Antioxidant potential of pentoxifylline on spermatozoa of small ruminants
Mazen Alomar
Division of Animal Production, Department of Agriculture, Atomic Energy Commission, P. O. Box 6091, Damascus, Syria
ABSTRACT
Objective: To investigate antioxidant potential of pentoxifylline on spermatozoa of small ruminants including rams and bucks.
Methods: The levels of hydrogen peroxide (H2O2) production in ram and buck spermatozoa incubated with 0 (control), 4 and 8 mM of pentoxifylline were measured after 45-min incubation. Then,the motility parameters of ram and buck spermatozoa incubated with 0 (control), 1 mM of H2O2, 1 mM of H2O2 plus 4 mM of pentoxifylline, and 1 mM of H2O2 plus 8 mM of pentoxifylline were analysed. H2O2 was estimated using a fluorometric assay and spermatozoa motility characteristics were analyzed using computer aided sperm analyzer.
Results: Pentoxifylline significantly decreased the levels of H2O2 produced from both ram and buck spermatozoa (P<0.05), and significant lower rates of H2O2 formation were noted when 8 mM of pentoxifylline was added (P<0.05). The values of all sperm motility parameters for the two species significantly decreased after incubation with H2O2 (P<0.05). In contrast, when the spermatozoa were incubated with both H2O2 and two concentrations of pentoxifylline, the motility values rose significantly compared to those incubated with H2O2 alone (P<0.05). For both ram and buck sperm samples, the rapid and static subpopulation motility parameters were the most affected categories by pentoxifylline addition compared to the medium and slow categories.
Conclusions: Pentoxifylline possesses an antioxidant capacity proved by its ability of reducing H2O2 levels as well as by increasing motility values of stressed spermatozoa. Therefore, pentoxifylline could be recommended as antioxidant additive for spermatozoa of small ruminants under stress conditions.
KEYWORDS: Pentoxifylline; Spermatozoa; Ram; Buck;Motiltiy; Hydrogen peroxide; H2O2; Antioxidants
Significance
Pentoxifylline is one of the most popular compounds used to stimulate sperm motility of human and animal species. In our present study, pentoxifylline could reduce the formation rates of hydrogen peroxide from the spermatozoa of small ruminants including rams and bucks. This compound could be used as antioxidant additive for spermatozoa under stress.
1. Introduction
It is well known that there are a number of chemicals that can stimulate spermatozoa motility in vitro and in vivo. One of the most popular compounds used to enhance sperm motility in different semen suspensions for human, sheep, goat, canine and equine is pentoxifylline[1-5]. This substance is a xanthines derivative phosphodiesterase inhibitor and has positively affected spermatozoa function by a build-up of cyclic adenosine monophosphate (cAMP)through inhibition of cAMP phosphodiesterase enzyme[6]. Elevated cAMP levels increase the rate of glycolysis, which produces adenosine triphosphate used to express sperm motility[7]. It must be noted that the rise of cAMP can also cause an increase in the cAMP-dependent processes of spermatozoa such as capacitation and acrosome reaction events[8,9]. On the other hand, the application of pentoxifylline in clinical procedures had led to reduce lipid peroxidation associated sperm membrane damage and DNA apoptosis[10]. Ponce et al[11] reported that pentoxifylline would preserve membrane integrity of mouse sperm tail. Moreover,pentoxifylline was used as a cryoprotectant agent for human spermatozoa[12].
Like other aerobic cells, spermatozoa were able to produce reactive oxygen species (ROS) in metabolic pathways and they were highly susceptible to oxidative stress which may finally cause a significant loss in their physiological function[13]. Antioxidants neutralize oxidative stress and seminal plasma of animal species contain several groups of these substances in order to maintain the redox balance[14]. Anyhow, sperm defence system may not be fully defensible to prevent the damages of free radicals and other ROS members, especially when sperms are under stress conditions. For that, the addition of different antioxidants to semen media was very important to provide an appropriate protection to spermatozoa[15,16].In this regard, pentoxofylline may present one of the interesting antioxidant agents which could be used in the different semen media. The ability of pentoxifylline to scavenge hydroxyl radicals(HO.) has been previously demonstrated[17]. Furthermore, it has also been reported that pentoxifylline has inhibitory effect on the generation of superoxide anion (O2-.) in human leukocytes in vitro and in vivo[18]. However, regarding the literature, no reports had previously noted the effects of pentoxifylline on the formation of hydrogen peroxide (H2O2) from the spermatozoa of small ruminants.In fact, development of semen media for small ruminants' species including ram and buck is an important part for the different assisted reproductions technologies including artificial insemination and in vitro fertilization. Moreover, the addition of pentoxifylline to semen media seems very promising to develop the processes of spermatozoa preservation for small ruminants.
