Dietary copper supplementation modulates performance and lipid metabolism in meat goat kids
ZHANG Yan-mei ,AO De ,LEI Kai-wen ,Lin XI ,Jerry W.SPEARS ,SHI Hai-tao ,HUANG Yan-ling,YANG Fa-long
1 Key Laboratory of Qinghai-Tibetan Plateau Animal Genetic Resource Reservation and Exploitation of Ministry of Education/Key Laboratory of Animal Science of State Ethnic Affairs Commission,College of Animal and Veterinary Sciences,Southwest Minzu University,Chengdu 610041,P.R.China
2 Department of Animal Science,North Carolina State University,Raleigh 27695,USA
Abstract Forty-eight male Lezhi black goat kids with similar body weight ((12.09±1.70) kg) and age ((60±5) d) were used to determine the effect of dietary copper (Cu),in the form of reagent grade Cu sulfate (CuSO4·5H2O),on performance,serum lipid profile,and the relative mRNA abundance of genes involved in lipid metabolism.Goat kids were stratified by body weight and randomly assigned to one of 4 treatment groups.Each treatment consisted of 12 replicate pens with each pen containing one goat kid.Treatment groups received the basal diet with no supplemental Cu (control),basal diet plus 10 mg of Cu kg–1 of dry matter (DM),basal diet plus 20 mg of Cu kg–1 of DM,or basal diet plus 30 mg of Cu kg–1 of DM.Goats were housed individually in pens and fed a high-concentrate pelleted diet for 60 d.Average daily gain,average daily feed intake and feed:gain of goats were not affected by dietary Cu supplementation(P>0.10).No differences were detected in serum total cholesterol,triglyceride,and high density lipoprotein cholesterol concentrations of goat kids fed with different Cu concentrations (P>0.05).However,serum low density lipoprotein cholesterol concentrations decreased linearly (P=0.01) as the concentration of dietary Cu increased.Intramuscular fat content of longissimus muscle increased (P=0.002) quadratically and liver Cu concentrations increased (P<0.001)linearly as dietary Cu concentration increased.Compared with the control,dietary supplementation of 20 mg Cu kg–1 DM decreased the relative mRNA abundance of fatty acid-binding protein 4 (P=0.01) and lipoprotein lipase (P=0.05),and tended to decrease the relative mRNA abundance of carnitine palmitoyltransferase I (P=0.06) in longissimus muscle of goats.The relative mRNA abundance of peroxisome proliferator-activated receptor alpha (P<0.001),carnitine acetyltransferase (P=0.001),and carnitine palmitoyltransferase I (P=0.001) were also decreased in liver by Cu supplementation.These results indicate that dietary supplementation of Cu modified lipid metabolism by increasing muscular fat and decreasing serum low density lipoprotein cholesterol,and the modification might be associated with the reduction of relative mRNA abundance of genes for oxidation of long-chain fatty acid in muscle and liver of Lezhi black goat kids.
Keywords: copper,gene expression,goats,lipid metabolism,growth performance
1.Introduction
Copper is an essential trace element for a number of physiological functions in the body (Davis and Mertz 1987).The NRC (2007) reported that the Cu requirement of goats was 15–25 mg kg–1dry matter (DM).Supplementation of Cu in diets of ruminants at or over the level of NRC requirement decreased backfat depth in lambs (6.7vs.16.7 or 26.7 mg Cu kg–1DM;Chenget al.2008),steers (4.9vs.14.9 or 24.9 mg Cu kg–1DM;Engle and Spears 2000) and Boer×Spanish goats (17vs.117 or 217 mg Cu d–1;Solaimanet al.2006),and increased unsaturated fatty acids concentrations inlongissimusmuscle (LM) (Engle and Spears 2000).Similar results were reported in non-ruminants as well.Feeding a high concentration of Cu altered lipid and cholesterol metabolism in broilers (125 mg Cu kg–1;Pesti and Bakalli 1996) and finishing pigs (250 mg Cu kg–1;Amer and Elliot 1973).These results suggested that dietary Cu level impacted fatty acids (FA) metabolic status in domestic animals.
We found that the addition of 20 mg Cu kg–1DM to a basal diet containing 14.3 mg Cu kg–1DM increased backfat depth,intramuscular fat (IMF) content,and polyunsaturated fatty acids concentrations in loin muscle of Jianyang male goats (Huanget al.2013).However,the underlying mechanism of how Cu affects lipid metabolism in meat goats has not been investigated further since then.Expression changes in a large number of genes were identified in cells and tissues with supplemental Cu using genome-wide approaches(Gonzalezet al.2008).The effect of Cu on lipid metabolism may be associated with the relative mRNA abundance of genes involved in lipid metabolism.However,there is no literature available in this area.We hypothesized that the changes in lipid metabolism observed in meat goats fed diet with Cu supplementation could be related to a modification in gene expression.To test our hypothesis,the effect of dietary supplementation of Cu on lipid profiles in serum and relative mRNA abundance of genes involved in lipid metabolism in muscle and liver was evaluated in Lezhi black meat goats.
