)community barplot analysis.Relative abundance of(D)g_Lactobacillus,(E)g_Lachnospiraceae_NK4A136_group,(F)g_Enterorhabdus,and(G)g_Eubacterium_xylanophilum_group.Results are presented as means±SD,n=6-8 for each group.*P<0.05,**P<0.01,and***P<0.001,compared to control.Ctrl:control;PRS-75:mice pre-administered with R.serra at a dose of 75 mg/kg before DSS treatment;RS-150:mice pre-administered with R.serra at a dose of 150 mg/kg before DSS treatment;COMP:component.
3.3.The water extract of R.serra ameliorated DSS-induced colitis in mice
A DSS-induced colitis mouse model was established,and the experimental procedure followed is presented in Fig.2A.Compared to the control group,colitis mice(DSS group)showed severe clinical symptoms,including significant body weight loss(Fig.2B),increased DAI scores(Fig.2C),and shortened colon length(Figs.2D and E).As shown in Fig.2F,H&E staining revealed that the colonic tissue sections of the DSS group showed a marked increase in inflammatory cell infiltration,goblet and epithelial cell disappearance,crypt abscesses,muscle layer thickening and detachment,and even ulcer formation.Moreover,histopathological analysis revealed remarkable colonic injury in colon tissue(Fig.2G).Strikingly,a pre-administration of theR.serra(PRS;the mice were administered with the water extract ofR.serrabefore DSS was administered to induce colitis)water extract significantly protected the mice against DSS-mediated body weight loss,shortened colon length,and inhibited colon tissue damage.In this study,R.serrashowed a preferable effect on alleviating the symptoms of DSSinduced colitis.When compared to the positive control 5-ASA,the first-line treatment option for IBD,R.serratreatment exhibited a similar efficacy in protecting against DSS-induced colitis[32].Taken together,these results indicated that the water extract ofR.serracould effectively ameliorate DSS-induced colitis in mice.
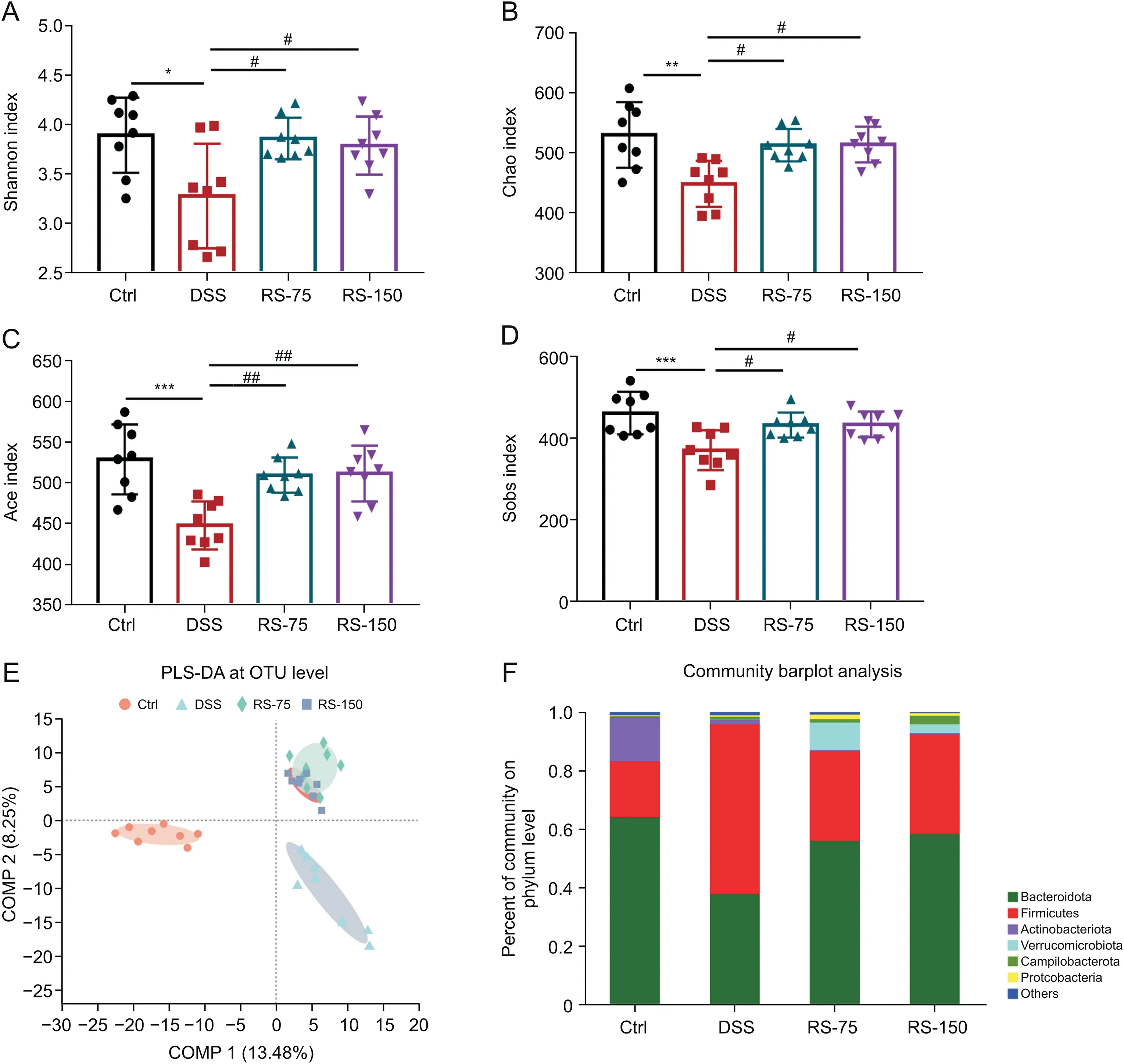
