Occult hepatitis B—the result of the host immune response interaction with different genomic expressions of the virus
lNTRODUCTlON
More than 40 years from its first description in the late 1970s[1],occult hepatitis B virus(HBV)infection(OBI)continues to be one of the most challenging topics in the field of viral hepatitis.OBI represents an important public health problem because of its many implications.
Harry limped up to the batter s box. I noticed he had his usual bat. I called a time out, ran up to him and whispered, Harry, this is the time for the magic bat. Give it a try. Just keep your eyes open and swing.
Since the introduction of hepatitis B surface antigen(HBsAg)testing in the routine screening of blood donors in the early 1970s,the incidence of transfusion-transmitted hepatitis has been dramatically reduced;however,it did not eliminate this unwanted event,and neither associating alanine aminotransferase level measurement nor anti HBc testing did.The majority of transmissions is attributable to occult hepatitis B.HBV remains the most frequent transfusion-transmitted viral infection[2].
OBI may also be the cause of HBV transmission in organ transplants or can represent a problem in patients receiving immunosuppressive therapy for various conditions,chemotherapy,or anti-CD-20 therapy because of the risk of reactivation.
The persistence of OBI may lead to the development of liver cirrhosis and,eventually,to hepatocellular carcinoma(HCC)[3].OBI can be one of the possible causes of cryptogenic liver disease.
When the children moved to a town five hours away, my husband was understandably devastated3. In order to maintain regular communication with the kids, we contacted Cyberspace4 and promptly5 set up an e-mail and chat-line service. This technology, combined with the telephone, would enable us to reach them on a daily basis by sending frequent notes and messages, and even chatting together when we were all on-line.
In people co-infected with hepatitis C virus(HCV)who have OBI,curing HCV with current directacting antiviral medication can lead to HBV reactivation,although this happens rarely in patients with OBI,mostly in those with overt HBV infection[4].The risk of HBV reactivation is also documented in human immunodeficiency virus(HIV)co-infected patients,especially after withdrawal of antiretrovirals that are also active on HBV[5].
SOURCES AND SELECTlON CRlTERlA
The first article considered to refer to occult B hepatitis,although it did not identify it by this specific term,dates back to 1979.It retrospectively analyzed sera from 128 donors from 1971 to 1977,who were chosen because their blood recipients developed clinically recognizable posttransfusion hepatitis.“The detection of hepatitis B core antibody(anti-HBc)alone in nine of 29 implicated donors with HBV markers,suggests that some HBsAg-negative donors implicated in the transmission of hepatitis B may be low-level carriers potentially detectable using tests for anti-HBc.However,the total absence of HBV markers in many implicated donors probably indicates that such donors did not transmit HBV infection”was one of that study’s[1]conclusion,and today,we know that their assumptions were not completely correct,although they identified an important category of patients.When we refer to that study,we have to keep in mind that it was a retrospective study;furthermore,the sensitivity of the serological tests used at that time was low,and molecular biology testing was not available.
We prioritized studies performed in humans,chimpanzees,or humanized chimeric mice,when available,but we also included those performed in other animal models,such as woodchucks and mice or
primary hepatocytes or cell lines.We looked into the most recent sources first,but if data from older sources were still available,we also cited such data.For the best quality of clinical evidence,we prioritized guidelines,technical reviews,or high-quality prospective observational studies or their meta-analyses,when available.
DEFlNlTlON OF OCCULT HEPATlTlS B lNFECTlON
The definition that resulted from the first Taormina workshop on occult hepatitis B was as follows: "Presence of HBV DNA in the liver(with detectable or undetectable HBV DNA in the serum)of individuals testing HBsAg negative by currently available assays"[6].The revision made 10 years later in the re-edited workshop was only slightly different:“the presence of replication-competent HBV DNA[
,episomal HBV covalently closed circular DNA(cccDNA)]in the liver and/or HBV DNA in the blood of people who test negative for HBsAg by currently available assays”;the latter emphasizes the importance of the fact that HBV DNA should be competent to replicate[7].There is another change of optics between the two workshops;in the first one,patients with
gene mutations that make HBsAg not detectable by usual commercially available detection assays but with HBV DNA levels comparable to those of the overt infections were called false OBIs[6],whereas in the 2018 workshop,these patients were considered a subset of OBI.
We searched PubMed for studies published in English between January 1979(when the first reference to OBI was considered to be found)and December 2019.We initially used the following search terms in combination with the term“HBV”or“hepatitis B”:“l(fā)ifecycle,”“persistence,”“natural history,”“guidelines,”“cccDNA,”“integrated DNA,”“immunity,”“immune system,”“innate immunity,”“adaptive immunity,”“pathogenesis,”“physiopathology,”and then with“OBI HBV”or“Occult hepatitis”+“HBV”:“definition,”“cccDNA,”“mutations,”“HBV variants,”“immune system,”“innate immunity,”“adaptive immunity,”“physiopathology,”“pathogenesis,”“mechanism,”“viral factors,”“host factors,”“epigenetics,”“miRNA.”
Over time,several definitions have been proposed for OBI.
OBI can be seropositive when either the anti-HBc and/or the hepatitis B surface antibody(anti-HBs)is positive(without prior hepatitis B vaccination)or seronegative and when both anti-HBc and anti-HBs are negative.Up to 20% of OBI are seronegative[3,8].
The latest issue of the Asian Pacific Association for the Study of the Liver clinical practice guidelines on the management of hepatitis B[8]and the latest European Association for the Study of the Liver clinical practice guidelines on the management of hepatitis B virus infection[9]recognize occult hepatitis B as a particular form of evolution of HBV infection,characterized by the absence of secreted HBsAg and the presence of HBV DNA either in the liver or in the blood of the patient.American Association for the Study of Liver Diseases(AASLD)guidelines identify a category of patients who test positive for anti-HBc antibodies but negative for HBsAg and,among them,a sub-category of patients who may or may not be HBV DNA positive - these groups may be at risk for reactivation or for developing HCC;however,AASLD guidelines do not define the category specifically as OBI[10].
TYPES OF OBlS
According to the 2019 Taormina workshop[7],OBIs can be categorized mainly into seropositive and seronegative.The seropositive status may be achieved either after the resolution of acute hepatitis(which is the case in more than 95% of immune-competent adults)or after a chronic HBV infection(with or without liver injury),either spontaneously or after antiviral treatment(that with actual substances rarely achieves this functional cure).In these cases,we have to ensure that the most sensitive HBsAg kits are used to rule out false OBIs.HBV DNA can be found intermittently in the blood of these patients,usually at levels below 200 IU/mL[3,7].The seronegative status may be the consequence of an OBI that progressively lost anti-HBc and anti-HBs antibodies or might be negative from the beginning(a situation called primary occult infection and demonstrated in woodchucks infected with small amounts of viral particles[11]).
A special category of OBI is represented by HBV genetic variants in which the HBs antigen is not recognized by available assays.The main cause of this situation is a mutation in the
-gene(S-escape mutants),but a mutation in the
-gene promoter or a splice variant can also be considered[7].This type of OBI is mainly seropositive;in a study regarding this issue,out of 99 patients with OBI and mutant HBV variants,only 3 patients were seronegative[3].At the previous Taormina workshop,this type of OBI was considered false[6].HBV DNA can have levels comparable with those of the overt HBV infection in this subtype of OBI,except in the one with splice variants,in which HBV DNA levels are low or undetectable[7].The above pathways to OBI are summarized in Figure 1.
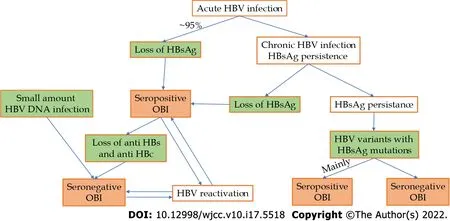
The term“functional cure”refers to OBIs in which HBsAg is not detectable as a result of the immune system’s action and not because of the situations in which HBsAg is present,mutant,and not detected by conventional commercially available assays.
