Oxidative stress and female reproductive disorder: A review
Kalaivani Manokaran, Pavithra Bhat, Deepak Nayak, Ravisankar Baskaran, Prabu Paramasivam, Shiek Fareeth Ahmed,Keerthi Priya, Karkala Sreedhara Ranganath Pai?, Vignesh Balaji E
1Department of Medical Laboratory Technology, Manipal College of Health Professions, Manipal Academy of Higher Education, Manipal-576104, India
2Department of Pathology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal-576104, India
3Department of Endocrinology, Dr ALM PGIBMS, Taramani, University of Madras, Chennai, India
4School of Medicine, Department of Neurology, University of New Mexico Health Sciences Center, University of New Mexico, Albuquerque, NM 87131, U.S.A.
5Faculty of Allied Health Science, Chettinad Hospital and Research Institute, Chennai, 603103, India
6Department of Pharmacology, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal-576104, India
ABSTRACT
Oxidative stress arises from an imbalance between the body’s prooxidants and antioxidants. Recently, oxidative stress has been proven a contributing factor to many female reproductive disorders including infertility, preeclampsia, endometriosis and polycystic ovarian syndrome. Herein, we review the mechanistic role of oxidative stress in inducing the most common female reproductive disorders. The current review has also highlighted the protective role of vitamin C, necessary for certain female reproductive hormone secretion by the antral follicle and corpus luteum and also essential for collagen production in ovarian tissue remodeling after ovulation, in alleviating oxidative stress and thus improving female reproductive outcomes.
KEYWORDS: Oxidative stress; Gynaecological disorders;Infertility; Endometriosis; Preeclampsia; Vitamin C
1. Introduction
Oxidative stress occurs when the proportion of pro-oxidant and antioxidant equilibrium is disrupted. The increased reactive oxygen species (ROS) and the insufficient production or availability of endogenous and exogenous antioxidants in the body lead to oxidative stress[1]. ROS are essential for carrying out various biological functions in the body. ROS in excess react with intracellular macromolecules leading to oxidative stress which is involved in the development of many disorders,including cardiovascular disorder, cancer, atherosclerosis, and diabetes[2]. ROS influence both male and female reproduction[3].
A considerable amount of ROS are essential for reproductive processes such as folliculogenesis, ovulation, steroidogenesis,fertilization, and implantation[4]. The higher levels of ROS initiate various infertility disorders such as preeclampsia, endometriosis,and polycystic ovarian syndrome (PCOS), and are often associated with miscarriage[4]. Oxidative stress mediates inflammation, genetic and epigenetic changes, endothelial dysfunction, and loss of cellular integrity of somatic and germ cells[5]. The oxidative stress disrupts various pathways and mechanisms, leading to the pathogenesis of various female reproductive disorders[1,6].
One of the possible ways to minimize ROS and its harmful effects is to enhance the natural antioxidant defense mechanism of the body. Antioxidants are the substances that scavenge the free oxygen and nitrogen radicals generated from metabolic processes and repair the tissue damage caused by harmful radicals[7,8]. Total antioxidants(enzymatic and exogenous antioxidants) present in the body fluids like peritoneal and follicular fluid play a principal role in stabilizing the effect of reactive radicals in reproductive health.Vitamin C, also termed ascorbic acid, provides antioxidant properties either through diet or supplementation[9]. The impact of vitamin C on the reproductive health of both men and women is considered important due to collagen synthesis[10], steroid hormone production, and antioxidant properties[11]. Few studies show the positive impact of the supplementation of vitamin C in treating reproductive disorders, which could be utilized as a therapeutic approach to treat gynaecological disorders. Our review aimed to investigate oxidative stress-induced common female reproductive disorders by which antioxidant therapy like vitamin C could help in treating these disorders.
2. ROS-induced female reproductive disorders
ROS are the oxygen free radicals formed as intermediates during metabolism[1]. ROS molecule initiate a cascade of reactions with the macromolecules like membrane lipids, nucleic acids of DNA,carbohydrates, proteins, and various other molecules within the cell[12-14]. These cascade reactions result in lipid peroxidation,DNA damage, loss of membrane integrity, interruption of protein biosynthesis, and exhaustion of adenosine triphosphate energy[15],thereby disrupting various mechanisms and pathways and eventually leading to cellular damage, which is termed as oxidative damage.