Fig.6.R.serra modulated the gut microbiota diversity and structure in colitic mice.(A)Shannon index;(B)Chao index;(C)Ace index;(D)Sobs index;(E)partial least squares discriminant analysis(PLS-DA)at the operational taxonomic unit(OTU)level;and(F)abundance of bacteria at the phylum level.Results are presented as means±SD,n=8 for each group.*P<0.05,**P<0.01,and***P<0.001,compared to control;#P<0.05 and ##P<0.01,compared to dextran sulfate sodium(DSS).Ctrl:control;DSS:DSS-induced colitis mice without other treatment;RS-75:DSS-induced colitis mice treated with R.serra at a dose of 75 mg/kg;RS-150:DSS-induced colitis mice treated with R.serra at a dose of 150 mg/kg;COMP:component.
3.4.R.serra decreased the levels of inflammatory factors in colitis mice
Intestinal microbiome imbalance,severe mucosal damage,and inflammation are closely associated with dysregulation of the immune response and levels of pro-inflammatory factors,which could exacerbate IBD inflammation[7].The levels of pro-and antiinflammatory factors in both serum and colonic tissue were evaluated to explore the anti-inflammatory effect ofR.serraon acute intestinal inflammation in DSS-induced colitis mice.Myeloperoxidase(MPO),a glycosylated enzyme,is a marker for inflammation,oxidative damage,and mucosal injury status in patients with IBD[33].Fig.3A shows the serum MPO level of each treatment group.MPO level was significantly increased in the DSS group and was significantly decreased by R.serra treatment.Moreover,the proinflammatory factors IFN-γand IL-17 are markers for inflammation that are closely associated with the progression of IBD.As shown in Figs.3B-I,R.serra treatment markedly decreased the levels of IL-17 and IFN-γin the serum and colonic tissue and increased the levels of anti-inflammatory factors IL-4 and TGF-β1.Similar effects were observed in the 5-ASA group.All these results suggested that R.serra alleviated intestinal inflammation in DSSinduced colitis mice by decreasing the levels of pro-inflammatory factors and promoting the expressions of anti-inflammatory factors.
