Progress in interventional radiology treatment of pulmonary embolism: A brief review
INTRODUCTION
Venous thromboembolism, clinically presenting as deep vein thrombosis or pulmonary embolism (PE), is the third most frequent acute cardiovascular syndrome globally, after myocardial infarction and stroke[1]. Approximately one-third of all patients with a new diagnosis of venous thromboembolism have PE, with or without deep vein thrombosis[2]. PE can be defined as the occlusion of the pulmonary arteries or its branches with embolic material (thrombus, air, fat or amniotic fluid) that originates elsewhere in the body. Most commonly, the cause is a thrombus arising from the deep veins of the lower extremities, which travels to the pulmonary circulation.
Diagnosis of PE can be subtle, as there are no specific symptoms, and clinical presentation varies widely, ranging from asymptomatic to sudden cardiac death, which is seen in 25%-30% of patients[3]. There have been many advances in the field of PE in the recent decades. The development of new diagnostic and therapeutic strategies, including medical and surgical treatment as well as endovascular therapy, has led to an increasing complexity of patient treatment and, consequently, to the need of optimizing the management of this serious condition.
PHYSIOPATHOLOGY
PE, by definition, is characterized by the presence of emboli in the pulmonary arterial circulation. Most emboli originate as thrombi in the deep veins of the lower extremities; the most common site of thrombosis is represented by the calf veins, followed by femoro-popliteal veins and iliac veins. Less frequently, emboli arise from upper extremity veins and are typically associated with central venous catheters, intracardiac devices, malignancy or venous trauma. A smaller percentage of PE is caused by pelvic deep vein thrombosis, but they are generally associated with a predisposing factor such as pelvic infection, pelvic surgery or pregnancy[4]. When 25%-30% of the pulmonary vasculature is obliterated by a thrombo-embolus, pulmonary artery pressure begins to increase. However, the mechanical obstruction is not the only element leading to pulmonary hypertension: the disruption of the alveolarcapillary membrane by the thrombi results in a decrease of oxygen diffusion, with subsequent hypoxia and release of vasoconstrictors that contribute to the acute development of pulmonary hypertension[5]. The increase of pression in the pulmonary artery determines heterogeneity of pulmonary perfusion, leading to the simultaneous presence of hypo- and hyperperfused areas; there will be an imbalance between ventilation and perfusion, generating hypoxemia[6].
Moreover, PE can have significant cardiac and hemodynamic consequences, related to the size of emboli and the presence or absence of underlying cardiopulmonary disease. In healthy patients, the mean pulmonary artery pressure can be up to 40 mmHg acutely; right ventricle (RV) failure ensues when 50%-75% of pulmonary arteries are obstructed[7]. When the degree of pulmonary artery obstruction exceeds 50%-75%, the right heart dilates and the combination of the increased wall stress and cardiac ischemia impair RV function and left ventricular (LV) output, leading to hypotension[8]. The presence of pre-existing cardiopulmonary disease results in diminished pulmonary vascular reserve and hemodynamic compromise at a lower level of pulmonary arterial obstruction.
PULMONARY EMBOLISM RISK STRATIFICATION
The American Heart Association (AHA) and the European Society of Cardiology (ESC) classified PE according to its severity, identifying three main categories[1,9].
Patients with massive (AHA) or high risk (ESC) PE present with hypotension, defined as a systolic blood pressure lower than 90 mmHg, or a drop of > 40 mmHg for at least 15 min or need for vasopressor support.
Submassive (AHA) or intermediate risk (ESC) classifications slightly differ as, according to AHA, patients with submassive PE present with an RV strain with no hypotension. RV strain is defined as: RV dysfunction on echocardiography or computed tomography pulmonary angiography, and RV injury identified by an increase in cardiac biomarkers as troponins or brain natriuretic hormone. On the other side, the ESC criteria for intermediate-risk PE include patients with a simplified Pulmonary Embolism Severity Index score ≥ 1, regardless of RV strain. The Pulmonary Embolism Severity Index score is based on the patient’s age, comorbidities, heart rate, blood pressure and oxygen saturation. Moreover, the ESC subclassifies intermediate-risk patients in two groups based on RV dysfunction and RV injury (intermediate risk-high) or only one or neither of these findings (intermediate risk-low).