ROS are produced endogenously by various cellular compartments involved in aerobic respiration, such as the electron transport chain of mitochondria, endoplasmic reticulum, and nuclear membrane[16].Metabolic pathways such as xanthine oxidases, cytochrome P450,and nicotine amide dinucleotide phosphate oxidases, are other primary sources of ROS production[16]. Psychological stress[17],lifestyle factors such as drugs, alcohol, smoking, and sedentary lifestyles promote the higher production of ROS[18-20]. Superoxide anion, hydrogen peroxide, hydroxyl radical, peroxyl, and hydroperoxyl are biologically significant ROS that cause oxidative stress[1].
ROS have a significant role in controlling the expression of specific genes and proteins that regulate cellular biological functions such as proliferation, differentiation, and apoptosis[21,22]. ROS in the female reproductive system are involved in tissue remodeling, hormone signaling, and cyclic endometrial alterations during menstruation.In the ovary, ROS regulate the germ cell function, maturation of ova, follicle synthesis and maturation, ovulation, tubal function,and steroid hormone production by ovaries[23]. ROS also influence the disintegration of the corpus luteum, implantation, and normal parturition[23,24]. The concentration of ROS and antioxidants in the ovaries, follicular fluid, and peritoneal fluid determines the oocyte quality, fertilization of ova with the sperm, implantation, and the development of an embryo[6]. Certain studies conducted in in-vivo and in-vitro models have shown the involvement of the ROS in vascular endothelial growth factor signaling, which promotes the angiogenesis process[25-27]. Furthermore, it implicates the role of ROS in folliculogenesis and early-stage embryonic development.
The dominant follicle selection is governed by the amount of ROS and antioxidant content in the microenvironment of follicular fluid.The presence of a considerable amount of ROS in mature Graafian follicles potentially induces ovulation, and any alteration in this ROS level may affect ovulation[28,29]. ROS initiate apoptosis in growing follicles by the upregulation of catalase, glutathione, and estrogen synthesis, which inhibits the apoptosis in the mature Graafian follicle that undergoes the ovulation process[30]. The physiological role of ROS in female reproduction is well illustrated in Figure 1. A higher concentration of ROS is found in reproductive disorders that include preeclampsia, PCOS, endometriosis, embryonic resorption,infertility, poor pregnancy outcomes, and intrauterine growth restriction, and fetal mortality[31,32].
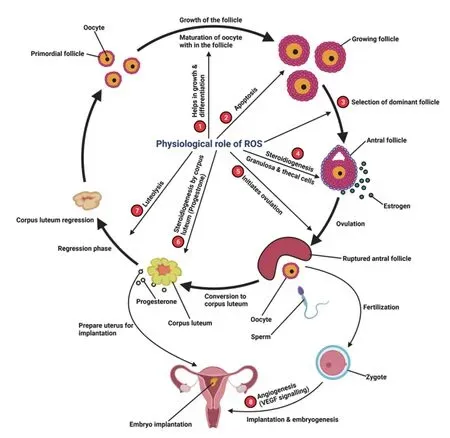
Figure 1. The physiological role of reactive oxygen species (ROS) in female reproduction. 1. Growth and differentiation of primordial follicle to growing follicle as well as growth of oocyte within the follicle; 2. Apoptosis of all follicle except the Graafian follicle; 3. Selection of dominant follicle that undergoes ovulation; 4. Ovarian steroidogenesis from the granulosa and theca cells within the antral follicle; 5. Initiates ovulation; 6. Progesterone synthesis from the corpus luteum; 7. Functional luteolysis of corpus luteum; 8. Vascular endothelial growth factor (VEGF) signaling initiated by ROS which is necessary in angiogenesis, a crucial step in embryogenesis and implantation.