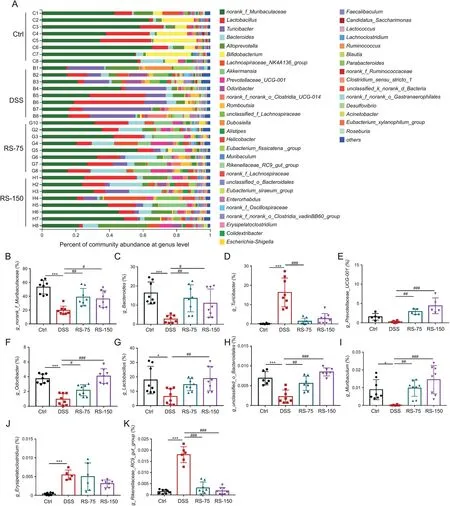
Fig.7.Gut microbial alterations at the genus level.(A)Community barplot analysis at the genus level.Relative abundance of(B)g_norank_f_Muribaculaceae,(C)g_Bacteroides,(D)g_Turicibacter,(E)g_Prevotellaceae_UGC-001,(F)g_Odoribacter,(G)g_Lactobacillus,(H)g_unclassified_o_Bacteroidales,(I)g_Muribaculum,(J)g_Erysipelatoclostridium,and(K)g_Rikenellaceae_RC9_gut_group.Results are presented as means±SD,n=6-8 for each group.*P<0.05,**P<0.01,and***P<0.001,compared to control;#P<0.05,##P<0.01,and###P<0.001,compared to dextran sulfate sodium(DSS).Ctrl:control;DSS:DSS-induced colitis mice without other treatment;RS-75:DSS-induced colitis mice treated with R.serra at a dose of 75 mg/kg;RS-150:DSS-induced colitis mice treated with R.serra at a dose of 150 mg/kg.
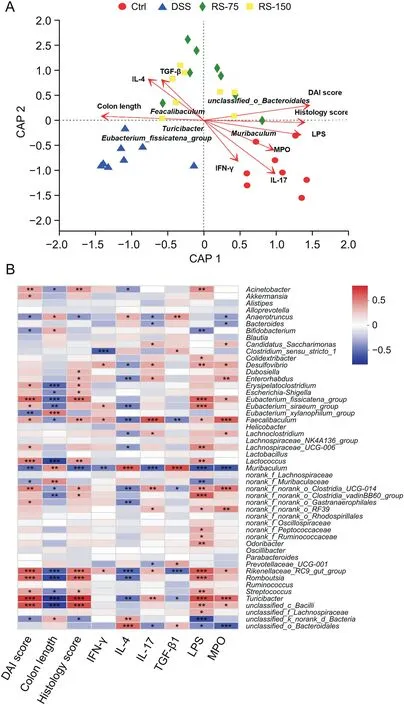
Fig.8.Correlation analysis.(A)Distance-based redundancy analysis.(B)Spearman correlation heatmap.Results are presented as means±SD,n=6-8 for each group.*P<0.05,**P<0.01 and***P<0.001.DAI:disease activity index;IFN:interferon;IL:interleukin;TGF:transforming growth factor;LPS:lipopolysaccharide;MPO:myeloperoxidase;Ctrl:control;DSS:dextran sulfate sodium(DSS)-induced colitis mice without other treatment;RS-75:DSS-induced colitis mice treated with R.serra at a dose of 75 mg/kg;RS-150:DSS-induced colitis mice treated with R.serra at a dose of 150 mg/kg.