The aim of the present study was firstly to examine the antioxidant ability of pentoxifylline by analysing H2O2formation from both ram and buck spermatozoa incubated with and without pentoxifylline and secondly to show different motion characteristics of spermatozoa incubated with both H2O2and pentoxifylline.
2. Material and methods
2.1. Animals
The present study was carried out at the Division of Animal Production, Der Al-Hajar Research Centre, about 33 km South-East of Damascus, Syria. Five adult Awassi rams and five Shami bucks(two local Syrian small ruminant species) were used. The animals were aged between 2 and 3 years and weighing (91.3±8.8) kg for the rams and (70.7±7.1) kg for the bucks. Animals were fed a diet based on concentrate, wheat straw and barley while water was available ad libitum.
2.2. Semen collection
In this study, a total of 60 ejaculates were collected during the breeding season (in August, 30 ejaculates from the rams and 30 ejaculates from the bucks). Semen samples were collected with the aid of an electro-ejaculator (Minitube-Electro Ejaculator, Tiefenbach,Germany) administrating a series of cycles pulses of short electrical stimuli with each cycle delivering a slightly higher intensity (from 0 Volt to 20 Volt maximum) until semen obtention. To diminish the effect of individual variation between the bucks and the rams, a pool of ejaculates from five animals of each species was used in each assay. All ejaculations with no or poor motility status (with less than 60% of motile spermatozoa) were immediately excluded before conducting the analyses.
2.3. Chemicals and medium preparation
Pentoxifylline (C13H18N4O3) was obtained from Dr. Ehrenstorfer Gmbh (LGC laboratory-Augsburg-Germany) and all other chemicals were purchased from Roth (Carl Roth Gmbh-Karlsruhe-Germany).Tris based medium was prepared as a 300 mOsm/kg solution which contained the following: 2.44 g Tris (hydroxymethyl)aminomethane, 1.36 g citric acid monohydrate and 1.00 g glucose in 100 mL of distilled water and held constant at pH 7.
2.4. Experimental design
Four experiments were conducted in the present study. In the first and second experiments using the mix of fresh semen samples collected from the rams and the bucks, the dynamic of H2O2generation from both rams and bucks spermatozoa, incubated in Tris medium with 0 (control), 4 and 8 mM pentoxifylline, respectively,during 45 min was defined by fluorometric assay. After conducting several preliminary experiments, we fund that the range between 4 to 8 mM was the most affected concentrations on the rams and bucks spermatozoa. Each experiment was repeated for three times.
In the third and fourth experiments, fresh semen from the same rams and the bucks (25×106spermatozoa/mL for each species) were incubated at 37 ℃ for 45 min in Tris medium containing 0 (control),1 mM H2O2, 1 mM H2O2with 4 pentoxifylline, and 1 mM H2O2with 8 mM pentoxifylline. Motility characteristics of the control and treated groups for the two species were assessed using computerassisted sperm analysis (CASA) system. Each experiment was repeated for three times.
2.5. Measurement of H2O2 formation
Amplex red (10-acetyl-3, 7-dihydroxyohenoxazine, Molecular Probes) was used to monitor H2O2production. In the presence of horseradish peroxidase, amplex red reacts with H2O2in a 1:1 stoichiometric reaction to produce resorufin, a highly fluorescent end product. A stock of amplex red was prepared in dimethyl sulfoxide (10 mM), while horseradish peroxidase was prepared in phosphate buffer (450 unit/mL, pH 7.5). Both stocks were stored at -20 ℃ until the assay. Twenty μL of the sperm suspensions containing 6×106without pentoxifylline or with 4 and 8 mM of pentoxifylline in Tris medium were added to 96-well plates (Nunc,Roskilde, Denmark). H2O2standards (50.00, 25.00, 12.50, 6.25 and 3.12 μM) were prepared extemporaneously in the same medium and 80 μL containing 40 μM amplex red and 1 unit of horseradish peroxidase/mL were added to each well. Sperm samples, standard solutions and blanks were assayed in duplicate. The generation of H2O2was measured using a fluorimeter (Fluoroskan-Ascent, Thermo Fisher Scientific, Vantaa, Finland; excitation wavelength: 530 nm;emission wavelength: 590 nm). Fluorescence was recorded at 15 min intervals for 45 min. The concentration of H2O2was determined from the standard curve for each measured time point and expressed in μM.