HEPATlTlS B VlRUS LlFECYCLE AND PERSlSTENCE
HBV is a member of the Hepadnaviridae family,and its complete HBV virion consists of an outer envelope,an inner nucleocapsid,and a 3.2 kb partially double-stranded DNA,known as relaxed circular DNA(RC-DNA),which is covalently connected with the DNA polymerase.The HBV genome contains four overlapping open reading frames,namely,preS1/S2/S,pre-core/core,polymerase,and X domains,which encode seven viral proteins[12].Of these proteins,four are of major importance:(1)The viral polymerase,that has a role in viral replication and packaging;(2)The small(S),medium(M),and large(L)surface antigens,polypeptides that constitute the HBsAg,that is part of the viral envelope and play a major role in viral entry;(3)HBV core protein(HBc),part of the viral capsid(that play a role in viral replication and packaging);and(4)The X protein(HBx),which has various functions,one of them being the regulation of viral genome transcription.HBx functions may vary with the stage of the HBV infection[12-14].
HBV virions bind initially to hepatocytes by interacting with heparan sulfate proteoglycans for virus docking and subsequently with the recently discovered functional receptor - sodium taurocholate cotransporting polypeptide[13,15].After endocytosis,the nucleocapsid is released in the cytoplasm and transported to the nucleus,where RC-DNA is released and converted(some say repaired)by host factors into cccDNA[12,14].When RC-DNA is not completely converted to cccDNA,the aberrant double-stranded linear DNA of HBV can be used for viral integrations into the host genome[16].The chromatinized cccDNA,which results after a complex multiple-step process,is a mini-chromosome that serves as a template for the pregenomic RNA and subgenomic RNA transcripts,encoding all viral proteins[12-14,17].The pregenomic RNA is the template for the generation of the progeny HBV RCDNA,and for this to occur,it has to interact with its own translation products,HBV polymerase,and the core protein,thus forming the nucleocapsid;the latter matures through complex processes and can either be enveloped to form HBV virions or can be re-imported into the nucleus to be converted into cccDNA from its RC-DNA in order to maintain a stable pool of cccDNA[12,14,17].HBs-coated mature nucleocapsids containing RC-DNA are released from infected cells
host cellular multivesicular body function[14,18]and can infect other hepatocytes.Other sub-viral particles,such as HBsAg,which can be produced in excess,are released by similar pathways as subviral noninfectious HBsAg particles[13,14,18].
After exposure to HBV,over 95% of immune-competent adults can eliminate HBsAg and HBV DNA from circulation,in many cases with HBs and HBe antigen seroconversion(loss of these antigens with the appearance of corresponding antibodies);despite this,HBV DNA can persist in the liver in the form of cccDNA or be integrated in the genome for years or a lifetime.This is called a functional cure and is a type of OBI.By contrast,infections in newborns with HBeAg expressing HBV strains lead to chronic overt infections in over 90% of cases.
The persistence of viable HBV virus particles is maintained through cccDNA or genomic material persistence(integrated HBV DNA fragments).We should keep in mind that laboratory techniques that are used to characterize HBV persistence are not perfect;they lack sensitivity,they may fail in recognizing HBsAg mutant variants,and they cannot distinguish the origin of HBsAg - whether it is from cccDNA or integrated HBV DNA.
HOST lMMUNE RESPONSE TO HBV
Both innate and adaptive immune responses are important in the course of HBV infection.
Innate immunity represents the first line in host defense,playing an important role in the resolution of a viral infection either through its direct activity or by initiating and regulating adaptive immunity.HBV,similar to many other pathogens,is recognized through germline-encoded pattern recognition receptors that are present either on the cell surface or within some intracellular compartments.The activation of these receptors normally triggers the recruitment of different types of adaptor molecules that eventually activate the signaling pathways of nuclear factor-kb and interferon(IFN)regulatory factors.Finally,this leads to the production of interferon-stimulated genes(ISGs)and different inflammatory cytokines,interferons(type I/III IFNs),and chemokines[19,20].Studies on experimentally infected chimpanzees and human patients with acute infection showed that HBV does not induce type I/III IFNs and also does not significantly increase ISGs,suggesting that the receptors are unable to recognize HBV or that HBV can actively block these pathways[21,22].In vitro studies(on HBV-infected cultures of human hepatocytes)or studies on liver specimens collected by biopsy from patients with chronic HBV infection showed similar results - ISG levels are not increased compared with those of the controls[23,24].Therefore,the term stealth virus was coined for HBV[22,23].
Natural killer(NK)cells are also important parts of the innate immune system,and they normally provide a rapid response when viral invasion is recognized.Their activity also seems to be impaired in HBV-infected hosts,and the mechanisms that could be involved include the reduction of the expression of NK group receptors 2D(NKG2D)and 2B4(NKG2B4)(activating receptors),which consequently reduces NK cells’ capacity to produce INFs and mediate cytotoxicity;the suppression of the expression of the major complexes of histocompatibility class I-related molecules A and B;and the increased expression of T cell immunoglobulin- and mucin-domain-containing molecule-3 in circulating NKs[25,26].
Nevertheless,the innate immune system eventually responds to the presence of HBV through both its components(circulating and intra-hepatic)and its cells;macrophages,monocytes,NK cells,dendritic cells(DC),myeloid-derived suppressor cells,and innate lymphoid cells start producing different signals that will lead to the activation of the adaptive immune system[27].The adaptive immune system acts mainly through specially developed subsets of T and B cells that are created to recognize and destroy HBV-infected hepatocytes.Prolonged exposure to viral components,such as HBsAg,HBeAg,and HBxAg,leads to immune system exhaustion and downregulation of host response[28].
Regulatory T cells(Tregs)normally play an immune suppressive role by suppressing DCs,NK cells,CD4 cells,and CD8 T cells[27,29].They perform their role by producing immunosuppressive mediators,IL-10 and TGF-β,and through direct contact[27].During acute HBV infection,Tregs protect the liver from exceedingly severe immune-mediated liver damage[30].On the other hand,the same Tregs seem to play a role in promoting chronic infection,as they are found in larger amounts in patients with chronic hepatitis B than in those with acute hepatitis B or HBe-negative HBV infection(formerly inactive carriers).
CD4 T cells play a central role in HBV infection management by manifesting their functions,including the activation of innate immune cells,B cells,and cytotoxic T cells.They promote antibody production and generate signals to attract neutrophiles at the site of infection[31].CD4 T cells also contribute to the selection and maintenance of HBV-specific CD8 T cells[32].On the other hand,besides the above-mentioned functions,CD4 T cells are involved in the pathogenesis of HBV chronic infection by producing and promoting inflammation and fibrosis[27].CD8 T cells are the actual effectors that perform viral clearance by inhibiting viral replication and contributing to the apoptosis of infected liver cells.In patients who achieve a functional cure,a polyclonal and multi-specific HBV CD8 T cell response can be identified,whereas in those with chronic hepatitis B,CD8 T cells display a narrow spectrum of epitopes and a consequently weak response[27,33,34].
One day, he told her not to come by anymore. He told her he was being shipped to another concentration camp. As he walked away with tears streaming down his face, he wondered if he d ever see her again. She was the only kind soul he d seen across the fence.
B cells are essentially seen as cells that produce antibodies and elements that can differentiate into plasma cells,providing long-term immunity.Lately,a new function of B cells has been identified - the regulatory function.This B subtype is called B regulatory cells(Bregs).Bregs produce IL-10,a mediator that serves as a downregulatory agent for other immune cells and promotes immune tolerance[35].In HBV-infected patients,Bregs are considered to promote viral replication and liver fibrosis.They could also be responsible for HBV flares by suppressing CD8 cells[36].The antibody production function of B cells is critical for the management of HBV infection;these cells can produce antibodies against different viral proteins,including but not limited to HBsAg,HBcAg,and HBeAg.HBs antibodies are critical for controlling or preventing viral infection.They are produced by B cells stimulated by specific T helper cells(follicular helpers)mainly through IL-21.Impairment of this chain at any of its links leads to persistent HBV infection[31].