Low risk patients, according to both AHA and ESC, do not meet criteria for the abovementioned risk categories.
MEDICAL AND SURGICAL TREATMENT
Severe PE leads to hypoxaemia due to the ventilation-perfusion mismatch. Therefore, it is advised to use oxygen in patients with oxygen saturation < 90%. High-flow oxygen and mechanical ventilation should be taken in consideration when extreme hemodynamic instability is present (
cardiac arrest), even though obtaining a good hypoxemia correction is not completely possible without PE reperfusion techniques[10,11]. Intubation should be considered in patients who are not manageable with noninvasive ventilation[1].
Regarding reperfusion treatment, systemic thrombolysis leads to fast improvement of the pulmonary obstruction and cardiovascular parameters in patients with PE compared to medical treatment alone[20,21]. The best results are obtained when reperfusion treatment starts 48 h after symptoms onset; however thrombolysis could be useful even after 6-14 d[22]. Intravenous administration of recombinant tissuetype plasminogen activator is preferred to first generation thrombolytic agents (
urokinase)[23].
A relatively new device for treatment of PE is Aspirex (Straub, Wangs, Switzerland). Launched in mid-2010, the Aspirex catheter acts as an Archimedean screw that rotates inside the catheter lumen; this spiral mechanism provides an aspiration supplied by an active motor. Clinical results are promising; however, only recent studies with small cohorts of patients demonstrated its safety and efficacy, and there is a lack of randomized studies supporting this evidence[31]. Two European case series have been reported, with complete thrombus clearance observed in 83% to 88% of patients with intermediate- and high-risk PE[31,32].
Temporary extracorporeal membrane oxygenation could be used in patients with a high-risk PE, cardiac arrest and circulatory collapse, but its use needs to be further tested with clinical trials[15,16].
Acute PE may lead to cardiac arrest, in which case the current advanced life support guidelines have to be followed[17].
Catheter directed thrombolysis (CDT) gives the advantage of locally delivering a high concentration of fibrinolytic agent to a great clot surface. This way, fibrinolytic dose can be greatly reduced compared to the systemic one, and side effects are therefore lower. A routine use diagnostic angiography catheter with multiple holes can be used to deliver the fibrinolytic agent and increase its local blood concentration. This could enhance the efficiency of fibrinolysis, reducing the risk of bleeding. Each pulmonary artery is catheterized with a multihole catheter, and a fibrinolytic agent such as tissue plasminogen activator is injected through the clot at a rate of 1 mg/h for 24 h in case of a unilateral PE (single device) and 1 mg/h for 12 h if bilateral PE (double device) (SEATTLE II Trial)[25]. A more recent trial, the OPTALYSE PE trial, analyzed the possibility to further lower the dose of tissue plasminogen activator with shorter infusions. The total dose was significantly lower, ranging from 4 to 12 mg per lung, and shorter infusion times (2 to 6 h)[26].
Vitamin K antagonists are vastly used for oral anticoagulation in recent years; when vitamin K antagonists are used, low-molecular weight heparin or unfractionated heparin should be continued along with oral anticoagulants for more than 5 d until the International Normalized Ratio value reaches 2-3 for 2 d[19].
Then Trusty John was quite delighted, and brought her to the ship; and the King, when he beheld19 her, saw that she was even more beautiful than her picture, and thought every moment that his heart would burst
4.Ass: An ass is a hardy and sure-footed animal smaller and with longer ears than the horse (WordNet). The miller would find the ass useful by providing the power to operate his mill. The ass could also be used to deliver the flour produced at the mill.Return to place in story.
Surgical embolectomy in patients with acute PE is performed through cardiopulmonary bypass, with incision of the pulmonary arteries and clots removal. This approach is advised in high-risk PE and in selected intermediate-risk patients[1,24].