2.6. Motility analyses
The motility characteristics of the spermatozoa were assessed by CASA technique using Hamilton-Thorne motility analyzer(HTM version 12.3, Beverly, USA). Spermatozoa concentration of the two small ruminant species for the control and the treated groups were 25×106spermatozoa/mL. Five microliter aliquots of diluted semen were placed in the system slide and loaded into the analyzer. At least three fields were counted for each sample. The motility characteristics included in the analysis were: motility (%),curvilinear velocity (VCL, μm/s), average path velocity (VAP, μm/s),straight line velocity (VSL, μm/s), and progressive motility (%; VAP≥75 μm/s and straightness STR≥ 80%).
Spermatozoa subpopulations were defined in four categories by CASA system: rapid (4): fraction of all cells moving with VAP> path velocity (VAP=25 μm/s); medium (3): fraction of all cells moving with VAP cutoff (5 μm/s) < VAP < path velocity (VAP=25 μm/s); slow (2): fraction of all cells moving with VAP < VAP cutoff (5 μm/s) or VSL < VSL cutoff (11 μm/s ); static (0-1): fraction of all cells that is not moving at all.
The Hamilton-Thorne motility analyzer settings used for buck spermatozoa were: negative phase contrast optics at a recording rate of 60 frame/s, temperature of analysis 37 ℃, light adjustment 90-110, minimum cell size 5 pixels, non motile head size 10 pixels,non motile head intensity 80, low VAP cut off 20 μm/s, low VSL cutoff 5 μm/s, static size limit 0.60/4.32 (min/max), and static intensity limit 0.20/1.92 (min/max). While the Hamilton-Thorne motility analyzer settings used for ram spermatozoa were: negative phase contrast optics at a recording rate of 60 frame/s, temperature of analysis 37 ℃, light adjustment 90-110, minimum cell size 5 pixels, non motile head size 10 pixels, non motile head intensity 80,low VAP cutoff 21.9 μm/s, low VSL cut off 6.0 μm/s, static size limit 0.60/8.00 (min/max), and static intensity limit 0.25/1.50 (min/max).
2.7. Statistical analysis
Statistical analysis was conducted with the Minitab program(Minitab, Coventry, United Kingdom). The normality of values distribution was first tested with the Shapiro-Wilk test. Data were subjected to a factorial analysis of variance, general linear model procedure followed by multiple pairwise comparisons using a post-hoc (Tukey test). Data were expressed as mean and standard deviation. A P-value less than 0.05 was statistically significant.
2.8. Ethics statement
The study was approved by the Local Scientific and Ethical Committee of the Atomic Energy Commission of Syria, Damascus,Syria (permit number 36-Z/M 3-2020).
3. Results
3.1. Effects of pentoxifylline on H2O2 generation
Figure 1 and 2 show H2O2levels generated from the ram and buck spermatozoa incubated with 0 (control), 4 and 8 mM of pentoxifylline. The amounts of H2O2increased significantly with time for the control and also for the spermatozoa incubated with 4 and 8 mM pentoxifylline (all P<0.05). Compared to the control,pentoxifylline was able to significantly reduce the levels of H2O2formation at the three incubation points times (15, 30 and 45 min)of the first and second experiments (all P<0.05). Clear significant differences between the 4 and 8 mM concentrations were noted for the two species and these differences were more obvious at the 45 min end time point, whereas the 8 mM level caused the lower formation rate of H2O2for both ram and buck spermatozoa.