It has been proven that Th17 and Treg cells,differentiated from CD4+T cells,are closely related to the occurrence,development,and prognosis of IBD[34].Th17 cells play a pro-inflammatory role by secreting the pro-inflammatory factors IL-21,IL-17,IFN-γ,and IL-22,while Treg cells play important anti-inflammatory and immunoregulatory roles by producing the anti-inflammatory factors IL-4,IL-10,and TGF-β[35,36].A balance between Th17 and Treg cells is required to regulate the intestinal immune responses[36].In this study,the effect of the water extract ofR.serraon Th17/Treg cell balance in DSSinduced colitis mice was evaluated(Figs.3J and K).R.serratreatment significantly decreased the percentage of IL17A+CD4+Th17 cells in MLNs and increased that of CD25+Foxp3+Treg cells.These values were consistent with the levels of inflammatory factors evaluated in the serum and colon of colitis mice.
3.5.R.serra preserved intestinal barrier function and reduced gut permeability
Previous studies have reported that intestinal epithelial layer injury in the gastrointestinal tract is one of the symptoms of IBD[3].Therefore,the integrity of intestinal barrier function is critical for maintaining gut health.Tight junction proteins,including ZO-1,claudins,occludin,and tricellulin,are directly responsible for intestinal barrier function and can decrease gut permeability and defense against pathogenic molecules such as LPS[12,37,38].Therefore,the expressions of occludin and ZO-1 in colonic tissue were evaluated through IHC analysis(Figs.4A-D).As shown in Figs.4A and B,mice in the DSS group exhibited deteriorated intestinal epithelial layer with a decrease in occludin and ZO-1 expressions,which were markedly improved byR.serratreatment.
The microbial endotoxin LPS is an indicator of gut permeability.Because of the disruption of the gut mucosa,LPS is transported to the systemic circulation and continuously stimulates the immune system[38].As shown in Fig.4E,serum LPS levels,which were increased by DSS,were significantly decreased byR.serrain mice.Furthermore,tight junction proteins can prevent the permeation of LPS from the intestinal tract into the blood[39].Hence,the increased levels of LPS in the DSS group might be associated with the decreased expressions of ZO-1 and occludin.AsR.serracan ameliorate DSS-mediated changes in the levels of ZO-1,occludin,and LPS,it might play a key role in repairing the intestinal barrier and reducing the level of external endotoxins.
3.6.R.serra alleviated DSS-induced gut dysbiosis
Increasing evidence has proven that the intestinal flora is closely associated with the pathogenesis of IBD[13].The gut microbiota plays a key role in defending against pathogens and regulating immune responses.However,alterations of the intestinal microbiome can drive the activation and differentiation of immune cells[4,7].Modulating the structure of the gut microbiota can be a promising therapy for IBD.To determine whetherR.serracan regulate the diversity and structural composition of the intestinal microbiome,we studied mouse fecal bacterial DNA by performing high-throughput gene sequencing analysis of 16S rRNA.
First,the gut microbial composition was evaluated before DSS induction in mice.Fecal specimens were collected after one week of oral pretreatment withR.serraat a dose of 75 and 150 mg/kg in normal C57BL/6J mice.Compared with the control group,mice in the PRS group showed similar richness and diversity of the gut microbiota in terms of the OTU level,which was determined by analyzing theα-diversity estimators(Chao,Shannon,Ace,and Sobs indices)(Figs.S2A-D).Additionally,aβ-diversity analysis of the OTU level and PCoA and PLS-DA results were performed to evaluate the structural changes of the gut microbiota.As shown in Figs.5A and B,a significant separation between the microbiota of the PRS group and that of the control group was observed.Furthermore,a community barplot analysis at the phylum level indicated that Firmicutes,Bacteroidetes,and Campilobacterota were the dominant members of the gut microbiota(Fig.S2E and F).Meanwhile,Lachnospiraceae_NK4A136_groupandLactobacilluswere significantly increased,whileEnterorhabdusandEubacterium_xylanophilum_groupwere significantly decreased(Figs.5D-G)through pretreatment withR.serra.Lachnospiraceae_NK4A136_groupandLactobacilluswere beneficial microbial species[4,7],whileEnterorhabdusandEubacterium_xylanophilum_groupwere found to be positively related to metabolic diseases and colon cancer[40,41].It has been reported that theLachnospiraceae_NK4A136_groupplays an important role in anti-inflammation by alleviating the symptoms of colonic inflammation and inhibiting the expressions of pro-inflammatory factors such as TNF-α,IL-6,and IL-12[41-43].Generally,Lactobacilluscan protect against pathogen colonization in the gut by creating a barrier[42].In conclusion,although there was no significant variation in the richness and diversity of the gut microbiota between mice pretreated withR.serraand control mice,R.serraincreased the relative abundance of beneficial bacteria.