ENDOVASCULAR TREATMENTS: CURRENT EVIDENCE AND FUTURE PERSPECTIVES
Catheter directed thrombolysis
Moreover, in patients with intermediate to high risk of PE, it is advised to start subcutaneous anticoagulation while waiting for diagnostic tests, usually with low-molecular weight heparin, fondaparinux or unfractionated heparin[18]. Clinical trials with non-vitamin K antagonist oral anticoagulants are ongoing.
FlowTriever
System (Inari Medical) is another aspiration device. Its mechanism features three selfexpanding nitinol mesh disks designed to engage, disrupt and deliver the clot to the Triever Aspiration Catheter for extraction. It has been evaluated in a recent single-arm multicenter trial involving 106 patients (FLARE Study) and appears safe and effective in patients with acute intermediate-risk PE, with significant improvement in RV/LV ratio and minimal major bleeding[35]. In 2021 Inari Medical, Inc. announced enrollment of the PEERLESS randomized controlled trial comparing the clinical outcomes of patients with intermediate-high risk PE treated with the company’s FlowTriever system
CDT (NCT05111613). PEERLESS is a prospective, multicenter trial that will include up to 700 patients and 60 centers in the United States and Europe. It will be the first ever randomized controlled trial to compare mechanical thrombectomy to catheter-directed thrombolysis for the treatment of PE and aims to provide definitive data on interventional treatment options for these patients.
Efficient systemic administration of heparin is continued throughout the endovascular fibrinolysis procedure. Despite the lack of randomized trial studies comparing endovascular and systemic thrombolytic therapy, several comparative studies have been carried out. In a meta-analysis of Bloomer
[27], the rate of intracranial hemorrhage with CDT was 0.35%, which is significantly lower than that reported with systemic thrombolytics in other randomized trials (1.46%). Bloomer
[27] also found that the rate of major bleeding or vascular complication was 4.65%, and the observed mortality rate was 3.4% (12.9% in the massive PE group, 0.74% in the submassive PE group).
The Indigo mechanical aspiration system (Penumbra, Alameda, United States) is an aspiration thrombectomy catheter system. A large caliber (8 French) catheter with a directional soft tip, allows easy aspiration of the clots in the pulmonary arteries due to the great suction power of a suction pump. Several studies are being performed to evaluate safety and efficacy of this device. The recent Indigo Aspiration System for Treatment of Pulmonary Embolism Trial (EXTRACT-PE), a prospective multicenter study on 119 patients demonstrated a significant reduction in the RV/LV ratio and a low major adverse event rate in submassive PE patients treated with the Indigo CAT8 aspiration system, with a reduction of administered intraprocedural thrombolytic drugs, which were avoided in 98.3% of patients[33]. The Indigo CAT8 received Food and Drug Administration approval for PE treatment in December 2019. The system is being monitored to assess its safety even in real-world clinical practice, showing a low incidence of reports linked to the product[34].
Mechanical thrombectomy
In cases of massive PE, the first aim should be to quickly declot the affected pulmonary artery to decrease pulmonary hypertension and the risk of RV failure. Initial fragmentation or thrombectomy by different devices (Table 1) can help reduce the thrombotic load and improve reperfusion. In addition, fragmentation of the clot exposes a greater surface of the thrombus, increasing the efficacy of local or systemic therapies[29].
“ How did your mommy and I get so lucky to get the best one?”Before he had time to finish, I would say, “You got me!” And then he would continue, “The best little girl in the whole wide world, and we got you.”
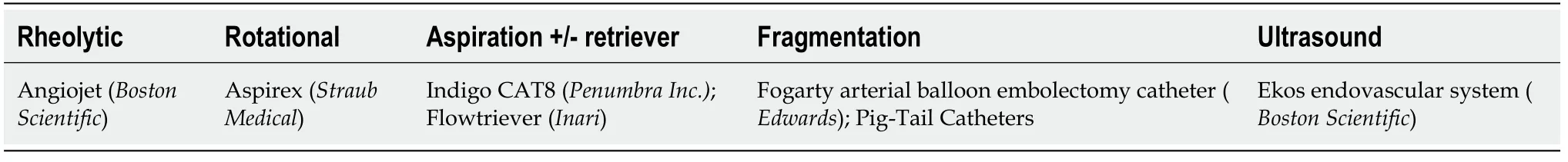
Current catheters for mechanical thrombectomy or endovascular aspiration are classified based on the mechanism of action.