Also of interest are human apolipoprotein B mRNA-editing catalytic polypeptide-like enzymes(APOBECs),essential components of our innate immune system,which can inhibit a wide range of viruses using mainly de-amination processes[37].By exerting their activity,APOBECs can lead to the selection of HBsAg mutants(
,G145R),which are not detectable by some commercially available assays[38]and can produce replication-incompetent transcripts(splice variants),which can also elude some HBsAg detection kits[39].Furthermore,APOBECs can inhibit DNA-RNA hybridization;they can increase susceptibility to nuclease digestion and decrease protein processing,leading to OBI[37,39].
The interaction of viral factors with the immune system of the host can lead to various outcomes,and the following are the five phases of the natural evolution of HBV infection[10,11]:(1)HBeAg-positive chronic infection(immune tolerance);(2)HBeAg-positive chronic hepatitis(immune clearance/chronic hepatitis);(3)HBeAg-negative chronic infection(inactive carriers);(4)HBeAg-negative chronic hepatitis(chronic hepatitis);and(5)HBsAg-negative phase(OBI).
MECHANlSMS THAT LEAD TO OBl
At the end of the class, I helped Katie dismount. Color in her cheeks now, she smiled radiantly and arched her thin arms around Stripe s lowered neck. He kept his head down. Burying her face in his mane, Katie murmured softly, I love you, Stripe. I stood motionless a few feet away, touched by a moment of uncommon25 beauty.
Mutations that affect the posttranslational production of virus envelope proteins.For example,Nglycosylation in the position N146 of the S domain in wild-type virus may lead to an escape variant[50].
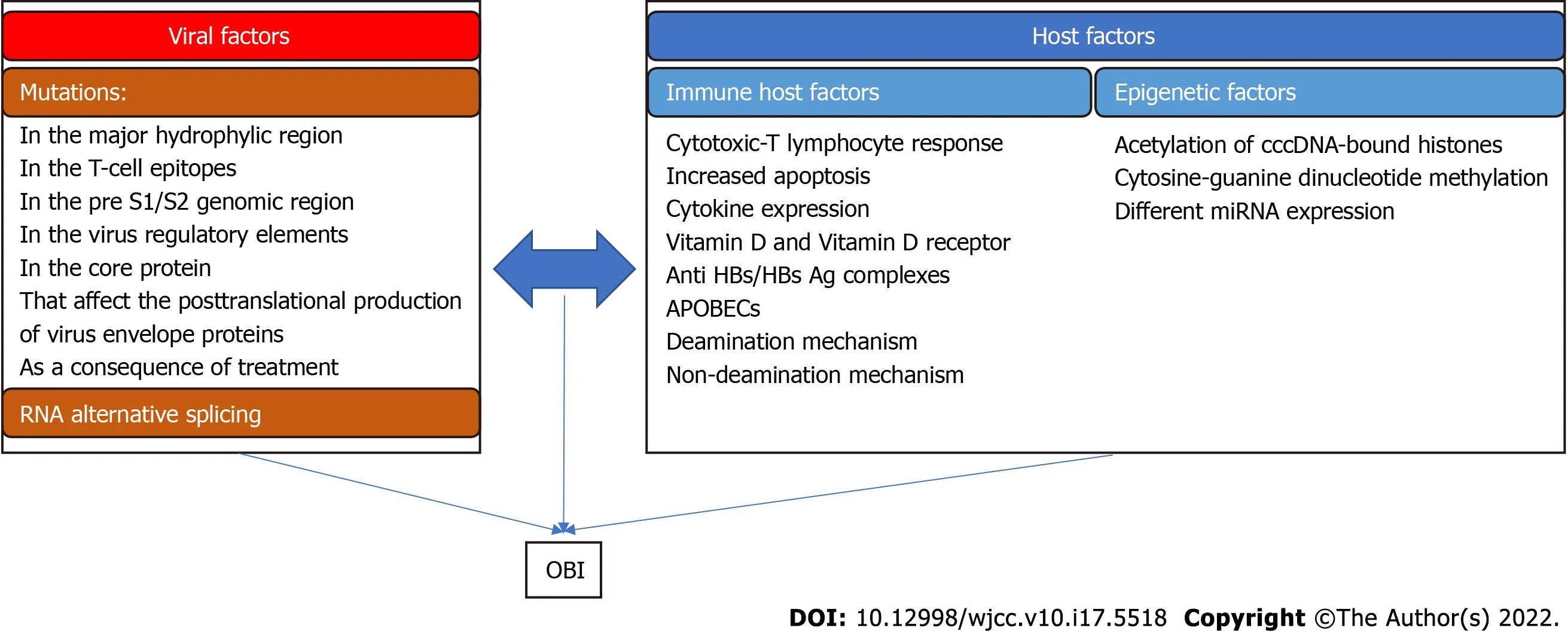
Viral factors
The factors related to HBV that result in OBIs are mainly related to the situation in which HBsAg is not recognized by available kits because of various mutations of the virus.
HBV variants may show different types of mutations:
In the major hydrophilic region(MHR/aminoacids 99-169)of the S protein.G119R,Q129R,T140I,and D144A are the mutations of this region found in one study,affecting mainly the“a”determinant of MHR(aminoacids 124-147),which contains a cluster of B-cells epitopes[42].P120T and S143L are other mutations associated with OBI[43].Another study looked even further and differentiated between OBIs in different genotypes.In this case,sM103,sS113,sS114,sG130,sS132,and sK160 appear specific to OBI with genotype B;sD102 and sW165 appear specific to OBI with genotype C;and sT118,sP135,and sS154 appear common to both genotypes[12].E2 mutations(E2G/A/V/D)can also influence the detection of HBsAg,leading to OBI[12,44].
In the T-cell epitopes.Positions 41,44,48,93,96,97,171,175,176,178,185,190,207,and 213 are affected and may generate immune-escape variants,with some not being recognized even by the host’s circulating HBs antibodies.These positions are outside the MHR,in the N-terminal and C-terminal regions of the S domain[40,45].
In the pre S1/S2 genomic region.The following pre-S1 mutations were found in one study: F25L,A28T,K57T,del 57-99,P65L,S78N,P89T,N98NK,N98NT,N98I,G102R;the following were found for pre S2: del 9-22,A11T,P36Q,and P54Q[42].Mutations in this genomic region can affect antigenicity,immunogenicity,cell elimination,and/or expression of HBsAg,leading to the failure of its detection or reducing or even inhibiting the replication and/or secretion of virions and thus having a negative effect on HBsAg detection[46].
In virus regulatory elements.Gene promoter regions are essential sites in DNA recognized by proteins for the downstream processes of replication and transcription.Alterations in these regions can lead to the down- or upregulation of the respective genes[40].One study showed that a 129 bp in-frame deletion in the S promoter region is associated with reduced levels of middle and small surface protein transcripts,resulting in a marked reduction in the expression of the two proteins.In infections with these mutants,a large amount of surface proteins accumulates inside the hepatocytes[47].
That was a marvellous flute! Its sound was as thrilling as thewhistle of a steam engine; in fact it was much stronger, for itsounded and was heard in the yard, in the garden, in the wood, andmany miles round in the country; at the same time a storm rose androared; Everything in the right place
In the core protein.Generally,studies have focused on the mutations occurring in the S and pre-S regions,but at least two studies have shown that core protein mutations can also lead to occult HBV infection.The W62R mutation in the core protein significantly reduces HBcAg and HBeAg production during HBV replication,potentially contributing to the occurrence of OBI[48,49].
And how should I know that thou speakest truth? answered Tsar Dolmat. Hadst thou come to me first I would have given thee the Fire Bird with honor. How will it be with thee now when I send into all Tsardoms, declaring how thou hast acted shamefully29 in my borders? However, Tsarevitch Ivan, I will excuse thee this if thou wilt30 serve me a certain service. If thou wilt ride across three times nine countries to the thirtieth Tsardom of Tsar Afron, and wilt win for me from him the Horse with the Golden Mane, which his father promised me and which is mine by right, then will I give to thee with all joy the Fire Bird. But if thou dost not serve me this service, then will I declare throughout all Tsardoms that thou art a thief, unworthy to share thy father s honors.
Mutations that appear as a consequence of treatment with nucleotide/nucleoside analogs and may affect both viral polymerase and S protein[51].Lamivudine-associated polymerase gene mutations M204I and L180M/M204I,corresponding to sI195M and sW196S in HBsAg,have been shown to be associated with reduced binding to HBs antibodies,and these mutants may not be correctly identified by HBsAg detection kits[39].