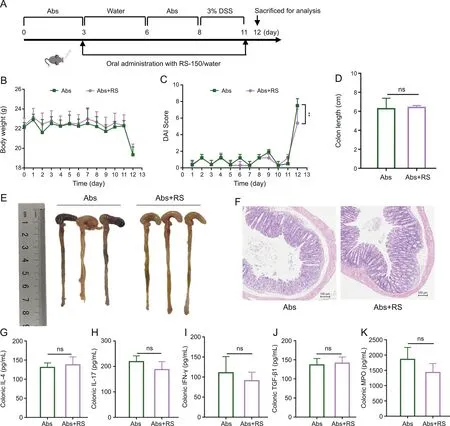
Fig.9.Therapeutic effect of R.serra on dextran sulfate sodium(DSS)-induced colitis mice was microbiota dependent.(A)Schematic illustration of the animal experiment design.C57BL/6J mice were pretreated with a broad-spectrum oral antibiotic(Abs)cocktail(100 mg/Kg of vancomycin,200 mg/Kg of ampicillin,200 mg/Kg of metronidazole,and 200 mg/Kg of neomycin sulfate)at days 0-3 and 6-8 and then administered with 3%DSS(m/V)for 4 days(days 8-11).The Abs and Abs+R.serra(RS)group were orally administered water and R.serra(150 mg/kg/day)(RS-150)from day 3 to day 11.(B)Body weight changes of each group were monitored daily and expressed as a percentage of initial weight.(C)Disease activity index(DAI)score.(D)Colon length.(E)Morphology of colons.(F)Typical hematoxylin and eosin(H&E)stained colon tissue sections.Colonic levels of(G)interleukin(IL)-4,(H)IL-17,(I)interferon(IFN)-γ,(J)transforming growth factor(TGF)-β1,and(K)myeloperoxidase(MPO).Abs group:Abs-treated mice administered with water;Abs+RS:Abstreated mice administered with R.serra extract.P values were analyzed using unpaired t-tests.**P<0.01;ns:not significant.
Subsequently,fecal specimens of the DSS-induced colitis mice were collected for 16S rRNA analysis afterR.serratreatment.The results showed that DSS treatment significantly reduced the Shannon(Fig.6A),Chao(Fig.6B),Ace(Fig.6C),and Sobs(Fig.6D)indices,indicating that the richness and diversity of the gut microbiota were decreased.WithR.serratreatment,these indices were increased,suggesting a recovery of intestinal microbial richness and diversity.Furthermore,a PLS-DA plot of the OTU level revealed that theR.serra-treated group was separated from the DSS group moving toward the control group based on the first two components(Fig.6E).It thus suggests thatR.serracan at least partially recover microbial diversity and composition in colitis.
Fig.6F shows a community barplot analysis of the gut microbiota at the phylum level.The abundance of Bacteroidota was much lower in the DSS group than in the control group,but its level was recovered withR.serratreatment.In contrast,compared with the control group,the abundance of Firmicutes was increased in the DSS group and was recovered afterR.serratreatment.