AngioJet (Boston Scientific, Massachusetts, United States) working mechanism is determined by aspiration of the thrombus using the Venturi-Bernoulli effect. It creates a suction effect with highpressure jets in the catheter’s distal holes. Various complications (
, bradycardia and heart attack, severe hemoptysis, kidney failure as well as intra- and periprocedural deaths) were reported during the use of this device[30]; hence, the use of AngioJet as a first-approach treatment should be avoided. Currently the main indication of this product remains treatment of peripheral venous districts.
Acute RV failure is a cause of death in high-risk PE patients due to the reduction of cardiac output. When low central venous pressure is present, modest fluid challenge (< 500 mL) could be an option, increasing cardiac index in these patients[12]. On the other hand, fluid challenge could also over-distend the RV, leading to a reduction of cardiac output. Therefore, it is recommended to use it wisely[13]. If signs of elevated central venous pressure are present, no volume loading is advised. Vasopressors are often necessary in association with reperfusion treatment (medical, surgical or interventional). Norepinephrine leads to an improvement in coronary perfusion and ventricular systolic interaction, without changing pulmonary vascular resistance[14]; the use of norepinephrine should be limited in patients with cardiogenic shock.
In addition, results of an American national registry enrolling 3107 patients who underwent systemic fibrinolytic treatment and 1319 patients undergoing CDT showed that the systemic thrombolysis group had increased rates of bleeding-related mortality (18.1%
8.4%), general mortality (14.9%
6.12%) and rehospitalization (10.6%
7.6%)[28]. According to these data, the risk of fatal bleeding is lower during CDT than in cases of systemic thrombolysis. This can be due to the higher (approximately four-fold) dose of fibrinolytic agent used in systemic thrombolysis. However, as these data are extracted from a national registry and not from randomized studies, they should cautiously be taken in consideration. The ongoing PE-TRACT and HI-PEITHO studies are designed to overcome this issue.
I never liked being alone. It was too quiet, disconcerting(). Ever since I was a little girl, I felt uncomfortable on my own. Even as an adult I found it distressing1.
Illustrators often provide a portrait of Puss in his boots. You can see several illustrators visions of Puss on the Illustrations of Puss in Boots page. The image is still popular in novelty and gift items today.
The lady slowly glanced up, her large eyes filled with such fear, sadness and pain that I was frightened by her stare. I gulped and then, hesitantly, began putting the items back into her cart.
So he went in with her, and in the castle was a great hall paved with marble, and many servants, who flung wide the doors; And the walls were all bright with beautiful hangings, and in the rooms were chairs and tables of pure gold, and crystal chandeliers hung from the ceiling, and all the rooms and bed-rooms had carpets, and food and wine of the very best were standing on all the tables, so that they nearly broke down beneath it
The EKOSonic system (Boston Scientific, Massachusetts, United States) is an ultrasound-assisted catheter-directed thrombolysis system, which was specifically indicated for treatment of PE. The ultrasound waves that depart from the interior of the 5.4 French catheter can reach and treat the whole thrombus; in addition, fibrinolytic agent infusion can be performed from the catheter, combining the two treatment modalities. The functioning tip of the catheter can be of different lengths, with a range from 6 to 50 cm. Although it has been associated with a relatively safe and effective profile, the clinical benefits of this treatment when compared to classical CDT has yet to be proven[25]. Ultrasound-assisted thrombolysis was shown in a randomized trial named ULTIMA to determine faster decreases of the RV/LV ratio in patients with acute onset of intermediate-risk PE when compared to medical treatment, with no occurrence of major bleeding. However, the authors did not observe variations in 90-d patient mortality[36].