Although a greater genetic variability in the
gene of HBV isolated from OBIs was found compared with overt infection,it has also been proven that the majority of OBI patients are not infected with mutant variants,suggesting that the mechanisms of OBI are not mainly viral mutations[40].MHR variants and,generally,
gene mutants may escape anti-HBs antibodies and may not be recognized by available kits,thus representing a serious health problem because they could infect even vaccinated persons[39].The same mutants are implicated in reinfections following liver transplantation,despite correct Hepatitis B Immune Globulin(HBIG)prophylaxis.Stopping HBIG administration after the procedure allows the mutant HBV to revert into the wild type,suggesting that HBIG may favor the selection of MHR mutants[52].In patients who present the reactivation of HBV infection from OBI during or after immunosuppressive therapy,the heterogeneity of reactivated HBV has been reported to be significantly lower than that from HBsAg-positive carriers,suggesting that OBI individuals are infected with HBV populations of low genomic heterogeneity in their liver[53].
RNA alternative splicing is an important posttranscriptional mechanism that enables single genes to produce multiple proteins.RNA splicing contributes to mRNA and protein diversities.It regulates gene expressions,providing an important causative relationship link between genetic variation and disease[54,55].Splicing has been shown to have a significant effect on gene expression in HBV,and its implication in the occurrence of OBI has been claimed.A G-to-A mutation at position 458 of the surface gene altered the splicing of the
gene mRNA because nucleotide 458 is close to the 5’ splice site of
gene mRNA.The mutation prevents the splicing of the pre-S2/S mRNA from positions 458 to 1305,and the two analyzed patients did not express pre-S2/S mRNA and HBsAg[56].Another group found another mutation mechanism based on splicing,which is specific to genotype D.They describe an evolutionary branch in which the acceptor site at nucleotide 202 and the donor site at nucleotide 2986 are involved in a splice event,resulting in the loss of the spacer region from the viral polymerase gene while retaining the original reading frame.As a result,polymerase functions are not affected,but the expression of the small,middle,and large surface proteins is.Reduced HBsAg expression in the infection with HBV with this mutation leads to OBI[57].
Despite all the above arguments,it is important to note that most OBI patients are not infected with specific mutants.Mutant populations,especially pre-S/S variants,can be found in people with overt infections.Occult HBV genotypes are more often perfectly able to replicate,and their heterogeneity is similar to those from overt infections.In vitro studies have shown that HBV taken from the host’s environment is going back to the wild type,being able to normally synthesize proteins and replicate.A similar situation is described above regarding post-liver transplantation from OBI donor reactivation of HBV in its wild type[40,51,52].
Host factors
The first evidence,though indirect,that the host immune system is important in OBI is the possibility of HBV infection reactivation in patients subjected to immune suppression,regardless of the possible virus mutations.
A long-term follow-up study has shown that cytotoxic-T lymphocyte(CTL)response following an acute HBV infection persists for decades after serological recovery.CTL response is directly correlated with the presence of HBV DNA in the serum of these patients[58].Therefore,it is possible to hypothesize that during the occult phase of the infection,HBV can still synthesize very small amounts of antigens that are not detectible by available kits but are sufficient to maintain an HBV-specific T cell response.This assumption is confirmed by the findings showing that,apart from HBV cccDNA molecules,all viral HBV transcripts(including the pregenomic RNA)can also be detected and quantified in the livers of OBI individuals[40,58].Some other studies have reported similar vigorous T cell responses in OBI[59,60].The presence or absence of serologic HBV markers defined two profiles of HBV-specific T-cell responses in occult infection.Anti-HBc-positive patients showed a T-cell response typical of protective memory,with robust
expansion and IFN-γ production by HBV-specific T cells,suggesting that this condition represents a resolved infection with immune-mediated virus control.By contrast,HBV-specific T cells in anti-HBc-negative patients did not readily expand and produce interferon-gamma in vitro,suggesting the possibility of less complete maturation of protective memory[60].It has been demonstrated that clearance of more than 90% of intrahepatic HBV DNA does not require lysis of HBV-infected hepatocytes,suggesting that some noncytolytic immune responses are critical in the clearance of acute HBV infection.It has also been shown that even HBV cccDNA is susceptible to these noncytolytic mechanisms.A noncytolytic HBsAg-specific T-cell response has been suggested as the potential mechanism for occult HBV infections associated with very low and undetectable levels of HBsAg[39].
45. Finger: The forces of the unconscious that can emerge without warning and hinder efforts of the conscious are represented by the finger (Olderr 1986).Return to place in story.
One study that examined cytokine expression in OBI compared with chronic HBV hepatitis found that interleukin 2,interleukin 4,and IFN-β responses were low in both situations.The authors also found that significantly lower levels of the soluble form of the anti-apoptotic regulator Fas(sFas)were detected in occult HBV infection than in chronic HBV infection(
= 0.01)[61].As a marker of apoptotic inhibition,decreased sFas during occult HBV infection would indicate that apoptosis occurs at higher rates in occult compared with chronic HBV infection and,therefore,may contribute to HBsAg clearance and HBV replication downregulation.Another study showed that reduced expression of CXCL12,a chemokine that modulates apoptosis,may play a role in occult HBV infection[62].Increased apoptosis may thus play a role in the occurrence of OBI[39,61,62].A more recent study found that in patients with OBI and chronic HCV infections compared with monoinfected(HCV)patients and healthy donors,the levels of TNF-α,IL-10,IL-6,IL-4,and IL-2 were increased[63].Vitamin D3 and vitamin D receptor(VDR)regulate several cytokines and are important determinants of anti-HBV response.They also modulate HBV loads and HBV protein expression[64,65].The polymorphisms in the T/T allele of exon 9 of VDR are possibly associated with OBI,and VDR and its functional polymorphisms are likely to be related to the occurrence of OBI in some patients[66].
Emerging data suggest that microRNAs(miRNAs)play vital roles in the occurrence and development of HBV infection,particularly in OBI occurrence.MiR-199a-3p and miR-210 were found to efficiently reduce HBsAg expression,and quantification of HBV DNA by real-time PCR showed that both miRNAs suppressed viral replication[83].In another study,miR-125a-5p was found to interact with the viral sequence and to suppress HBsAg expression and release[84].MiR-141 was identified to repress HBV expression,and synthetic miR-141 could also significantly suppress HBV expression and replication by targeting peroxisome proliferator-activated receptor alpha[85].In a more recent study,miRNAs,including hsa-miR-25-3p,-486-5p,-92a-3p,and -1-3p,showed the ability to distinguish OBI from healthy controls efficiently,with an area under the curve value of 0.874,0.776,0.886,and 0.807,respectively.In total,32 differentially expressed miRNAs were identified between OBI and the healthy controls by miRNA sequencing[86].Compared with the case of the healthy controls,plasma miR-451a and miR-340-3p were significantly upregulated in OBI,making the authors propose these markers for distinguishing OBI from healthy donors[86].
In 1965, my world was suddenly uprooted7. I found myself alone with two young sons when my husband wanted a divorce. I was fortunate to receive a full scholarship to the University of Connecticut in the field of special education. I decided8 to sell the furniture and household items and return to my home state with just our clothes.
The physiological function of apolipoprotein B mRNA-editing enzyme catalytic polypeptides is cytidine deamination[68].The expression of APOBEC3G in cells replicating HBV resulted in a 50-fold reduction in HBV DNA levels.Both deamination-dependent and deamination-independent mechanisms of inhibition of HBV replication have been reported for APOBECs[69].Both mechanisms have also been implicated in the APOBEC-induced inhibition of HBV replication[70].APOBEC deamination-dependent activity may lead to HBsAg mutants,as mentioned above[38,39].IFN-alpha can upregulate APOBEC3A in HBV-infected cells in which HBV core protein mediates the interaction of APOBEC3A with HBV cccDNA,resulting in cytidine deamination,apurinic/apyrimidinic site formation,and,finally,cccDNA degradation[70].Deamination-independent processes of APOBECs lead to decreased HBV DNA production and to a decrease in HBV protein synthesis[37,38].