2.Materials and methods
2.1.Animals,diets and experimental design
A total of 48 male Lezhi black goat kids (average body weight (BW) (12.09±1.70) kg,and age 2 mon) were used in this experiment.The Lezhi black goat was developed from crossbreeding Nubian with Chinese local breeds,and is one of the major goat breeds in China and and Southeast Asia.All goat kids were gradually transitioned from an initial forage-based diet to the high-concentrate pelleted basal diet,containing 10.3 mg Cu kg–1DM by analysis,during the first 7 d adaptation period and fed the high-concentrate pelletd basal diet for another 7 d adaption to lower the Cu concentration in the body.After a 14-d adaptation period,the goat kids were weighed on 2 consecutive days and randomly assigned by BW to 1 of 4 treatments (12 replicate pens for each treatment,one goat per pen) in a completely randomized design:no supplemental Cu (control,10.3 mg Cu kg–1DM by analysis),10 mg supplemental Cu kg–1DM (21.2 mg Cu kg–1DM by analysis),20 mg supplemental Cu kg–1DM (30.9 mg Cu kg–1DM by analysis),and 30 mg supplemental Cu kg–1DM (41.6 mg Cu kg–1DM by analysis).The maximum level of total Cu allowed in goat diets in China and the European Union (EU) is 35 mg kg–1DM.Therefore,only the analyzed Cu content(41.6 mg kg–1DM) of the 30 mg kg–1supplemental Cu treatment exceeded the China and EU regulations.All diets (pelleted total mixed rations) were formulated to meet or exceed all nutrient requirements for goats (NRC 2007) with the exception of Cu.The basal diet was mixed in a single batch and divided into 4 aliquots.Each aliquot was then mixed with the appropriate amount of supplemental Cu from reagent grade CuSO4·5H2O according to the dietary treatment.Ingredients and chemical compositions of the basal diet are listed in Table 1.All goat kids were housed individually in 1.9 m×2.1 m wooden pens equipped with wooden feeders and a stainless watering system,and were allowedad libitumaccess to the experimental diets and tap water containing no detectable Cu for 60 d.Diets were delivered twice daily (0800 h and 1600 h) in amounts to allow goat kidsad libitumaccess to feed.Feed offered and refusals were recorded daily in order to calculate average daily feed intake (ADFI).
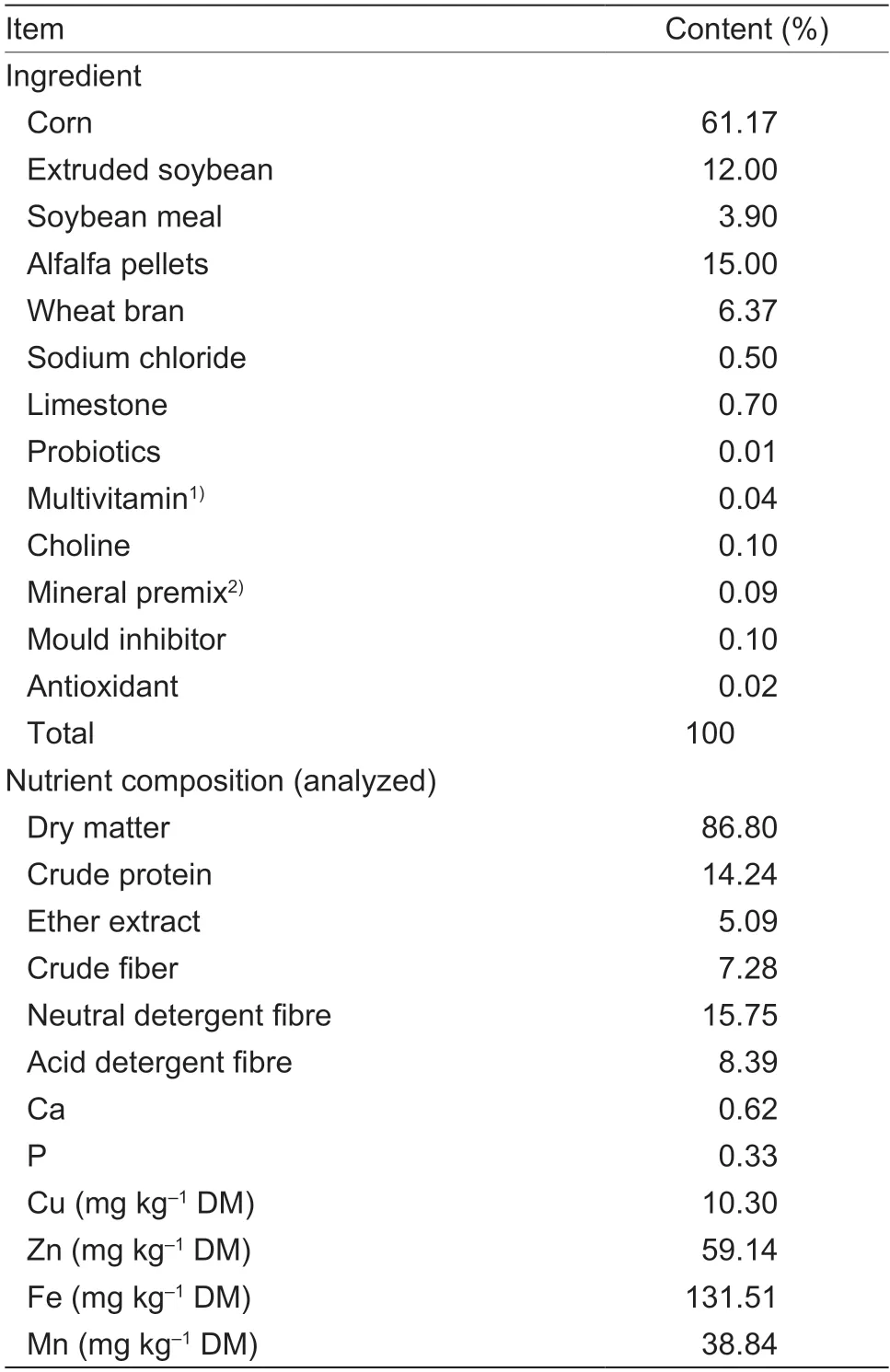
Table 1 Composition and nutrient concentrations of basal diet(air-dry basis)