She bade him be of good courage, looked to see that she had the three nuts which she had found beside her mother s grave, mounted her horse, and rode out into the forest
CONCLUSION
Actual ESC guidelines indicate that in high-risk or intermediate/high-risk patients (with RV dysfunction at transthoracic ultrasonography or at computed tomography pulmonary angiography or Pulmonary Embolism Severity Index greater than 1 and positive troponin test), reperfusion treatments should be performed, in association with prompt hemodynamic support[1]. However, systemic thrombolysis is actually considered as the first indication, and as literature evidence states surgical pulmonary embolectomy is recommended in patients with high-risk PE in whom systemic thrombolysis is contraindicated or has failed (level of evidence I). Percutaneous catheter-directed treatment has level of evidence IIa and therefore should be conditionally considered after failure or infeasibility of the abovementioned medical and surgical therapies[2].
Set up of a multidisciplinary team and of management protocols for high-risk and intermediate/highrisk patients with PE should be considered, to promptly and correctly address every PE case.
New perspectives
The 2021 announcement of the multicentric prospective PEERLESS randomized controlled trial comparing aspiration thrombectomy
catheter-directed thrombolysis in up to 700 patients will provide real-life data on interventional radiology treatments for patients with intermediate/high-risk PE. At the same time, ultrasonography-assisted thrombolysis is proving valuable in intermediate/high-risk PE patients with good results and low complication rates[36]. However, more prospective studies are needed to shed light on the best interventional radiology treatment for this critical condition as well as to give the right place in the guidelines to these endovascular and mini-invasive techniques, on par to medical and surgical treatments.
One Friday evening I came home from work to find a big beautiful German shepherd on our doorstep. This wonderful strong animal gave every indication that he intended to enter the house and make it his home. I, however, was wary4. Where did this obviously well-cared-for dog come from? Was it safe to let the children play with a strange dog? Even though he seemed gentle, he still was powerful and commanded respect. The children took an instant liking5 to German and begged me to let him in. I agreed to let him sleep in the basement until the next day, when we could inquire around the neighborhood for his owner. That night I slept peacefully for the first time in many weeks.
FOOTNOTES
Posa A designed the research study; Posa A, Barbieri P, Tanzilli A and Mazza G performed the research and wrote the manuscript; Posa A, Iezzi R, Manfredi R and Colosimo C revised the manuscript; All authors have read and approved the final manuscript.
All the Authors have no conflict of interest to disclose.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Italy
Alessandro Posa 0000-0001-9617-3413; Roberto Iezzi 0000-0002-2791-481X; Riccardo Manfredi 0000-0002-4972-9500.
Liu JH
Clark was much older-seventy-eight to Allison s thirty-five. They were married. They were both quite tall and looked something alike in their facial features. Allison wore a natural-hair wig5. It was a thick blonde hood8 around her face. She was dressed in bright-dyed denims today. She wore durable9 clothes, usually, for she volunteered afternoons at a children s daycare center.
Filipodia
Liu JH
1 Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jiménez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Ní áinle F, Prandoni P, Pruszczyk P, Righini M, Torbicki A, Van Belle E, Zamorano JL; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS).
2020; 41: 543-603 [PMID: 31504429 DOI: 10.1093/eurheartj/ehz405]
2 Ortel TL, Neumann I, Ageno W, Beyth R, Clark NP, Cuker A, Hutten BA, Jaff MR, Manja V, Schulman S, Thurston C, Vedantham S, Verhamme P, Witt DM, D Florez I, Izcovich A, Nieuwlaat R, Ross S, J Schünemann H, Wiercioch W, Zhang Y. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism.
2020; 4: 4693-4738 [PMID: 33007077 DOI: 10.1182/bloodadvances.2020001830]
3 Morrone D, Morrone V. Acute Pulmonary Embolism: Focus on the Clinical Picture.
2018; 48: 365-381 [PMID: 29737640 DOI: 10.4070/kcj.2017.0314]
4 Turetz M, Sideris AT, Friedman OA, Triphathi N, Horowitz JM. Epidemiology, Pathophysiology, and Natural History of Pulmonary Embolism.