Some of the mechanisms that control HBV transcription or replication can be influenced in some cases by the modification of gene expression rather than by the alteration of the DNA sequence itself.This is called epigenetic modification.Epigenetic modifications can alter the expression pattern of a gene without changing its nucleotide sequence.Many studies have revealed that epigenetic mechanisms are important for the occurrence of OBI[39,71,72].
In recent years,significant advances have been made in understanding the HBV lifecycle and the molecular mechanisms leading to the persistence of the virus in the occult state.These factors are mainly related to the host immune system and,to a smaller proportion,to the virus[40].Some external factors can contribute to the appearance of OBI by interfering either with the host immune system or with the lifecycle of HBV.Of these,notable are HIV and HCV co-infections[3,40].Furthermore,coinfection with Schistosoma mansoni was found to inhibit HBV replication[39,41].
HBV cccDNA minichromosomes are located in the nucleus of infected hepatocytes and can be associated with histones,such as H1,H2A,H2B,H3,and H4[72],or non-histone proteins(HBV core proteins)[73].The acetylation status of cccDNA-bound histones H3 and H4 regulates HBV replication,while the recruitment of histone deacetylase 1 correlates with low HBV replication[74].In the presence of histone deacetylase inhibitors(valproic acid or trichostatin A),high HBV transcript levels and increased HBV replication are correlated with an increase in acetylated histones bound to cccDNA[74].IFN-α can inhibit cccDNA-based RNA transcription by inducing the hypoacetylation of cccDNA-bound histones.This mechanism could be implicated not only in OBI but also in an active epigenetic long-term control of cccDNA activity after IFN-α therapy[75,76].
Along with histones,HBx protein can be recruited to cccDNA,and an HBx mutant has been shown to induce rapid hypoacetylation of histones,thus reducing HBV pregenomic RNA and HBV regulation[77,78].
Our windows looked into the tan-yard, which was divided into two parts by a partition of planks5; in one half were many skins and hides, raw and tanned
Besides acetylation,methylation is another epigenetic mechanism considered to be involved in OBI occurrence.Cytosine-guanine dinucleotide(CpG)methylation in a gene promoter region rich in CpGs(CpG island)acts like a switch,silencing the gene[79].It was already demonstrated a long time ago that HBV DNA integrated into the host genome is methylated,leading to the loss of HBV core protein in PLC/PRF/5.Methylation of HBV DNA is an epigenetic mechanism that modifies HBV proteins,interferes HBV replication,and impairs HBV virion production,possibly leading to occult HBV infection[80].Methylation of CpG island 2 in the HBV genome is frequently detected in occult HBV infection[81].Hypermethylated HBV DNA sequences are often found in HCC patients with occult HBV infection[82].
With regard to antibodies,one study found that positive anti-HBs(≥ 10 mIU/mL)were more frequent in HBsAgNx[ARCHITECT HBsAg NEXT(sensitivity 0.005 IU/mL)]- negative than in HBsAgNx-positive nucleic acid testing yield samples(
= 0.0014),while there was no significant difference for the HBsAgNx-negative
HBsAgNx-positive OBI samples(
= 0.0748)[42].HBsAgNx is a“supersensitive”assay and its use in this study has been shown to improve HBsAg detection with 22.6% as compared to standard tests[42].The masking of HBsAg by anti-HBs has been proposed as one reason for the lack of detection of OBI even if anti-HBs is undetectable[67].Data from the first study[42]suggest that anti-HBs levels over 300 mIU/mL may affect the detection of samples with extremely low viral loads(median viral load: 4.42 IU/mL)and that the detection of such samples would require at least a 20000-fold excess of HBsAg to reach the detection limit of the HBsAgNx assay.Whether anti-HBs might be a consequence of vaccination in these cases or produced as a normal response to the immunogenic stimulus could be the subject of another discussion.
The above depicted mechanisms that may lead to OBI are summarized in Figure 2.
It was on a farm in the United States later that I enjoyed for the first time a picnic on the grass in the setting sun and then a concert in the moonlight
CONCLUSlON
OBI remains one of the most challenging problems in the hepatology field.It is a public health problem and a subject that needs further research in the future.OBI is currently diagnosed using PCR and realtime PCR assays.However,all efforts should be made to exclude false negative HBsAg,and new standardized methods must be developed to correctly identify OBI.Some of the studies mentioned above have found different markers(especially in the miRNA field)that could be used in the future for this purpose.Facts regarding OBI have become clearer in recent years;the factors that determine this outcome are now better understood,with host factors(immune or epigenetic)being identified as seemingly the main contributors.Viral factors are important but account for only a minority of OBIs.Some external factors can contribute to the appearance of OBI by interfering either with the host immune system or with the lifecycle of HBV.Of these,HIV and HCV co-infections are notable.Coinfection with Schistosoma mansoni was also found to inhibit HBV replication.Future research in this domain and increased awareness regarding this topic must be encouraged,as this particular form of evolution of HBV infection is still far from being completely understood and controlled.
His mother then said, You know, what we have here is a failed experiment in how to effectively carry a big milk bottle with two tiny hands. Let s go out in the back yard and fill the bottle with water and see if you can discover a way to carry it without dropping it. The little boy learned7 that if he grasped the bottle at the top near the lip with both hands, he could carry it without dropping it. What a wonderful lesson!
Presently the old witch sat down to supper and Vasilissa brought all she had cooked, enough for five grown men, and set it before her, and brought beer and honey, and then she herself stood silently waiting. The Baba Yaga ate and drank it all, every morsel, leaving not so much as a crumb38 of bread; then she said snappishly: Well, why dost thou say nothing, but stand there as if thou wast dumb?
FOOTNOTES
Gherlan GS reviewed the literature,wrote the paper and elaborated the figures.
Gherlan GS has no conflict of interest to declare.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial(CC BYNC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Romania
George Sebastian Gherlan 0000-0003-3994-5020.
Notwithstanding, the youth felt his spirits return to him when he entered the lower regions of the castle, for in the kitchen the most tempting5 and delicious food was spread out, the cellars were full of the most costly6 wine, and the store-room crammed7 with pots of every sort of jam you can imagine
Gong ZM
A
Gong ZM
1 Tabor E,Hoofnagle JH,Smallwood LA,Drucker JA,Pineda-Tamondong GC,Ni LY,Greenwalt TJ,Barker LF,Gerety RJ.Studies of donors who transmit posttransfusion hepatitis.
1979;19: 725-731[PMID: 230620 DOI: 10.1046/j.1537-2995.1979.19680104098.x]
2 Niederhauser C,Mansouri Taleghani B,Graziani M,Stolz M,Tinguely C,Schneider P.Blood donor screening: how to decrease the risk of transfusion-transmitted hepatitis B virus?
2008;138: 134-141[PMID: 18330733]
3 Makvandi M.Update on occult hepatitis B virus infection.
2016;22: 8720-8734[PMID: 27818588 DOI: 10.3748/wjg.v22.i39.8720]
4 Mücke MM,Backus LI,Mücke VT,Coppola N,Preda CM,Yeh ML,Tang LSY,Belperio PS,Wilson EM,Yu ML,Zeuzem S,Herrmann E,Vermehren J.Hepatitis B virus reactivation during direct-acting antiviral therapy for hepatitis C: a systematic review and meta-analysis.
2018;3: 172-180[PMID: 29371017 DOI: 10.1016/S2468-1253(18)30002-5]
5 Puoti M,Torti C,Bruno R,Filice G,Carosi G.Natural history of chronic hepatitis B in co-infected patients.
2006;44: S65-S70[PMID: 16338021 DOI: 10.1016/j.jhep.2005.11.015]
6 Raimondo G,Allain JP,Brunetto MR,Buendia MA,Chen DS,Colombo M,Craxì A,Donato F,Ferrari C,Gaeta GB,Gerlich WH,Levrero M,Locarnini S,Michalak T,Mondelli MU,Pawlotsky JM,Pollicino T,Prati D,Puoti M,Samuel D,Shouval D,Smedile A,Squadrito G,Trépo C,Villa E,Will H,Zanetti AR,Zoulim F.Statements from the Taormina expert meeting on occult hepatitis B virus infection.