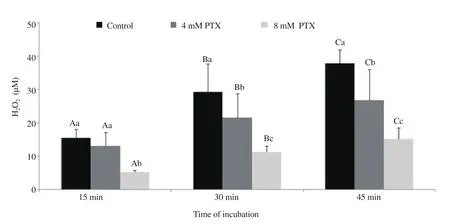
Figure 1. Generation of hydrogen peroxide (H2O2; mean±SD) by ram spermatozoa during 45 min of incubation in Tris medium with and without pentoxifylline (PTX). Different letters (A-C) within different time points for each treatment denote significant difference (P<0.05). Different letters (a-c)within different treatments for each time point denote significant difference (P<0.05).
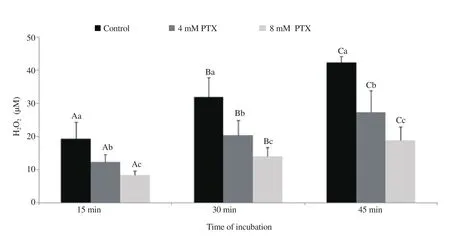
Figure 2. Generation of hydrogen peroxide(H2O2; mean±SD) by buck spermatozoa during 45 min of incubation in Tris medium with and without pentoxifylline (PTX). Different letters (A-C) within different time points for each treatment denote significant difference (P<0.05). Different letters (a-c)within different treatments for each time point denote significant difference (P<0.05).
3.2. Effects of pentoxifylline on motility characteristics
Table 1 and 2 show CASA motility values of ram and buck sperm samples incubated with 1 mM of H2O2, 1 mM of H2O2+ 4 mM pentoxifylline, and 1 mM of H2O2+ 8 mM pentoxifylline in Tris medium. One mM of H2O2was able to reduce all the values of motility characteristics for ram and buck spermatozoa (all P<0.05). After 45 min of H2O incubation with both 4 and 8 mM of pentoxifylline, sperm motility, progressive motility, VAP, VSL and
VCL values had significantly increased compared to the samples incubated with 1 mM H2O2alone (all P<0.05). However, all these values remained below the motility values of the control samples.
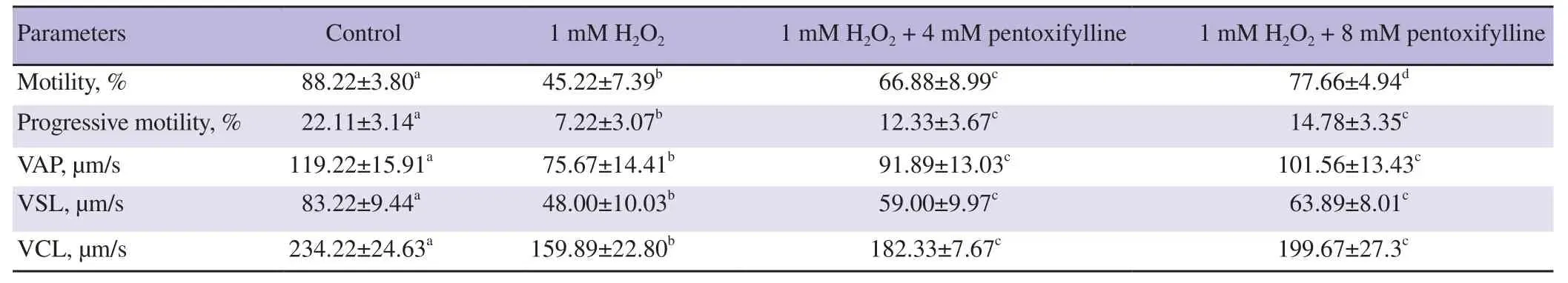
Table 1. Effects of hydrogen peroxide and pentoxifylline on the motility characteristics of spermatozoa samples of ram.