As shown in Fig.7A,remarkable alterations in the gut microbiota at the genus level were observed.The relative abundance ofnorank_f_Muribaculaceae(Fig.7B),Bacteroides(Fig.7C),Prevotellaceae_UCG-001(Fig.7E),Odoribacter(Fig.7F),Lactobacillus(Fig.7G),unclassified_o_Bacteroidales(Fig.7H),andMuribaculum(Fig.7I)were decreased by DSS treatment,while that ofTuricibater(Fig.7D)andRikenellaceae_RC9_gut_group(Fig.7K)were increased.The relative aboundance of g_Erysipelatoclostridiumwas not significantly different between DSS and RS groups(Fig.7J).R.serratreatment was able to normalize the ratio of these bacterial genus.Moreover,as revealed by the community barplot analysis at the genus level,the familyMuribaculaceae,which are propionateproducing intestinal bacteria,were predominant members of the gut microbiota in healthy mice.It has been proven that propionate,a short-chain fatty acid,can improve intestinal barrier function and reduce oxidative stress and inflammation in DSS-induced colitis[44].Moreover,LactobacillusandBacteroidesare also considered to exhibit anti-inflammatory activity and promote recovery of the intestinal mucosa[12,13,45].Lactobacilluscan reduce the expressions of pro-inflammatory factors,modulate oxidative stress,and prevent metabolic disorders[46,47].We found thatR.serraplayed a role in restoring the balance of the gut microbiota by elevating the abundance ofnorank_f_Muribaculaceae,Lactobacillus,Prevotellaceae_UCG-001,andBacteroides.
3.7.Results of the Db-RDA analysis and Spearman correlation
To test whether the therapeutic effect ofR.serraon DSS-induced colitis mice is associated with inflammatory factors,immune cells,and the gut microbiota,Db-RDA and Spearman correlation heatmap analyses were performed(Fig.8).Db-RDA analysis revealed thatR.serratreatment was positively correlated with histology score,DAI score,colon length,and the anti-inflammatory factors IL-4 and TGFβ.Furthermore,Spearman correlation heatmap analysis revealed that DAI score and histology score were highly correlated withTuricibacter,unclassified_c_Bacilli,Romboutsia,Rikenellaceae_RC9_gut_group,Lactococcus,andEubacteriun_fissicatena_group.The antiinflammatory factors IL-4 and TGF-β1 were significantly correlated withunclassified_o_BacteroidalesandMuribaculum,while the proinflammatory factors IFN-γand IL-17 were correlated withRikenellaceae_RC9_gut groupandFeacalibaculum.Indeed,Feacalibaculumhas been proven to be able to induce inflammation by destroying the intestinal barrier[48].Previous studies have reported that theRikenellaceae_RC9_gut groupcan contribute to intestinal inflammation by increasing intestinal permeability and oxidative stress and impacting energy metabolism[49,50].Moreover,there was a significant correlation between LPS and MPO withUtriculate,unclassified_c_Bacilli,Rikenellaceae_RC9_gut_group,norank_f_norank_o_Clostridia_UCG-014,Faecalibaculum,andEubacterium_fissic atena_group.
Above all,R.serramodulated the gut microbiota in DSS-induced mice by restoring its richness and diversity,increasing the abundance of beneficial bacteria,includingMuribaculaceae,Bacteroides,Lactobacillus,Lachnospiraceae_NK4A136_group,andPrevotellaceae_UCG-001,and decreasing the abundance of pathogenic bacteria,includingTuricibacter,Eubacterium_fissicatena_group,andEubacterium_xylanophilum_group.