2008;49: 652-657[PMID: 18715666 DOI: 10.1016/j.jhep.2008.07.014]
7 Raimondo G,Locarnini S,Pollicino T,Levrero M,Zoulim F,Lok AS;Taormina Workshop on Occult HBV Infection Faculty Members.Update of the statements on biology and clinical impact of occult hepatitis B virus infection.
2019;71: 397-408[PMID: 31004683 DOI: 10.1016/j.jhep.2019.03.034]
8 Sarin SK,Kumar M,Lau GK,Abbas Z,Chan HL,Chen CJ,Chen DS,Chen HL,Chen PJ,Chien RN,Dokmeci AK,Gane E,Hou JL,Jafri W,Jia J,Kim JH,Lai CL,Lee HC,Lim SG,Liu CJ,Locarnini S,Al Mahtab M,Mohamed R,Omata M,Park J,Piratvisuth T,Sharma BC,Sollano J,Wang FS,Wei L,Yuen MF,Zheng SS,Kao JH.Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update.
2016;10: 1-98[PMID: 26563120 DOI: 10.1007/s12072-015-9675-4]
9 European Association for the Study of the Liver.EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection.
2017;67: 370-398[PMID: 28427875 DOI: 10.1016/j.jhep.2017.03.021]
10 Terrault NA,Lok ASF,McMahon BJ,Chang KM,Hwang JP,Jonas MM,Brown RS Jr,Bzowej NH,Wong JB.Update on prevention,diagnosis,and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance.
2018;67: 1560-1599[PMID: 29405329 DOI: 10.1002/hep.29800]
11 Mulrooney-Cousins PM,Michalak TI.Asymptomatic Hepadnaviral Persistence and Its Consequences in the Woodchuck Model of Occult Hepatitis B Virus Infection.
2015;3: 211-219[PMID: 26623268 DOI: 10.14218/JCTH.2015.00020]
12 Wang J,Zhang P,Zeng J,Du P,Zheng X,Ye X,Zhu W,Fu Y,Candotti D,Allain JP,Li C,Li T.Occurrence of occult hepatitis B virus infection associated with envelope protein mutations according to anti-HBs carriage in blood donors.
2020;92: 38-45[PMID: 31877352 DOI: 10.1016/j.ijid.2019.12.026]
13 Wei L,Ploss A.Mechanism of Hepatitis B Virus cccDNA Formation.
2021;13[PMID: 34452329 DOI: 10.3390/v13081463]
14 Shi Y,Zheng M.Hepatitis B virus persistence and reactivation.
2020;370: m2200[PMID: 32873599 DOI: 10.1136/bmj.m2200]
15 Selzer L,Zlotnick A.Assembly and Release of Hepatitis B Virus.
2015;5[PMID: 26552701 DOI: 10.1101/cshperspect.a021394]
16 Yan H,Zhong G,Xu G,He W,Jing Z,Gao Z,Huang Y,Qi Y,Peng B,Wang H,Fu L,Song M,Chen P,Gao W,Ren B,Sun Y,Cai T,Feng X,Sui J,Li W.Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus.
2012;1: e00049[PMID: 23150796 DOI: 10.7554/eLife.00049]
17 Tuttleman JS,Pourcel C,Summers J.Formation of the pool of covalently closed circular viral DNA in hepadnavirusinfected cells.
1986;47: 451-460[PMID: 3768961 DOI: 10.1016/0092-8674(86)90602-1]
18 Nassal M.HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B.
2015;64: 1972-1984[PMID: 26048673 DOI: 10.1136/gutjnl-2015-309809]
19 Seeger C,Mason WS.Molecular biology of hepatitis B virus infection.
2015;479-480: 672-686[PMID: 25759099 DOI: 10.1016/j.virol.2015.02.031]
20 Shin EC,Sung PS,Park SH.Immune responses and immunopathology in acute and chronic viral hepatitis.
2016;16: 509-523[PMID: 27374637 DOI: 10.1038/nri.2016.69]
21 Dandri M,Bertoletti A,Lütgehetmann M.Innate immunity in hepatitis B and D virus infection: consequences for viral persistence,inflammation,and T cell recognition.
2021;43: 535-548[PMID: 34019142 DOI: 10.1007/s00281-021-00864-x]
22 Dunn C,Peppa D,Khanna P,Nebbia G,Jones M,Brendish N,Lascar RM,Brown D,Gilson RJ,Tedder RJ,Dusheiko GM,Jacobs M,Klenerman P,Maini MK.Temporal analysis of early immune responses in patients with acute hepatitis B virus infection.
2009;137: 1289-1300[PMID: 19591831 DOI: 10.1053/j.gastro.2009.06.054]
23 Wieland SF,Chisari FV.Stealth and cunning: hepatitis B and hepatitis C viruses.
2005;79: 9369-9380[PMID: 16014900 DOI: 10.1128/JVI.79.15.9369-9380.2005]
24 Mutz P,Metz P,Lempp FA,Bender S,Qu B,Sch?neweis K,Seitz S,Tu T,Restuccia A,Frankish J,D?chert C,Schusser B,Koschny R,Polychronidis G,Schemmer P,Hoffmann K,Baumert TF,Binder M,Urban S,Bartenschlager R.HBV Bypasses the Innate Immune Response and Does Not Protect HCV From Antiviral Activity of Interferon.
2018;154: 1791-1804.e22[PMID: 29410097 DOI: 10.1053/j.gastro.2018.01.044]
25 Sun C,Fu B,Gao Y,Liao X,Sun R,Tian Z,Wei H.TGF-β1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence.
2012;8: e1002594[PMID: 22438812 DOI: 10.1371/journal.ppat.1002594]
26 Wu J,Zhang XJ,Shi KQ,Chen YP,Ren YF,Song YJ,Li G,Xue YF,Fang YX,Deng ZJ,Xu X,Gao J,Tang KF.Hepatitis B surface antigen inhibits MICA and MICB expression
induction of cellular miRNAs in hepatocellular carcinoma cells.
2014;35: 155-163[PMID: 23917076 DOI: 10.1093/carcin/bgt268]
27 Ju Y,Hou N,Meng J,Wang X,Zhang X,Zhao D,Liu Y,Zhu F,Zhang L,Sun W,Liang X,Gao L,Ma C.T cell immunoglobulin- and mucin-domain-containing molecule-3(Tim-3)mediates natural killer cell suppression in chronic hepatitis B.
2010;52: 322-329[PMID: 20133006 DOI: 10.1016/j.jhep.2009.12.005]
28 Khanam A,Chua JV,Kottilil S.Immunopathology of Chronic Hepatitis B Infection: Role of Innate and Adaptive Immune Response in Disease Progression.
2021;22[PMID: 34071064 DOI: 10.3390/ijms22115497]
29 Bertoletti A,Kennedy PT.The immune tolerant phase of chronic HBV infection: new perspectives on an old concept.
2015;12: 258-263[PMID: 25176526 DOI: 10.1038/cmi.2014.79]
30 Gol-Ara M,Jadidi-Niaragh F,Sadria R,Azizi G,Mirshafiey A.The role of different subsets of regulatory T cells in immunopathogenesis of rheumatoid arthritis.
2012;2012: 805875[PMID: 23133752 DOI: 10.1155/2012/805875]
31 Stross L,Günther J,Gasteiger G,Asen T,Graf S,Aichler M,Esposito I,Busch DH,Knolle P,Sparwasser T,Protzer U.Foxp3+regulatory T cells protect the liver from immune damage and compromise virus control during acute experimental hepatitis B virus infection in mice.
2012;56: 873-883[PMID: 22487943 DOI: 10.1002/hep.25765]
32 Wang X,Dong Q,Li Q,Li Y,Zhao D,Sun J,Fu J,Meng F,Lin H,Luan J,Liu B,Wang M,Wang FS,He F,Tang L.Dysregulated Response of Follicular Helper T Cells to Hepatitis B Surface Antigen Promotes HBV Persistence in Mice and Associates With Outcomes of Patients.