2.2.Sample collection
At the end of the study,final BW was measured on 2 consecutive days.After an overnight period of feed withdrawal,jugular vein blood samples were collected in nonheparinized vacutainer tubes (Becton Dickenson,Franklin Lakes,NJ,USA).The samples were returned on ice to our laboratory and centrifuged at 2 500×g for 10 min.The serum was collected and stored at–20°C for lipid profile determinations.After collection of blood samples,10 goats from each treatment were selected and slaughtered by removing the goat kids with the highest and lowest body weight to better represent the average and reduce the work load.Two separated samples from both liver and LM tissues were obtained from each animal postmortem.One sample from each tissue was frozen in liquid nitrogen immediately and then stored at–80°C for analysis of mRNA relative abundance of genes involved in lipid metabolism.The other sample from each tissue was stored at–20°C for Cu concentration analysis in liver and IMF analysis in LM.The liver sample was collected from the same lobe and the LM sample was sliced from the 9th to 11th rib interface of the right side of the carcass.The relative mRNA abundance of peroxisome proliferator-activated receptor alpha (PPARα),carnitine palmitoyltransferase I (CPTI) and carnitine acetyltransferase (CRAT) were determined in liver and LM tissues.In addition of PPARα,CPTI and CRAT,the relative expression of FA synthase (FAS) and acetyl-CoA carboxylase (ACC) in liver and lipoprotein lipase (LPL) and FA-binding protein 4 (FABP4) in LM also were measured.
2.3.Laboratory analysis
Total cholesterol (TC),triglyceride (TG),low density lipoprotein cholesterol (LDLC),and high density lipoprotein cholesterol (HDLC) concentrations in serum were determined by assay kits (Nanjing Jiancheng Bioengineering Institute,Nanjing,China) using an automatic biochemistry analyzer (Synchron CX5 Pro,Beckman Coulter,Fullerton,CA,USA).The content of IMF was determined using the methods described previously (Huanget al.2013).Feed samples of the experimental diets and liver samples were dried at 100°C for 48 h,weighed,and then prepared for Cu analysis using a microwave digestor (Model: ETHOS T260,MILESTONE,Sorisole,Italy).Cu concentrations in diets and liver were determined by flame atomic absorption spectrophotometry (SOLAAR S2 Series;Thermo Electron,Cheshire,CT).