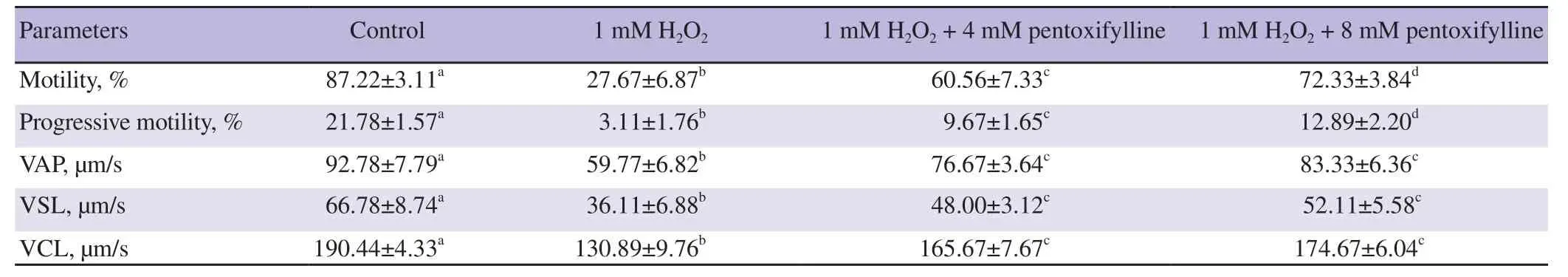
Table 2. Effects of hydrogen peroxide and pentoxifylline on the motility characteristics of spermatozoa samples of buck.
For both the rams and the bucks, no differences were noted for the values of VAP, VSL and VCL parameters between the samples treated with 1 mM H2O2+ 4 mM pentoxifylline and those treated with 1 mM of H2O2+ 8 mM pentoxifylline.
The motility categories distributions of ram and buck spermatozoa influenced by both H2O2and pentoxifylline are illustrated in Tables 3 and 4. In the case of ram samples treated by H2O2, 4 and 8 mM of pentoxifylline, the rapid, medium and static subpopulation categories had significantly differed from the control (all P<0.05).Compared to 1 mM H2O2level, pentoxifylline (4 and 8 mM) was able to significantly increase the rapid and medium categories and to decrease the static grade (Table 3). In the case of buck samples and after H2O2addition, the static category was significantly raised, while the rapid grade was significantly reduced compared to the control group (P<0.05). However, when pentoxifylline at 4 and 8 mM was added and compared to 1 mM H2O2level, the static category significantly decreased and the rapid category significantly rose(P<0.05) (Table 4).
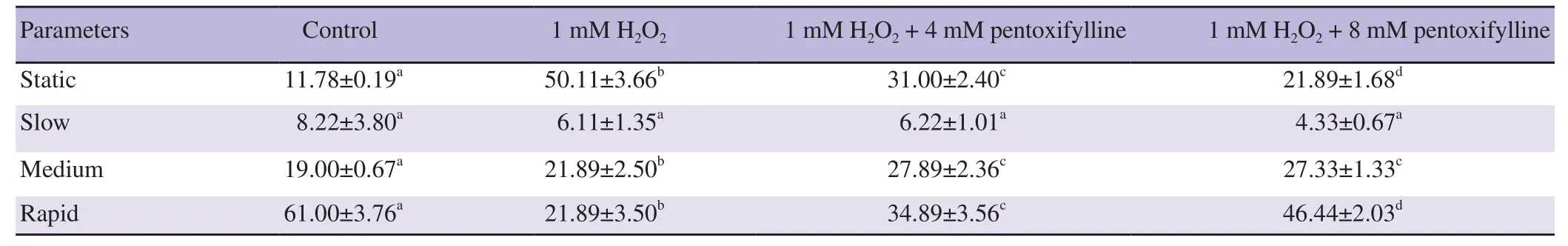
Table 3. Effects of hydrogen peroxide and pentoxifylline on the distribution percentage of motility subpopulations for ram sperm samples (%).
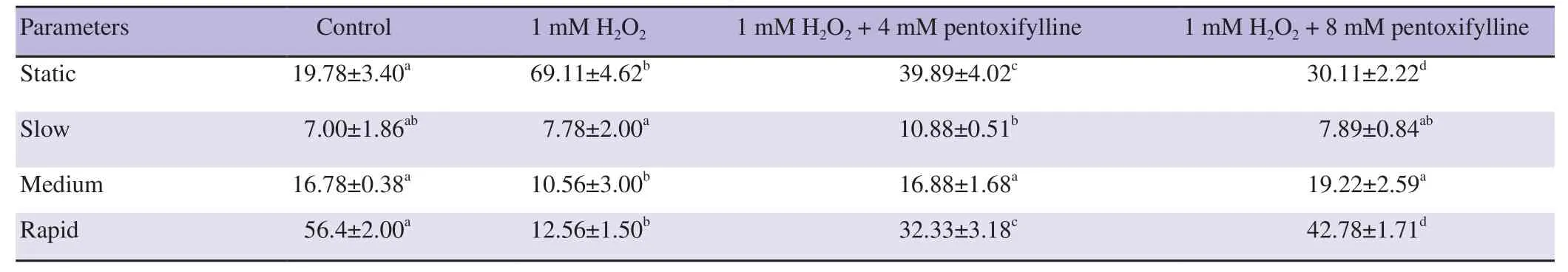
Table 4. Effects of hydrogen peroxide and pentoxifylline on the distribution percentage of motility subpopulations for buck sperm samples (%).