2018;154: 2222-2236[PMID: 29544722 DOI: 10.1053/j.gastro.2018.03.021]
33 Thimme R,Wieland S,Steiger C,Ghrayeb J,Reimann KA,Purcell RH,Chisari FV.CD8(+)T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection.
2003;77: 68-76[PMID: 12477811 DOI: 10.1128/jvi.77.1.68-76.2003]
34 Bertoletti A,Ferrari C,Fiaccadori F,Penna A,Margolskee R,Schlicht HJ,Fowler P,Guilhot S,Chisari FV.HLA class Irestricted human cytotoxic T cells recognize endogenously synthesized hepatitis B virus nucleocapsid antigen.
1991;88: 10445-10449[PMID: 1660137 DOI: 10.1073/pnas.88.23.10445]
35 Vazquez MI,Catalan-Dibene J,Zlotnik A.B cells responses and cytokine production are regulated by their immune microenvironment.
2015;74: 318-326[PMID: 25742773 DOI: 10.1016/j.cyto.2015.02.007]
36 Alatrakchi N.Bregs in Chronic HBV: Is It Time for Bragging Rights?
2015;60: 1115-1117[PMID: 25686744 DOI: 10.1007/s10620-015-3584-1]
37 Sadeghpour S,Khodaee S,Rahnama M,Rahimi H,Ebrahimi D.Human APOBEC3 Variations and Viral Infection.
2021;13[PMID: 34372572 DOI: 10.3390/v13071366]
38 Komatsu H,Inui A,Umetsu S,Tsunoda T,Sogo T,Konishi Y,Fujisawa T.Evaluation of the G145R Mutant of the Hepatitis B Virus as a Minor Strain in Mother-to-Child Transmission.
2016;11: e0165674[PMID: 27812178 DOI: 10.1371/journal.pone.0165674]
39 Samal J,Kandpal M,Vivekanandan P.Molecular mechanisms underlying occult hepatitis B virus infection.
2012;25: 142-163[PMID: 22232374 DOI: 10.1128/CMR.00018-11]
40 Raimondo G,Pollicino T.Occult HBV Infection.In: Liaw YF,Zoulim F.(eds)Hepatitis B Virus in Human Diseases.Molecular and Translational Medicine.Humana Press,Cham.2016: 277-301
41 McClary H,Koch R,Chisari FV,Guidotti LG.Inhibition of hepatitis B virus replication during schistosoma mansoni infection in transgenic mice.
2000;192: 289-294[PMID: 10899915 DOI: 10.1084/jem.192.2.289]
42 Kuhns MC,Holzmayer V,Anderson M,McNamara AL,Sauleda S,Mbanya D,Duong PT,Dung NTT,Cloherty GA.Molecular and Serological Characterization of Hepatitis B Virus(HBV)-Positive Samples with Very Low or Undetectable Levels of HBV Surface Antigen.
2021;13[PMID: 34696483 DOI: 10.3390/v13102053]
43 Elkady A,Iijima S,Aboulfotuh S,Mostafa Ali E,Sayed D,Abdel-Aziz NM,Ali AM,Murakami S,Isogawa M,Tanaka Y.Characteristics of escape mutations from occult hepatitis B virus infected patients with hematological malignancies in South Egypt.
2017;9: 477-486[PMID: 28396718 DOI: 10.4254/wjh.v9.i9.477]
44 Wang H,Liao F,Xie J,Gao W,Wang M,Huang J,Xu R,Liao Q,Shan Z,Zheng Y,Rong X,Li C,Fu Y.E2 Site Mutations in S Protein Strongly Affect Hepatitis B Surface Antigen Detection in the Occult Hepatitis B Virus.
2021;12: 664833[PMID: 34867835 DOI: 10.3389/fmicb.2021.664833]
45 Lazarevic I,Banko A,Miljanovic D,Cupic M.Immune-Escape Hepatitis B Virus Mutations Associated with Viral Reactivation upon Immunosuppression.
2019;11[PMID: 31450544 DOI: 10.3390/v11090778]
46 Zhu HL,Li X,Li J,Zhang ZH.Genetic variation of occult hepatitis B virus infection.
2016;22: 3531-3546[PMID: 27053845 DOI: 10.3748/wjg.v22.i13.3531]
47 Xu Z,Yen TS.Intracellular retention of surface protein by a hepatitis B virus mutant that releases virion particles.
1996;70: 133-140[PMID: 8523517 DOI: 10.1128/JVI.70.1.133-140.1996]
48 Chen J,Liu B,Tang X,Zheng X,Lu J,Zhang L,Wang W,Candotti D,Fu Y,Allain JP,Li C,Li L,Li T.Role of core protein mutations in the development of occult HBV infection.
2021;74: 1303-1314[PMID: 33453326 DOI: 10.1016/j.jhep.2020.12.023]
49 Quarleri J.Core promoter: a critical region where the hepatitis B virus makes decisions.
2014;20: 425-435[PMID: 24574711 DOI: 10.3748/wjg.v20.i2.425]
50 El Chaar M,Candotti D,Crowther RA,Allain JP.Impact of hepatitis B virus surface protein mutations on the diagnosis of occult hepatitis B virus infection.
2010;52: 1600-1610[PMID: 20815025 DOI: 10.1002/hep.23886]
51 Pollicino T,Cacciola I,Saffioti F,Raimondo G.Hepatitis B virus PreS/S gene variants: pathobiology and clinical implications.
2014;61: 408-417[PMID: 24801416 DOI: 10.1016/j.jhep.2014.04.041]
52 Ghany MG,Ayola B,Villamil FG,Gish RG,Rojter S,Vierling JM,Lok AS.Hepatitis B virus S mutants in liver transplant recipients who were reinfected despite hepatitis B immune globulin prophylaxis.
1998;27: 213-222[PMID: 9425940 DOI: 10.1002/hep.510270133]
53 Inuzuka T,Ueda Y,Morimura H,Fujii Y,Umeda M,Kou T,Osaki Y,Uemoto S,Chiba T,Marusawa H.Reactivation from occult HBV carrier status is characterized by low genetic heterogeneity with the wild-type or G1896A variant prevalence.
2014;61: 492-501[PMID: 24798622 DOI: 10.1016/j.jhep.2014.04.033]
54 Li YI,van de Geijn B,Raj A,Knowles DA,Petti AA,Golan D,Gilad Y,Pritchard JK.RNA splicing is a primary link between genetic variation and disease.
2016;352: 600-604[PMID: 27126046 DOI: 10.1126/science.aad9417]
55 Kelemen O,Convertini P,Zhang Z,Wen Y,Shen M,Falaleeva M,Stamm S.Function of alternative splicing.
2013;514: 1-30[PMID: 22909801 DOI: 10.1016/j.gene.2012.07.083]
56 Hass M,Hannoun C,Kalinina T,Sommer G,Manegold C,Günther S.Functional analysis of hepatitis B virus reactivating in hepatitis B surface antigen-negative individuals.
2005;42: 93-103[PMID: 15962285 DOI: 10.1002/hep.20748]
57 van Hemert FJ,Zaaijer HL,Berkhout B,Lukashov VV.Occult hepatitis B infection:An evolutionary scenario.
2008;5: 146[PMID: 19077239 DOI: 10.1186/1743-422X-5-146]
58 Rehermann B,Ferrari C,Pasquinelli C,Chisari FV.The hepatitis B virus persists for decades after patients' recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response.
1996;2: 1104-1108[PMID: 8837608 DOI: 10.1038/nm1096-1104]
59 Bes M,Vargas V,Piron M,Casamitjana N,Esteban JI,Vilanova N,Pinacho A,Quer J,Puig L,Guardia J,Sauleda S.T cell responses and viral variability in blood donation candidates with occult hepatitis B infection.
2012;56: 765-774[PMID: 22173156 DOI: 10.1016/j.jhep.2011.11.011]
60 Zerbini A,Pilli M,Boni C,Fisicaro P,Penna A,Di Vincenzo P,Giuberti T,Orlandini A,Raffa G,Pollicino T,Raimondo G,Ferrari C,Missale G.The characteristics of the cell-mediated immune response identify different profiles of occult hepatitis B virus infection.