2.4.Relative abundance of mRNA
Based on the IMF results in the present study and our previous study which also indicated that 20 mg kg–1DM or greater increased IMF in LM of goats (Huanget al.2013),the samples collected from the control and 20 mg Cu kg–1supplementation groups were chosen for analyzing the relative mRNA abundance of the selected genes.Total RNA was extracted from LM and liver samples using Trizol reagent (SK1321,Sangon,Shanghai,China).The integrity of total RNA was confirmed by agarose gel electrophoresis.The purity of total RNA was measured by using a SMA4000 micro-spectrophotometer(Merinton,USA) for the determination of OD260and OD280(OD260/280≥1.8;Chenet al.2016;Panet al.2016).Reverse transcription was conducted with RevertAid Premium Reverse Transcriptase (EP0733,Thermo Scientific?,USA).The resulting first-strand cDNA was diluted to 1:10 with ddH2O and used as a template for real-time quantitative PCR.RT-qPCR was performed in a quantitative thermal cycler (Light Cycler480 II,Roche,Switzerland) with SG Fast qPCR Master Mix (BBI,Roche,Switzerland),containing 10 μL SG Fast qPCR Master Mix,2 μL of cDNA,0.4 μL of each primer and 7.2 μL ddH2O.The forward and reverse primer sequences are shown in Table 2.Specific primers were synthesized by Sangon(Shanghai,China).The thermal cycling parameters were as follows: 3 min at 95°C,45 cycles at 95°C for 3 s,and 60°C for 30 s.All reactions were performed in duplicate and each reaction was verified to contain a single product of the correct size by agarose gel electrophoresis.The levels of relative mRNA abundance were determined by 2-ΔΔCtmethod normalized with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) level (Chenet al.2016;Huet al.2017).On the basis of the Ct values,GAPDH mRNA abundance was stable across treatments in this experiment.
2.5.Statistical analysis
Gene relative abundance data was analyzed usingt-test of SAS (SAS Inst.Inc.,Cary,NC).Other data analysis was conducted by ANOVA using the MIXED procedure of SAS with each replicate (each pen or individual goat) as the experimental unit.Treatment effects were separated using least squares means with the PDIFF option.Orthogonal comparisons were applied for linear and quadratic responses.The residual or standard error of each observation was estimated within each treatment by the difference between the predicted value,based on the regression equation,and the actual observed value.Residuals with 5 or more standard errors from zero were considered outliers and were not used in the statistical analysis (Brookset al.2016).Significance was declared atP≤0.05 and tendencies were discussed at 0.05<P<0.10.
3.Results
3.1.Growth performance
The ADFI (P=0.12),average daily gain (ADG) (P=0.18),and feed:gain (P=0.46) were not affected by dietary Cu supplementation (Table 3).
3.2.Serum lipid profile parameters
Serum TC,TG,and HDLC were not affected by Cu supplementation (P>0.05;Table 4).However,serum LDLC concentrations decreased linearly (P=0.01) as the concentration of dietary Cu increased.
3.3.Intramuscular fat and liver Cu concentration
Intramuscular fat content of LM increased (P=0.021)linearly as dietary Cu increased (Table 5).Goats receiving 20 or 30 mg Cu kg–1DM had higher (P<0.05)IMF than those from the control.No difference (P>0.05)was detected between 10 mg Cu kg–1group and control group.There were no differences (P>0.05) among Cusupplemented groups.Liver Cu concentration increased(P<0.001) linearly as dietary Cu increased.