3. Oxidative stress-induced female reproductive disorders
3.1. Infertility
Infertility is a clinical condition in which the couple cannot conceive despite constant sexual intercourse for more than a year[33].Various factors give rise to infertility, where male reproductive problems account for 30% of the cases[34], and female reproductive causes account for 40%-50%[35]. Excessive ROS generated in the male reproductive tract affect the sperm count per ejaculation and the motility of the sperm[20]. ROS also cause morphological defects in spermatozoa by inducing the oxidation of the fatty acid chain, thereby resulting in decreased functional spermatozoa in the semen[36].
In females, various oxidative stress mechanisms result in the pathogenesis of conditions like endometriosis[6], PCOS, luteal phase and tubal factor defects, and defective placentation[37], which are predisposing factors to infertility. ROS concentration and the antioxidants in the peritoneal cavity, follicular microenvironment,and ovary determine the quality and trait of the oocyte, zygote, and embryo[23,24,37-39]. Overaccumulation of pro-oxidants initiates the mitochondria-mediated caspase activation for the apoptosis in oogonial cells[40], which results in fertilization failure[41]. Even after successful fertilization, oxidative stress may cause embryopathy,defective implantation, miscarriage or pregnancy loss, and congenital disabilities in the neonates[4]. Gametes lose their membrane integrity due to lipid peroxidation leading to cellular enzyme inactivation and DNA damage, which induces apoptosis in gametes, ultimately resulting in infertility[42,38].
3.2. Endometriosis
Endometriosis is a reproductive disorder of fertile women characterized by the extra-uterine deposition and growth of endometrial tissues in the regions like the fallopian tube, ovaries,and peritoneal cavity, accompanied by severe abdominal and pelvic pain[43,39]. Some women may be asymptomatic with general complaints of irregular and painful menstruation and dyspareunia[44,45]. Endometriosis is caused by retrograde menstruation. The endometrial debris from the shedding of the lining during the menstruation is leaked out through the oviduct into the peritoneal region, initiating the pathogenesis of endometriosis[46,47].Consequently, endometrial tissue deposition, red blood cells from the bleeding lesions, and macrophages are embedded in the peritoneal region[32]. These factors are principal sources of ROS, which trigger the local inflammation in the peritoneal cavity and severe pelvic pain in the endometriosis patient.
Endometriosis is one of the contributing factors to the failure of conception[43] and results in complications such as miscarriage, fetal growth restriction, and fetal dysmorphogenesis[4]. Many studies have proven the significant changes in oxidative markers in patients diagnosed with endometriosis, implicating the role of oxidative stress in initiating the disorder[48,49]. Affected patients demonstrate an increased level of macrophages in the peritoneal fluid[50],indicating an increase in oxidative stress. Activated macrophageinduced oxidative stress inflammatory response plays a crucial role in advancing endometriosis[51]. The peritoneal fluid of endometriotic women showed elevated levels of iron, ferric, haemoglobin,and siderophage accumulation compared to healthy women[52].Additionally, the endometrial tissues implanted in the pelvic cavity are estrogen-dependent and respond to hormonal changes similar to the endometrium during the menstrual cycle stages. Some growth factors- and oxidative stress-mediated angiogenesis causes the proliferation of endometrial cells[53].
Studies showed elevated levels of malondialdehyde (MDA), ROS,and nitric oxide and lower total antioxidant activity in the follicular fluid of endometriotic patients than in the control group[54]. Overall,the presence of increased levels of oxidative biomarkers such as MDA, lipid hydroperoxides[55], and advanced oxidation protein products[56] in fluids like peritoneal fluid and follicular fluid is observed in endometriosis women[57]. Moreover, constant exposure of ovaries to inflammatory mediators in the pelvic and peritoneum in the endometriosis patient are likely to develop ovarian cancer[58].
3.3. Preeclampsia
Preeclampsia is a pregnancy disorder, which presents from the 2nd or 3rd trimester of the gestation period with the clinical manifestation of increased blood pressure and proteinuria (>300 mg/24 h)[16];preeclampsia is a 2-stage pathological process of oxidative stress initiated primarily by defective implantation of the placenta, which later advances to maternal vascular endothelial dysfunction. Both are major contributors to preeclampsia[59,60].