2008;134: 1470-1481[PMID: 18355815 DOI: 10.1053/j.gastro.2008.02.017]
61 Martin CM,Welge JA,Shire NJ,Shata MT,Sherman KE,Blackard JT.Cytokine expression during chronic versus occult hepatitis B virus infection in HIV co-infected individuals.
2009;47: 194-198[PMID: 19625194 DOI: 10.1016/j.cyto.2009.06.005]
62 Hassanshahi G,Arababadi MK,Khoramdelazad H,Yaghini N,Zarandi ER.Assessment of CXCL12(SDF-1α)polymorphisms and its serum level in posttransfusion occult HBV-infected patients in Southeastern Iran.
2010;41: 338-342[PMID: 20851290 DOI: 10.1016/j.arcmed.2010.07.001]
63 Ribeiro CRA,de Almeida NAA,Martinelli KG,Pires MA,Mello CEB,Barros JJ,de Paula VS.Cytokine profile during occult hepatitis B virus infection in chronic hepatitis C patients.
2021;18: 15[PMID: 33435966 DOI: 10.1186/s12985-021-01487-2]
64 Kong J,Grando SA,Li YC.Regulation of IL-1 family cytokines IL-1alpha,IL-1 receptor antagonist,and IL-18 by 1,25-dihydroxyvitamin D3 in primary keratinocytes.
2006;176: 3780-3787[PMID: 16517748 DOI: 10.4049/jimmunol.176.6.3780]
65 Huang YW,Liao YT,Chen W,Chen CL,Hu JT,Liu CJ,Lai MY,Chen PJ,Chen DS,Yang SS,Kao JH.Vitamin D receptor gene polymorphisms and distinct clinical phenotypes of hepatitis B carriers in Taiwan.
2010;11: 87-93[PMID: 19693091 DOI: 10.1038/gene.2009.65]
66 Arababadi MK,Pourfathollah AA,Jafarzadeh A,Hassanshahi G,Rezvani ME.Association of exon 9 but not intron 8 VDR polymorphisms with occult HBV infection in south-eastern Iranian patients.
2010;25: 90-93[PMID: 19793172 DOI: 10.1111/j.1440-1746.2009.05950.x]
67 Gerlich WH,Glebe D,Schüttler CG.Hepatitis B viral safety of blood donations: New gaps identified.
2018;3: 38[DOI: 10.21037/aob.2018.09.03]
68 Navaratnam N,Morrison JR,Bhattacharya S,Patel D,Funahashi T,Giannoni F,Teng BB,Davidson NO,Scott J.The p27 catalytic subunit of the apolipoprotein B mRNA editing enzyme is a cytidine deaminase.
1993;268: 20709-20712[PMID: 8407891]
69 Turelli P,Mangeat B,Jost S,Vianin S,Trono D.Inhibition of hepatitis B virus replication by APOBEC3G.
2004;303: 1829[PMID: 15031497 DOI: 10.1126/science.1092066]
70 Bonvin M,Greeve J.Hepatitis B: modern concepts in pathogenesis--APOBEC3 cytidine deaminases as effectors in innate immunity against the hepatitis B virus.
2008;21: 298-303[PMID: 18448976 DOI: 10.1097/QCO.0b013e3282fe1bb2]
71 Levrero M,Pollicino T,Petersen J,Belloni L,Raimondo G,Dandri M.Control of cccDNA function in hepatitis B virus infection.
2009;51: 581-592[PMID: 19616338 DOI: 10.1016/j.jhep.2009.05.022]
72 Zhang X,Hou J,Lu M.Regulation of hepatitis B virus replication by epigenetic mechanisms and microRNAs.
2013;4: 202[PMID: 24133502 DOI: 10.3389/fgene.2013.00202]
73 Bock CT,Schwinn S,Locarnini S,Fyfe J,Manns MP,Trautwein C,Zentgraf H.Structural organization of the hepatitis B virus minichromosome.
2001;307: 183-196[PMID: 11243813 DOI: 10.1006/jmbi.2000.4481]
74 Pollicino T,Belloni L,Raffa G,Pediconi N,Squadrito G,Raimondo G,Levrero M.Hepatitis B virus replication is regulated by the acetylation status of hepatitis B virus cccDNA-bound H3 and H4 histones.
2006;130: 823-837[PMID: 16530522 DOI: 10.1053/j.gastro.2006.01.001]
75 Belloni L,Allweiss L,Guerrieri F,Pediconi N,Volz T,Pollicino T,Petersen J,Raimondo G,Dandri M,Levrero M.IFN-α inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome.
2012;122: 529-537[PMID: 22251702 DOI: 10.1172/JCI58847]
76 Dandri M.Epigenetic modulation in chronic hepatitis B virus infection.
2020;42: 173-185[PMID: 32185454 DOI: 10.1007/s00281-020-00780-6]
77 Belloni L,Pollicino T,De Nicola F,Guerrieri F,Raffa G,Fanciulli M,Raimondo G,Levrero M.Nuclear HBx binds the HBV minichromosome and modifies the epigenetic regulation of cccDNA function.
2009;106: 19975-19979[PMID: 19906987 DOI: 10.1073/pnas.0908365106]
78 Gong Q,Chen S,Guo J,Sun H,Zheng G,Liu Q,Ren H,He S.Chromosome remodeling related to hepatitis B virus replication in HepG2 cells.
2011;30: 347-354[PMID: 21345131 DOI: 10.1089/dna.2010.1172]
79 Portela A,Esteller M.Epigenetic modifications and human disease.
2010;28: 1057-1068[PMID: 20944598 DOI: 10.1038/nbt.1685]
80 Miller RH,Robinson WS.Integrated hepatitis B virus DNA sequences specifying the major viral core polypeptide are methylated in PLC/PRF/5 cells.
1983;80: 2534-2538[PMID: 6302693 DOI: 10.1073/pnas.80.9.2534]
81 Vivekanandan P,Thomas D,Torbenson M.Hepatitis B viral DNA is methylated in liver tissues.
2008;15: 103-107[PMID: 18184192 DOI: 10.1111/j.1365-2893.2007.00905.x]
82 Kaur P,Paliwal A,Durantel D,Hainaut P,Scoazec JY,Zoulim F,Chemin I,Herceg Z.DNA methylation of hepatitis B virus(HBV)genome associated with the development of hepatocellular carcinoma and occult HBV infection.
2010;202: 700-704[PMID: 20653444 DOI: 10.1086/655398]
83 Zhang GL,Li YX,Zheng SQ,Liu M,Li X,Tang H.Suppression of hepatitis B virus replication by microRNA-199a-3p and microRNA-210.
2010;88: 169-175[PMID: 20728471 DOI: 10.1016/j.antiviral.2010.08.008]
84 Potenza N,Papa U,Mosca N,Zerbini F,Nobile V,Russo A.Human microRNA hsa-miR-125a-5p interferes with expression of hepatitis B virus surface antigen.
2011;39: 5157-5163[PMID: 21317190 DOI: 10.1093/nar/gkr067]
85 Hu W,Wang X,Ding X,Li Y,Zhang X,Xie P,Yang J,Wang S.MicroRNA-141 represses HBV replication by targeting PPARA.
2012;7: e34165[PMID: 22479552 DOI: 10.1371/journal.pone.0034165]
86 Hao QQ,Wang QH,Xia W,Qian HZ.Circulating miRNA expression profile and bioinformatics analysis in patients with occult hepatitis B virus infection.
2020;92: 191-200[PMID: 31513283 DOI: 10.1002/jmv.25594]
 World Journal of Clinical Cases2022年17期
World Journal of Clinical Cases2022年17期
- World Journal of Clinical Cases的其它文章
- Repetitive transcranial magnetic stimulation for post-traumatic stress disorder:Lights and shadows
- Response to dacomitinib in advanced non-small-cell lung cancer harboring the rare delE709_T710insD mutation:A case report
- Loss of human epidermal receptor-2 in human epidermal receptor-2+breast cancer after neoadjuvant treatment:A case report
- Tumor-like disorder of the brachial plexus region in a patient with hemophilia:A case report
- High-frame-rate contrast-enhanced ultrasound findings of liver metastasis of duodenal gastrointestinal stromal tumor:A case report and literature review
- Gitelman syndrome:A case report