4. Discussion
To the best of our knowledge, this is the first study which shows the antioxidant potential of pentoxifylline by demonstrating its ability in reducing H2O2formation from the spermatozoa of small ruminants' species. Live and dead ram and buck spermatozoa were capable of generating H2O2and the formation levels of this agent increased when both the numbers of spermatozoa and the time of incubation increased[19-21]. For that, there was a persistent need to provide semen media with antioxidants capable of protecting small ruminants' spermatozoa from any eventual damages caused by H2O2.
Several reports showed the important roles of antioxidants such as catalase, glutathione, taurine, cysteine, vitamins E, C and B12in spermatozoa protection[13,19,22,23]. Based on our primary results presented here, it seems that pentoxifylline could be included and introduced to these groups of effective antioxidants. Moreover, by using two models of semen from two different small ruminants'species, our present results support the antioxidant capacity of pentoxifylline which could not be only related to any specific species.
The ability of pentoxifylline to decrease ROS formation is not a new topic at least for human spermatozoa. Indeed, Gavella et al[24] reported that pentoxifylline was effective in reducing O2production. In general, an antioxidant property was found when men semen was supplemented by pentoxifylline[18,25]. In this respect, the results of McKinney et al[25] indicated that pentoxifylline was able to significantly control lipid peroxidation. It must be stressed out that in our study we demonstrated the antioxidant capacity of pentoxifylline by showing its ability in neutralizing H2O2formed from the small ruminants' spermatozoa, while all the previous studies have proven such capacity by decreasing the level of O2-.or HO.formed from human sperms. In fact, H2O2is not a free radical as the two previous mentioned ROS members. Moreover, H2O2is highly important as it could easily penetrate biological membranes[26]. This ROS agent was responsible for the loss of motility that occurred in response to stress challenge in spermatozoa[27,28], and in high concentration it can induce lipid peroxidation[29]. Furthermore, when H2O2was added to ram spermatozoa, it was able to significantly reduce the level of motility[30].
Probably, the most important question in the present study is how pentoxifylline would be able to neutralize H2O2. Pentoxifylline seems to be triggered by activated neutrophils in response to the superoxide produced by nicotinamide adenine dinucleotide phosphate oxidase[31]. On the other hand, it is not known whether the specific mechanism of pentoxifylline could be related to any possible ability of enhancing antioxidant systems in the semen of small ruminants. Anyhow, supplementary studies are very essential to explain the precise antioxidant mechanism action of pentoxifylline.Pentoxifylline was able to raise motility values of sperm samples stressed by a high level of H2O2. These are important results to support our previous finding about the antioxidant ability of this compound. Pentoxifylline has been previously used to increase sperm motility of different animal species including bovine, canine,sheep and goat[3,4,32-34]. However, our present study demonstrated the ability of this substance to raise the level of spermatozoa motility of small ruminants' spermatozoa subjected to a great deal of stress.Although the spermatozoa of the two ruminants' species were treated with the same concentrations of pentoxifylline in the same experimental conditions, our results revealed that pentoxifylline was more effective in raising motility and progressive motility of the stressed samples in buck spermatozoa than in those of ram ones. The reason of these differences is not clear; however, the different ability of forming ROS from the spermatozoa of the two species as well as the differences on the antioxidant content in semen could partly explain the reason of such differences.