Oxidative stress is a major contributor to the pathogenesis of preeclampsia. The study conducted by Ford & his colleague in 2009 demonstrated an increased concentration of oxidative stress markers like MDA in the plasma of preeclamptic patients[61], suggesting the role of oxidative stress in the etiology of the preeclampsia. Impaired placentation into the uterine wall is caused by defective remodeling of uteroplacental arteries and major blood vessels that supply nutrients to the placenta and growing fetus. This faulty remodeling results in decreased arterial blood flow. It causes reperfusion injuries,which creates a hypoxic condition that brings about an exaggerated generation of oxygen free radicals leading to oxidative stress[62].
The placental oxidative stress accompanies maternal endothelial lining dysfunction of blood vessels. Excessive ROS and the activated neutrophils, tissue necrosis factor-alpha (TNF-α) released by macrophages/monocytes cause activation of NF-κB gene expression that regulates the inflammation and apoptosis in the placental cells.As a consequence of this damage, the cellular debris and placentaderived factors such as soluble FMS-like tyrosine kinase-1 (sFlt-1)and soluble endoglin are active anti-angiogenic factors[63,64].Procoagulants, prostaglandins, and inflammatory cytokines acting on the maternal vascular endothelial cells provoke the oxidative stress and etiology of the preeclampsia[16].
Patients diagnosed with preeclampsia show a decreased antioxidant activity[65] and lower level of enzyme antioxidants like superoxide dismutase (SOD), thioredoxin, and glutathione[66]. The preeclamptic patients were also observed with decreased concentrations of dietary antioxidants like vitamin C and vitamin E, indicating overall diminished total antioxidant status in the preeclamptic women compared to normal pregnant women[67]. In addition to this, there is also an excessive generation of ROS in preeclamptic women,especially superoxide anion due to the overexpression of nicotine amide dinucleotide phosphate oxidases in the trophoblast[68] by TNF-α, oxidized low-density lipoprotein[1] and autoantibodies of angiotensin 1 receptor[69]. Besides, the deposition of advanced oxidation protein products from the oxidative damage of proteins results in the production of sFlt-1, an anti-angiogenic substance,thereby leading to the development of the preeclampsia[70]. Pregnant women, who are at a high risk of developing preeclampsia showed an elevated level of superoxide anion and decreased amount of SOD and catalase activity, indicating the decreased total antioxidant capacity[71].
3.4. PCOS
PCOS is a low-grade inflammatory disorder affecting 5%-10% of the pre-menopausal women worldwide, accompanied by hormonal dysregulation resulting in female infertility issues[72]. Major clinical features of PCOS include hyperandrogenaemia[73], which leads to excessive facial hair growth, acne, anovulation[74], and multiple cyst formation in the ovary[1,2]. Many studies observed significant oxidative stress markers fluctuation in the pathophysiology of PCOS[75]. PCOS-related clinical features result from the excessively generated oxygen free radicals[73].
In hyperglycaemic PCOS women, the inflammatory transcription factor NF-κB gene is switched 'on' due to increased propagation of ROS by the leucocytes, especially by macrophages/monocytes,which release TNF-α and other cytokines that create a general inflammatory response[76]. Affected patients demonstrate an elevated concentration of TNF-α, interleukin-6, interleukine-8, and C-reactive protein and other inflammatory markers in the blood,suggesting oxidative stress-induced low-grade inflammation[77].As discussed earlier, abundant ROS cause protein peroxidation.Advanced oxidation protein products are a known pro-inflammatory factor in PCOS patients[78]. TNF-α is known to play a crucial role in establishing insulin resistance[79]. Oxidative stress causes activation of various protein kinases in the cells that lead to a defect in insulin receptor substrate, thereby impairing the normal utilization of glucose by cells[80]. Due to oxidative stress, the normal tyrosine phosphorylation of insulin receptor substrate is replaced by serine/threonine phosphorylation by activated protein kinases, resulting in functional impairment and causing degeneration of insulin receptor substrate[81]. Thereby, insulin receptor substrate will not interact with insulin receptors, subsequently causing insulin resistance[82].Insulin resistance is associated with compensatory hyperinsulinemia to decrease blood glucose levels[83]. Therefore, insulin resistance and hyperglycemia are two main features of PCOS that enhance oxidative stress[80,73].