3.8.R.serra ameliorated DSS-induced colitis in mice in a microbiota-dependent manner
To further confirm whether the effect ofR.serraon DSS-induced colitis mice is microbiota dependent,we pretreated the C57BL/6 mice with a broad-spectrum oral antibiotic(Abs)cocktail to disrupt the intestinal microbiome before administering them with 3% DSS to induce acute colitis(Fig.9A).TheR.serra-untreated group was treated with an antibiotic cocktail(Abs group)as a control.Body weight changes and DAI score were recorded daily.Compared with the Abs group,antibiotic cocktail andR.serratreatment(Abs+RS group)did not significantly ameliorate the symptoms of DSSinduced colitis,manifested by similar body weight changes(Fig.9B)and colon length(Figs.9D and E).H&E staining of both groups revealed a partial crypt architecture disruption,inflammatory cell infiltration,and goblet cell loss(Fig.9F).However,the DAI score of the Abs+RS group on the last day was lower than that of the Abs group(Fig.9C).Moreover,determination of the levels of pro-and anti-inflammatory factors in colonic tissue(Figs.9G-K)revealed that the anti-inflammatory effect ofR.serrawas abolished in the absence of interaction with the gut microbiota.These results indicate thatR.serracan alleviate DSS-induced colitis by modulating the gut microbiome.
4.Conclusion
In conclusion,R.serraalleviated DSS-induced colitis in a gut microbiota-dependent manner.A total of 46 compounds,consisting of 19ent-kaurane diterpenoids,9 flavonoids,5 phenolic acids,3 steroids,and 10 other compounds,were identified in the water extract ofR.serrausing UPLC-LTQ-Orbitrap-MS.Additionally,in a DSS-induced colitis mouse model,R.serrawas shown to significantly improve colon length,upregulate the anti-inflammatory factors MPO,IL-17,IFN-γ,and LPS,downregulate the proinflammatory factors IL-4 and TGF-β,and restore Th17/Treg cell imbalance.R.serracould also preserve intestinal barrier function by increasing the level of the tight junction proteins ZO-1 and occludin in colonic tissue.Furthermore,R.serraaltered the gut microbiota composition by increasing bacterial richness and diversity,increasing the abundance of beneficial bacteria(Muribaculaceae,Bacteroides,Lactobacillus,Lachnospiraceae_NK4A136_group,andPrevotellaceae_UCG-001),and decreasing the abundance of pathogenic bacteria(Turicibacter,Eubacterium_fissicatena_group,andEubacterium_xylanophilum_group),which was further confirmed by the depletion of the gut microbiota.
CRediT author statement
Hongyi Li:Investigation,Data curation,Writing-Original draft preparation;Yi Wang:Methodology,Investigation,Data curation;Shumin Shao and Hui Yu:Resources;Deqin Wang and Chuyuan Li:Formal analysis,Data curation;Qin Yuan and Wen Liu:Investigation,Formal analysis,Data curation;Jiliang Cao:Investigation,Data curation;Xiaojuan Wang:Writing-Reviewing and Editing;Haibiao Guo:Resources;Xu Wu and Shengpeng Wang:
Conceptualization,Supervision,Writing-Reviewing and Editing.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the Science and Technology Development Fund of Macao,China(File No.:0151/2020/A3),Key Area Research and Development Program of Guangdong Province,China(Grant No.:2020B1111110003),the Research Fund of the University of Macau,Macao,China(File No.:MYRG2020-00141-ICMS),and the Research Fund of Southwest Medical University,China(Grant No.:2021ZKZD017).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2022.08.001.
 Journal of Pharmaceutical Analysis2022年6期
Journal of Pharmaceutical Analysis2022年6期
- Journal of Pharmaceutical Analysis的其它文章
- Corrigendum to“The potential of miRNA-based therapeutics in severe acute respiratory syndrome coronavirus 2(SARS-CoV-2)infection:A review”[J.Pharma.Anal.11(2021)265-271]
- Development of a surface plasmon resonance biosensor for accurate and sensitive quantitation of small molecules in blood samples
- Peptide-RNA complexation-induced fluorescence“turn on”displacement assay for the recognition of small ligands targeting HIV-1 RNA
- Fluorescent aptasensor for detection of live foodborne pathogens based on multicolor perovskite-quantum-dot-encoded DNA probes and dual-stirring-bar-assisted signal amplification
- Tumor-targeting intravenous lipid emulsion of paclitaxel:Characteristics,stability,toxicity,and toxicokinetics
- Metabolomic and elemental profiling of blood serum in bladder cancer