In the present study, we adopted two concentrations of pentoxifylline which were to some extent similar to those reported in other studies[25]. Addition of 3 and 6 mM pentoxifylline alone or with trehalose was able to enhance motility status and reduce the damage caused by cooling and cryopreservation processes of goat spermatozoa[35]. The effect of pentoxifylline on spermatozoa motility was related to the experimental conditions and the used concentration[36]. We previously observed a clear fluctuation of pentoxifylline effects on goat sperms motility and these effects were largely related to the used concentration and also the spermatozoa types whether they were fresh, chilled or frozen (data not shown).Anyhow, there has been clear disagreement regarding the effects of pentoxifylline in stimulating sperm velocity in vitro[37]. In humans, some investigations reported that pentoxifylline improves sperm motility[38], whereas others showed that the addition of this substance just stimulates a higher curvilinear velocity without affecting total motility[1]. In Boni and co-workers’ study[33],pentoxifylline did not improve total and progressive sperm motility;however, after two to three hours of incubation, VCL, VSL and VAP showed a significant increase. It is important to note that a balance must be achieved between antioxidant systems and the formation of ROS. Indeed, high concentrations of antioxidants can disrupt the functional integrity of the axosome and mitochondrial microtubules of spermatozoa associated with reducing of sperm motility by inhibiting ROS that is beneficial at physiological concentrations.Moreover, a high concentration of pentoxifylline caused a clear decrease in the quality of human spermatozoa[39] with detrimental effects on sperm membrane integrity[40]. Therefore, choosing the optimal concentration of pentoxifylline is very important in order to obtain the optimal result without compromising sperm motility,membrane integrity or fertility.
Another important finding generated from our present investigation was the clear effect of pentoxifylline on spermatozoa subpopulations.The presence of different motile sperm subpopulations based on individual motility characteristics in multiple mammals species using computer aided sperm analyser has been noted in several studies[41,42]. Our CASA system was able to identify four clear subpopulations categories based on the values of VAP parameter.The rapid grade in both ram and buck subpopulations was the most affected subpopulation by pentoxifylline treatment compared to the other three motility categories. In agreement with the present results, we previously noted higher proportions of goat spermatozoa with rapid pattern after the incubation with different antioxidants compared to control[16]. The slow and static subpopulations constituted by poorly and non motile spermatozoa could represent a late stage of sperms deterioration in any semen sample. Anyhow,the slow category was less affected by the pentoxifylline treatments,especially in the ram samples.
The current study is a preliminary study where we were able to prove the antioxidant activity of pentoxifylline in small ruminant's spermatozoa. But it is important to note that the antioxidant ability of pentoxifylline could be related to many influencing factors that cannot be only explained by the ability to reduce H2O2and the ability to improve the level of sperm motility under stress conditions.For that, there is a need for additional researches to explain this important property of this compound.
In conclusion, the outcomes of this study demonstrate the ability of pentoxifylline to reduce H2O2formation from the spermatozoa of small ruminants. Pentoxifylline is able to enhance the motility characteristics of stressed spermatozoa in both rams and bucks samples. The effects of pentoxifylline on the spermatozoa are clearly related to the used concentration. The use of pentoxifylline as an antioxidant could be recommended to support and to increase the level of motility of small ruminants' spermatozoa under stress conditions. This special effect of pentoxifylline may practically contribute to improving the results of in vitro and in vivo fertilization of the different assisted reproductive technologies.
Conflict of interest statement
The author declares that there are no conflicts of interest.
Funding
This study was funded by Atomic Energy Commission, Syria(Project: 36/ZM2 - 2021).
Author’s contributions
The conception and the design of the research, the revision of the intellectual content and the drafting of the paper were conducted by Mazen Alomar.
 Asian Pacific Journal of Reproduction2022年3期
Asian Pacific Journal of Reproduction2022年3期
- Asian Pacific Journal of Reproduction的其它文章
- Oxidative stress and female reproductive disorder: A review
- Investigation of FOXP3 (rs3761548) polymorphism with the risk of preeclampsia and recurrent spontaneous abortion: A systemic review and meta-analysis
- Sperm DNA fragmentation does not affect the clinical outcomes in the cumulative transfers of an ICSI cycle along with blastocyst transfers in couples with normozoospermic male patients
- Placental pathologies and fetal outcome in pregnant women with COVID-19: A retrospective study
- Effect of cholesterol-loaded cyclodextrin enriched extenders on the quality of prefrozen and frozen buffalo semen