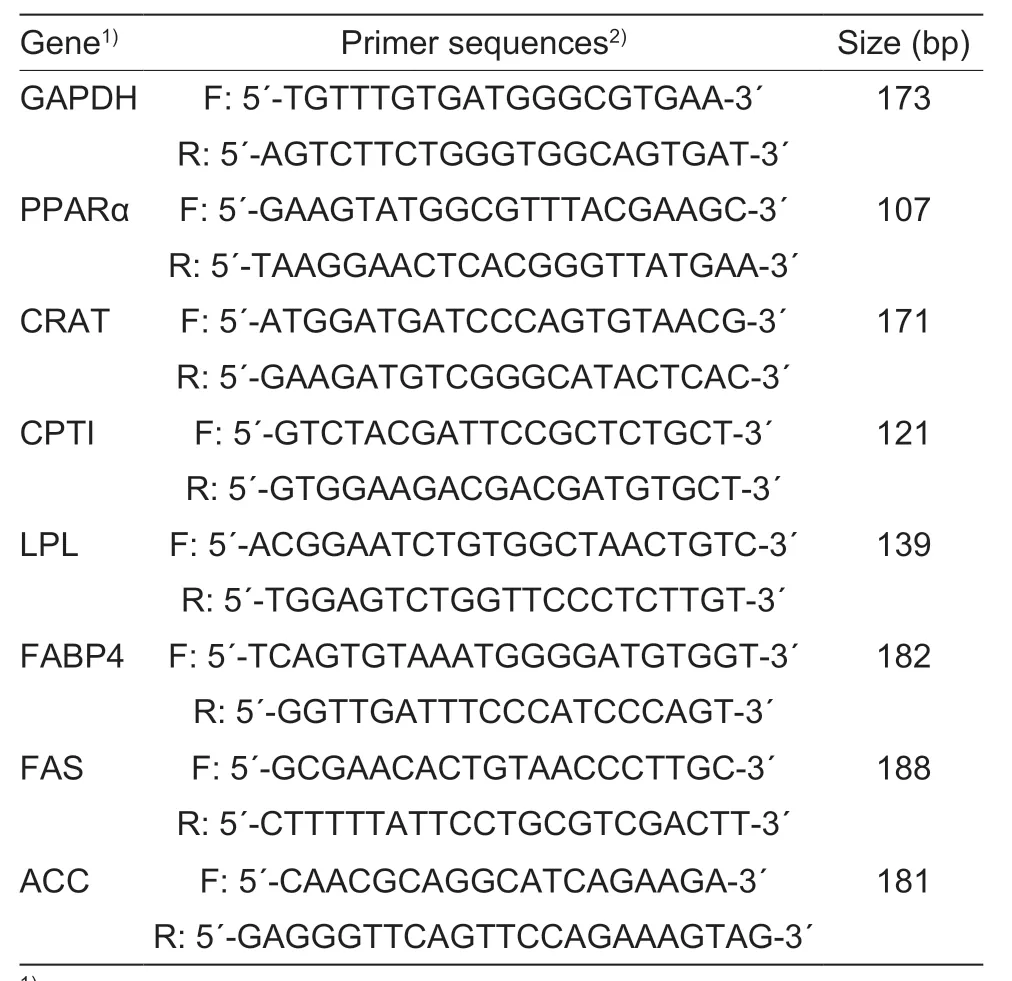
Table 2 Primer sequence for real-time PCR
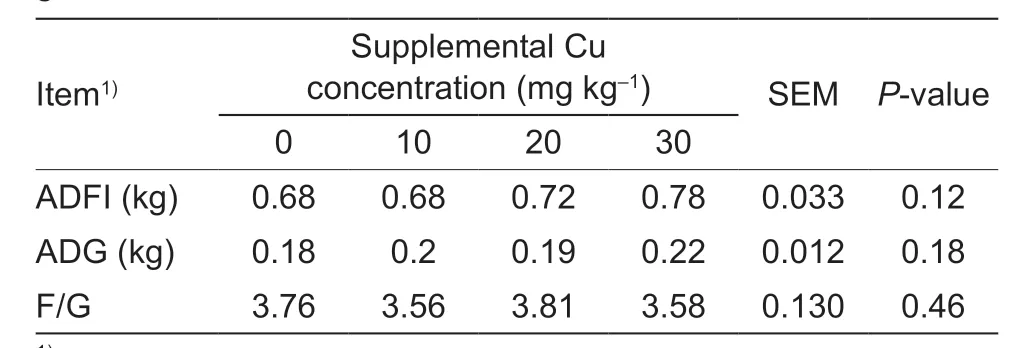
Table 3 Effect of dietary copper on growth performance of goat kids
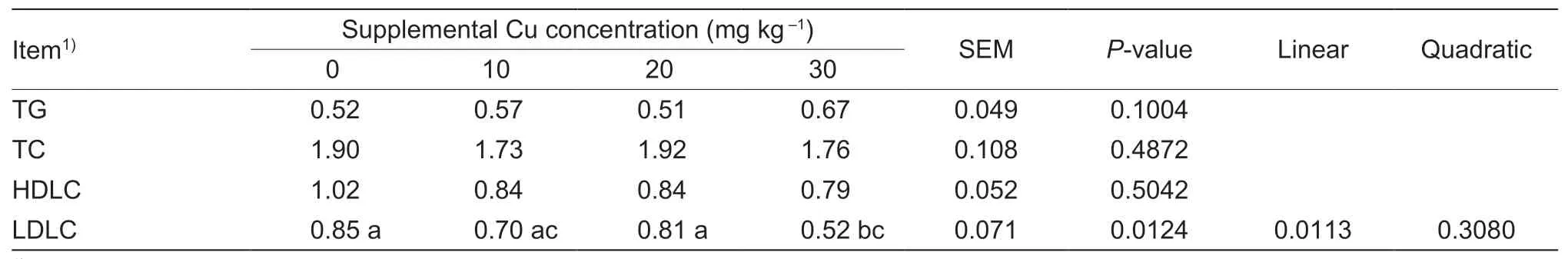
Table 4 Effect of dietary copper on serum lipid profile of goat kids (mmol L–1)
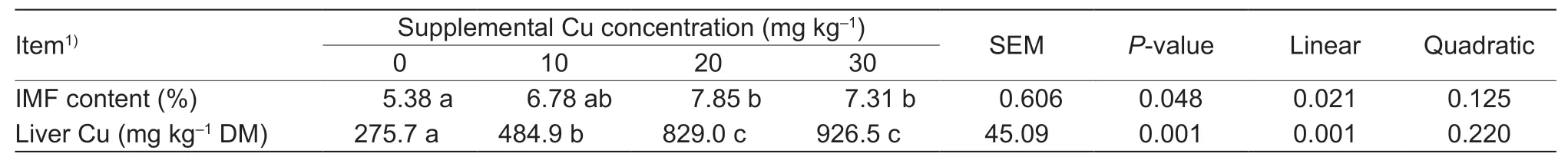
Table 5 Effect of dietary copper on intramuscular fat and liver copper concentration of goat kids
3.4.Relative mRNA abundance of genes associated with lipid metabolism in muscle
The results showed that dietary supplementation of 20 mg Cu kg–1DM did not affect (P>0.10) relative mRNA abundance of PPARα or CRAT in muscle (Table 6).Relative mRNA abundance of FABP4 (P=0.01) and LPL(P=0.05) were reduced in the goat kids supplemented with 20 mg Cu kg–1DM.The relative mRNA abundance of CPTI in muscle also tended (P=0.06) to be reduced by the dietary Cu supplementation.
3.5.Relative mRNA abundance of genes associated with lipid metabolism in liver