2018; 35: 92-98 [PMID: 29872243 DOI: 10.1055/s-0038-1642036]
5 Stratmann G, Gregory GA. Neurogenic and humoral vasoconstriction in acute pulmonary thromboembolism.
2003; 97: 341-354 [PMID: 12873915 DOI: 10.1213/01.ANE.0000068983.18131.F0]
6 Fernandes CJ, Luppino Assad AP, Alves-Jr JL, Jardim C, de Souza R. Pulmonary Embolism and Gas Exchange.
2019; 98: 253-262 [PMID: 31390642 DOI: 10.1159/000501342]
7 Matthews JC, McLaughlin V. Acute right ventricular failure in the setting of acute pulmonary embolism or chronic pulmonary hypertension: a detailed review of the pathophysiology, diagnosis, and management.
2008; 4: 49-59 [PMID: 19924277 DOI: 10.2174/157340308783565384]
8 Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism.
2002; 121: 877-905 [PMID: 11888976 DOI: 10.1378/chest.121.3.877]
9 Giri J, Sista AK, Weinberg I, Kearon C, Kumbhani DJ, Desai ND, Piazza G, Gladwin MT, Chatterjee S, Kobayashi T, Kabrhel C, Barnes GD. Interventional Therapies for Acute Pulmonary Embolism: Current Status and Principles for the Development of Novel Evidence: A Scientific Statement From the American Heart Association.
2019; 140: e774-e801 [PMID: 31585051 DOI: 10.1161/CIR.0000000000000707]
10 Messika J, Goutorbe P, Hajage D, Ricard JD. Severe pulmonary embolism managed with high-flow nasal cannula oxygen therapy.
2017; 24: 230-232 [PMID: 28452810 DOI: 10.1097/MEJ.0000000000000420]
11 Lacroix G, Pons F, D'Aranda E, Legodec J, Romanat PE, Goutorbe P. High-flow oxygen, a therapeutic bridge while awaiting thrombolysis in pulmonary embolism?
2013; 31: 463.e1-463.e2 [PMID: 23159426 DOI: 10.1016/j.ajem.2012.08.030]
12 Mercat A, Diehl JL, Meyer G, Teboul JL, Sors H. Hemodynamic effects of fluid loading in acute massive pulmonary embolism.
1999; 27: 540-544 [PMID: 10199533 DOI: 10.1097/00003246-199903000-00032]
13 Green EM, Givertz MM. Management of acute right ventricular failure in the intensive care unit.
2012; 9: 228-235 [PMID: 22805893 DOI: 10.1007/s11897-012-0104-x]
14 Ghignone M, Girling L, Prewitt RM. Volume expansion
norepinephrine in treatment of a low cardiac output complicating an acute increase in right ventricular afterload in dogs.
1984; 60: 132-135 [PMID: 6198941 DOI: 10.1097/00000542-198402000-00009]
15 Corsi F, Lebreton G, Bréchot N, Hekimian G, Nieszkowska A, Trouillet JL, Luyt CE, Leprince P, Chastre J, Combes A, Schmidt M. Life-threatening massive pulmonary embolism rescued by venoarterial-extracorporeal membrane oxygenation.
2017; 21: 76 [PMID: 28347320 DOI: 10.1186/s13054-017-1655-8]
16 Meneveau N, Guillon B, Planquette B, Piton G, Kimmoun A, Gaide-Chevronnay L, Aissaoui N, Neuschwander A, Zogheib E, Dupont H, Pili-Floury S, Ecarnot F, Schiele F, Deye N, de Prost N, Favory R, Girard P, Cristinar M, Ferré A, Meyer G, Capellier G, Sanchez O. Outcomes after extracorporeal membrane oxygenation for the treatment of high-risk pulmonary embolism: a multicentre series of 52 cases.
2018; 39: 4196-4204 [PMID: 30137303 DOI: 10.1093/eurheartj/ehy464]
17 Perkins GD, Olasveengen TM, Maconochie I, Soar J, Wyllie J, Greif R, Lockey A, Semeraro F, Van de Voorde P, Lott C, Monsieurs KG, Nolan JP; European Resuscitation Council. European Resuscitation Council Guidelines for Resuscitation: 2017 update.
2018; 123: 43-50 [PMID: 29233740 DOI: 10.1016/j.resuscitation.2017.12.007]
18 Soar J, Nolan JP, B?ttiger BW, Perkins GD, Lott C, Carli P, Pellis T, Sandroni C, Skrifvars MB, Smith GB, Sunde K, Deakin CD; Adult advanced life support section Collaborators. European Resuscitation Council Guidelines for Resuscitation 2015: Section 3. Adult advanced life support.
2015; 95: 100-147 [PMID: 26477701 DOI: 10.1016/j.resuscitation.2015.07.016]
19 Witt DM, Clark NP, Kaatz S, Schnurr T, Ansell JE. Guidance for the practical management of warfarin therapy in the treatment of venous thromboembolism.
2016; 41: 187-205 [PMID: 26780746 DOI: 10.1007/s11239-015-1319-y]
20 Goldhaber SZ, Haire WD, Feldstein ML, Miller M, Toltzis R, Smith JL, Taveira da Silva AM, Come PC, Lee RT, Parker JA. Alteplase
heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion.
1993; 341: 507-511 [PMID: 8094768 DOI: 10.1016/0140-6736(93)90274-k]
21 Kline JA, Nordenholz KE, Courtney DM, Kabrhel C, Jones AE, Rondina MT, Diercks DB, Klinger JR, Hernandez J. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 mo: multicenter double-blind, placebo-controlled randomized trial.
2014; 12: 459-468 [PMID: 24484241 DOI: 10.1111/jth.12521]
22 Daniels LB, Parker JA, Patel SR, Grodstein F, Goldhaber SZ. Relation of duration of symptoms with response to thrombolytic therapy in pulmonary embolism.
1997; 80: 184-188 [PMID: 9230156 DOI: 10.1016/s0002-9149(97)00315-9]
23 Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the "MOPETT" Trial).
2013; 111: 273-277 [PMID: 23102885 DOI: 10.1016/j.amjcard.2012.09.027]
24 Lee T, Itagaki S, Chiang YP, Egorova NN, Adams DH, Chikwe J. Survival and recurrence after acute pulmonary embolism treated with pulmonary embolectomy or thrombolysis in New York State, 1999 to 2013.
2018; 155: 1084-1090.e12 [PMID: 28942971 DOI: 10.1016/j.jtcvs.2017.07.074]
25 Piazza G, Hohlfelder B, Jaff MR, Ouriel K, Engelhardt TC, Sterling KM, Jones NJ, Gurley JC, Bhatheja R, Kennedy RJ, Goswami N, Natarajan K, Rundback J, Sadiq IR, Liu SK, Bhalla N, Raja ML, Weinstock BS, Cynamon J, Elmasri FF, Garcia MJ, Kumar M, Ayerdi J, Soukas P, Kuo W, Liu PY, Goldhaber SZ; SEATTLE II Investigators. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study.
2015; 8: 1382-1392 [PMID: 26315743 DOI: 10.1016/j.jcin.2015.04.020]
26 Tapson VF, Sterling K, Jones N, Elder M, Tripathy U, Brower J, Maholic RL, Ross CB, Natarajan K, Fong P, Greenspon L, Tamaddon H, Piracha AR, Engelhardt T, Katopodis J, Marques V, Sharp ASP, Piazza G, Goldhaber SZ. A Randomized Trial of the Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Intermediate-Risk Pulmonary Embolism: The OPTALYSE PE Trial.
2018; 11: 1401-1410 [PMID: 30025734 DOI: 10.1016/j.jcin.2018.04.008]
27 Bloomer TL, El-Hayek GE, McDaniel MC, Sandvall BC, Liberman HA, Devireddy CM, Kumar G, Fong PP, Jaber WA. Safety of catheter-directed thrombolysis for massive and submassive pulmonary embolism: Results of a multicenter registry and meta-analysis.
2017; 89: 754-760 [PMID: 28145042 DOI: 10.1002/ccd.26900]
28 Arora S, Panaich SS, Ainani N, Kumar V, Patel NJ, Tripathi B, Shah P, Patel N, Lahewala S, Deshmukh A, Badheka A, Grines C. Comparison of In-Hospital Outcomes and Readmission Rates in Acute Pulmonary Embolism Between Systemic and Catheter-Directed Thrombolysis (from the National Readmission Database).