The relative mRNA abundance of FAS and ACC in liver were not affect (P>0.10) by dietary Cu (Table 6).However,the relative mRNA abundance of PPARα,CRAT,and CPTI were decreased (P=0.001) by the dietary Cu supplementation.
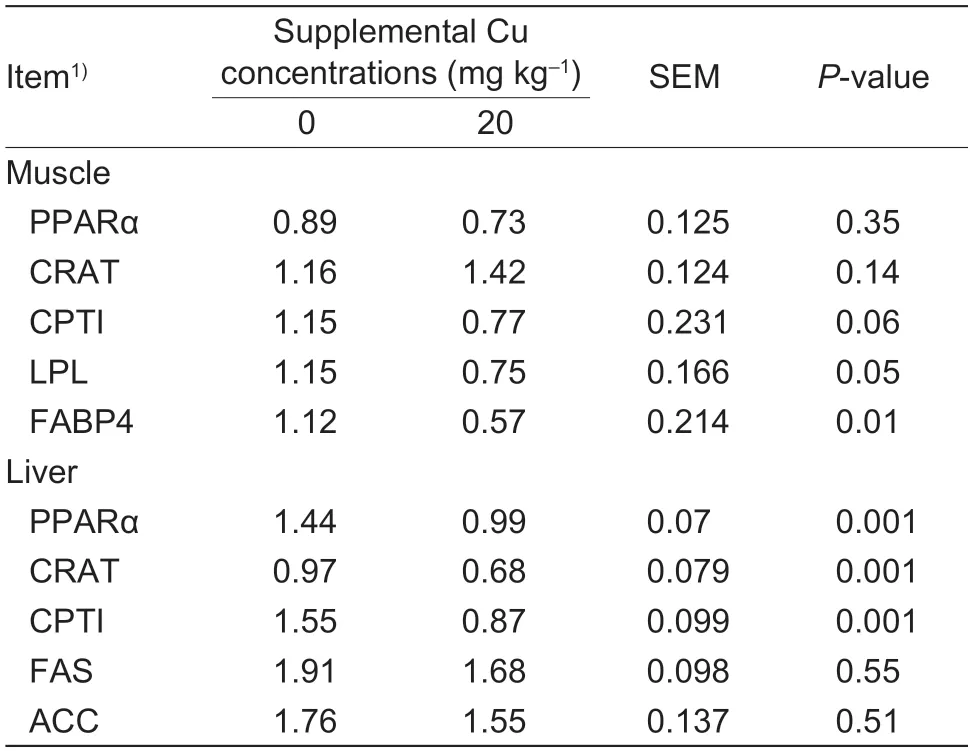
Table 6 Effect of dietary copper on relative mRNA levels of genes associated with lipid metabolism genes in the muscle and liver of goat kids
4.Discussion
The hypothesis that the changes in lipid metabolism observed in meat goats fed diet with Cu supplementation could be related to a modification in gene expression has been supported by the results of the present study.The findings from this study clearly demonstrated that changes in IMF in goats supplemented with 20 mg Cu kg–1wereassociated with reductions in relative mRNA abundance of enzymes involved in long-chain FA oxidation in muscle and liver.These findings could help us to better understand the role of Cu in lipid metabolism of meat goats.