2017; 120: 1653-1661 [PMID: 28882336 DOI: 10.1016/j.amjcard.2017.07.066]
29 Patel N, Patel NJ, Agnihotri K, Panaich SS, Thakkar B, Patel A, Savani C, Patel N, Arora S, Deshmukh A, Bhatt P, Alfonso C, Cohen M, Tafur A, Elder M, Mohamed T, Attaran R, Schreiber T, Grines C, Badheka AO. Utilization of catheter-directed thrombolysis in pulmonary embolism and outcome difference between systemic thrombolysis and catheter-directed thrombolysis.
2015; 86: 1219-1227 [PMID: 26308961 DOI: 10.1002/ccd.26108]
30 Kuo WT, Gould MK, Louie JD, Rosenberg JK, Sze DY, Hofmann LV. Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques.
2009; 20: 1431-1440 [PMID: 19875060 DOI: 10.1016/j.jvir.2009.08.002]
31 Dumantepe M, Teymen B, Akturk U, Seren M. Efficacy of rotational thrombectomy on the mortality of patients with massive and submassive pulmonary embolism.
2015; 30: 324-332 [PMID: 25683156 DOI: 10.1111/jocs.12521]
32 Bayiz H, Dumantepe M, Teymen B, Uyar I. Percutaneous aspiration thrombectomy in treatment of massive pulmonary embolism.
2015; 24: 46-54 [PMID: 25060976 DOI: 10.1016/j.hlc.2014.06.014]
33 Sista AK, Horowitz JM, Tapson VF, Rosenberg M, Elder MD, Schiro BJ, Dohad S, Amoroso NE, Dexter DJ, Loh CT, Leung DA, Bieneman BK, Perkowski PE, Chuang ML, Benenati JF; EXTRACT-PE Investigators. Indigo Aspiration System for Treatment of Pulmonary Embolism: Results of the EXTRACT-PE Trial.
2021; 14: 319-329 [PMID: 33454291 DOI: 10.1016/j.jcin.2020.09.053]
34 Sedhom R, Abdelmaseeh P, Haroun M, Megaly M, Narayanan MA, Syed M, Ambrosia AM, Kalra S, George JC, Jaber WA. Complications of Penumbra Indigo Aspiration Device in Pulmonary Embolism: Insights From MAUDE Database.
2022; 39: 97-100 [PMID: 34706845 DOI: 10.1016/j.carrev.2021.10.009]
35 Tu T, Toma C, Tapson VF, Adams C, Jaber WA, Silver M, Khandhar S, Amin R, Weinberg M, Engelhardt T, Hunter M, Holmes D, Hoots G, Hamdalla H, Maholic RL, Lilly SM, Ouriel K, Rosenfield K; FLARE Investigators. A Prospective, Single-Arm, Multicenter Trial of Catheter-Directed Mechanical Thrombectomy for Intermediate-Risk Acute Pulmonary Embolism: The FLARE Study.
2019; 12: 859-869 [PMID: 31072507 DOI: 10.1016/j.jcin.2018.12.022]
36 Kucher N, Boekstegers P, Müller OJ, Kupatt C, Beyer-Westendorf J, Heitzer T, Tebbe U, Horstkotte J, Müller R, Blessing E, Greif M, Lange P, Hoffmann RT, Werth S, Barmeyer A, H?rtel D, Grünwald H, Empen K, Baumgartner I. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism.
2014; 129: 479-486 [PMID: 24226805 DOI: 10.1161/CIRCULATIONAHA.113.005544]
 World Journal of Radiology2022年8期
World Journal of Radiology2022年8期
- World Journal of Radiology的其它文章
- Triple rule-out computed tomography angiography: Evaluation of acute chest pain in COVID-19 patients in the emergency department
- Imaging volumes during COVID-19: A Victorian health service experience
- Amebic liver abscess: Clinico-radiological findings and interventional management
- Advanced magnetic resonance imaging findings in salivary gland tumors
- Augmenting prostate magnetic resonance imaging reporting to incorporate diagnostic recommendations based upon clinical risk calculators