The ADFI,ADG and feed:gain were not affected by dietary Cu supplementation in present study.This implies that the basal diet,containing 10.3 mg Cu kg–1DM,was adequate for growth of goats during the 60-d study.Cu supplementation (30 or 60 mg Cu kg–1DM) to a basal diet containing 7.8 mg Cu kg–1did not affect gain or feed intake in native breed Greece kids during a 140-d study(Zervaset al.1990).Supplementation of 10 or 30 mg Cu kg–1DM to a basal diet containing 6.2 mg Cu kg–1DM also did not alter the performance of Boer×Brush kids in a 88-d study (Luginbuhlet al.2000).Similarly,adding Cu to a basal diet containing 14.3 mg Cu kg–1DM did not affect performance of Jianyang kids (Huanget al.2013).
Serum TC,TG,and HDLC concentrations were not affected by dietary Cu in the present study.Dietary Cu supplementation reduced TC in Liaoning Cashmere goats(Zhanget al.2012) and in Black Bengal goat kids (Dattaet al.2007;Mondalet al.2007),but not in Jianyang goats(Huanget al.2014).The response of serum TG to dietary Cu was consistent with that observed in previous studies with goats (Dattaet al.2007;Mondalet al.2007;Huanget al.2014).Serum LDLC from Lezhi black goat kids in this study decreased linearly as dietary Cu increased.This finding agreed with the observations reported previously in goats that received diets with the supplementation of Cu (Dattaet al.2007;Zhanget al.2012).Because Cu is an essential mediator for inducing LDL oxidation (Nakanoet al.2004),the decrease in LDLC could be due to an oxidation of LDL induced by dietary supplemental Cu.
Dietary Cu supplementation to the control diet increased IMF in LM of goats in the present study.This is in agreement with our previous study indicating that 20 mg kg–1DM or greater increased IMF in LM of goats(Huanget al.2013).In finishing steers,dietary Cu did not affect IMF (Engle and Spears 2000;Engleet al.2000a,b).Copper supplementation,viaan intraruminal Cu oxide bolus increased the protein content,but did not affect IMF in sternocephalicus or rectus abdominis muscles of deer (Serranoet al.2019).It is generally assumed that IMF content positively influences sensory quality traits,for instance,flavor,juiciness and tenderness of meat (Hocquetteet al.2010).Therefore,dietary Cu supplementation may modify goat meat characteristics.
As expected,liver Cu concentration increased linearly with the increasing dietary Cu (Table 5).Mondalet al.(2007)observed a similar increase in liver Cu in Black Bengal goat kids supplemented with 10,20 or 30 mg Cu kg–1DM for 90 d.The increase in liver Cu with increased dietary Cu was greater in the present study than that observed in Jianyang goats (Huanget al.2013) or Boer×Brush goats (Luginbuhlet al.2000).The greater increase in liver Cu with increasing dietary Cu may be due to the low forage level (15% alfalfa pellets) in the present study compared to previous studies where diets contained 70% (Luginbuhlet al.2000) or 37%hay (Huanget al.2013).
Based on the results of the current study,relative mRNA abundance of FABP4 and LPL were reduced in goats supplemented with 20 mg Cu kg–1DM,and the mRNA abundance of CPTI also tended to be reduced by Cu supplementation.The enzymes of these genes are involved in the oxidation of FA as an energy source for muscle.The FABP4 is one of the transport proteins that have been proposed to target FA β-oxidation pathways by facilitating the transfer of FA between extra-and intracellular membranes in muscle (Storch and Thumser 2010).The LPL is the enzyme localized on the outer surface of endothelial cells lining the capillaries of muscle and most other tissues and hydrolyzes blood TG to FA.The activity of LPL affects FA uptake following TG hydrolysis in tissues,while CPTI is involved in the transfer of long-chain FA into the mitochondria for β-oxidation(Newsholme and Leech 1983).Therefore,downregulation of FABP4,LPL,and CPTI in Cu-supplemented goats may indicate a metabolic shift from FA oxidation to greater glucose utilization by muscle.The greater IMF observed in Cu-supplemented goats could be explained by increased glucose uptake by muscle.It has been shown that glucose contributes a larger proportion of acetyl units to FA synthesis in IMF in ruminants than in subcutaneous adipose tissue (Smithet al.2009).
The relative mRNA abundance of FAS and ACC in liver were not affected by dietary Cu in the current study.This is not surprising since these two enzymes are involved in FA synthesis and adipose tissue is the principal site of FA synthesis goats (Liepaet al.1978).Dietary Cu supplementation reduced the relative mRNA abundance of PPARα,CRAT,and CPTI in liver.The PPARα is a transcription factor which controls the expression of genes involved in FA oxidation (Gutgesellet al.2009).The CRAT and CPTI are PPARα target genes that involved in the entry of long-chain FA into the mitochondria for oxidation.Collectively,downregulation of the message for these enzymes suggest that Cu supplementation may have reduced oxidation of long-chain FA by liver.In ruminants,considerable quantities of acetate and butyrate are produced from rumen fermentation.In a fed state acetate and butyrate would be available for oxidation as an energy source in liver.Acetate and butyrate can be transported into the mitochondria for oxidation without the carnitine transport system that involves CRAT and CPTI(Newsholme and Leech 1983).Studies in goats indicated that addition of 10,20 or 30 mg kg–1DM of Cu from copper sulfate increased ruminal concentrations of total volatile fatty acids and acetate as well as apparent totaltract digestibility of DM,organic matter and fiber (Mondalet al.2007;Zhanget al.2007).Vázquez-Armijoet al.(2011) observed thatin vitroruminal production of shortchain fatty acid and gas increased by the addition of 21.7 mg Cu kg–1DM when rumen inoculum was obtained from goats fed a control diet containing 10.3 mg Cu kg–1DM.The addition of 5,7.5,or 10 mg Cu kg–1DM,from coated Cu sulfate,to a control diet containing 8.5 mg Cu kg–1increased ruminal total volatile fatty acid concentrations and molar proportion of butyrate in dairy cows (Wanget al.2020).
The responses of gene expression to dietary supplemental Cu in muscle appeared to be different from liver,suggesting that there might be tissue specificity.Considering the metabolic characteristics between the two tissues,it would be very interesting to investigate the effect of Cu on the protein expression and activity of the enzymes involved in the lipid metabolism further.Due to the lack of specific antibody and limited fund,we have not determined the enzyme proteins and activities in this study.However,the tissue specificity and the role of dietary supplemental Cu in modulating the enzyme proteins and activitiesviagene expression will be tested in our future studies.
5.Conclusion
The results from the present study indicate that the addition of 20 or 30 mg Cu kg–1to a control diet (10.3 mg Cu kg–1DM by analysis) increased IMF in male Lezhi black goats.Consistent with our hypothesis,changes in IMF in goats supplemented with 20 mg Cu kg–1was associated with reductions in relative mRNA abundance of enzymes involved in long-chain FA oxidation in muscle and liver.Results suggest that greater IMF in Cusupplemented goats may be due to increased glucose utilization by muscle.The greater IMF content in Cusupplemented goats may improve meat quality.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31501977),the Sichuan Provincial Key R&D Project,China (22ZDYF0194),the Fundamental Research Funds for the Central Universities of Southwest Minzu University (2021PTJS20),and the Innovation Team Development Funds for Sichuan Mutton Sheep,China(cxtd2019–14).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Ethical approval
All experimental procedures were approved by the Animal Management Committee (in charge of animal welfare issue) of the Southwest Minzu University (SWUN,Chengdu,China) and performed in accordance with the ARRIVE guidelines for reporting animal research.
 Journal of Integrative Agriculture2023年1期
Journal of Integrative Agriculture2023年1期
- Journal of Integrative Agriculture的其它文章
- Less hairy leaf 1,an RNaseH-like protein,regulates trichome formation in rice through auxin
- Characterization of a blaCTX-M-3,blaKPC-2 and blaTEM-1B co-producing lncN plasmid in Escherichia coli of chicken origin
- Consumers’ experiences and preferences for plant-based meat food: Evidence from a choice experiment in four cities of China
- Farmers’ precision pesticide technology adoption and its influencing factors: Evidence from apple production areas in China
- Visual learning graph convolution for multi-grained orange quality grading
- lnfluence of two-stage harvesting on the properties of cold-pressed rapeseed (Brassica napus L.) oils