Increased insulin acts synergistically with the luteinizing hormone receptor or through insulin-like growth factor-1 receptors by stimulating the theca cells of growing follicles, which in turn causes increased androgen synthesis which causes insulin receptor substrateutism in women[84-86]. Additionally, insulin also increases cytochrome P450 expression, a primary element of ROS production[87]. Oxidative stress-mediated inflammatory environment and insulin resistance cause ovarian tissue remodeling[88] and modification of ion channels present in the cell membrane disrupting the calcium ion homeostasis in the ovary. It thereby causes follicular arrest, a cause of anovulation, and amenorrhea, which are the contributing factors of infertility among PCOS women[89]. Above discussed, pathogenesis strongly suggests the intimate connection of oxidative stress with PCOS. Besides, many studies show an increased level of lipid peroxidation index[90,91], increased nitric oxide levels[92], and decreased level of total antioxidant capacity[93]among affected women compared to the normal group. Excessive free radicals react with DNA causing mutation and aberrant DNA cross-linkage. These DNA modifications lead to epigenetic changes and abnormal coding of tumor suppressor genes[94], increasing the risk for reproductive tract-related cancers in PCOS patients.
4. Antioxidants: A defensive mechanism against ROS/oxidative stress
Antioxidants act as a reactive radical scavenger opposing ROS production and promoting the repair of oxidative damage to cellular morphology[37]. The antioxidants are of two forms, enzymatic(endogenous) antioxidants like glutathione, SOD, glutathione peroxidase (GPx), etc., and non-enzymatic (supplementary/dietary)antioxidants like vitamin A, vitamin C, vitamin E[95] and other natural compounds like polyphenols[96]. Trace elements that are essentially supplied through the diet, such as copper (Cu), zinc (Zn),manganese (Mn), and selenium (Se), act as cofactors in regulating the function of enzymatic antioxidant systems such as MnSOD, Se-GPX, etc.[97]. Glutathione, an endogenous antioxidant, is involved in neutralizing the free radicals and ROS as well as maintaining the exogenous antioxidants (vitamin A, C, E) in their reduced form[31].These exogenous supplementations of dietary antioxidants are essential in female reproductive processes to maintain antioxidant balance and stabilize the deleterious effects of ROS[8].
5. Vitamin C as an antioxidant in female reproductive disorders
Vitamin C, also known by the terms 'ascorbic acid', 'L-ascorbate' or'ascorbate', is one of the essential nutrients to be provided through the diet[98]. The recommended daily allowance of vitamin C for adults ranges from 75 to 90 mg[99]. Leukocytes, especially the neutrophils and lymphocytes, are primary cellular transporters of L-ascorbate circulating all around the body[100]. The amount of vitamin C consumed through the diet, its absorption in the intestine,the amount that is excreted, health status, lifestyle, and age are some factors that determine the amount of circulating vitamin C in the plasma. It varies from 50 μM to 150 μM[101,102]. Vitamin C levels in the plasma are inversely proportional to age i.e.; there is a 20% decrease in plasma concentration with an increase in age[101].In addition to its antioxidant property, vitamin C has a therapeutic effect in treating cancer[103].
Vitamin C in reproductive health is primarily due to its three primary functions, collagen biosynthesis[103], its role in the synthesis of steroid & peptide hormones[104], and its antioxidant property[105].Vitamin C scavenges free radicals and protects from the deleterious effect of ROS and metal-oxygen complexes on the gametes during gametogenesis and fertilization[104-106].
Vitamin C has a biological role in the menstrual cycle and helps in various reproductive processes like follicle development, production of the hormones by the ovary, tissue remodeling, repair of the follicle after ovulation, and protection of the oocyte from oxidative damage.The ovary is considered a pool of L-ascorbate, and its turnover[107,108]forms evidence for the influence of vitamin C in reproduction. Large measures of vitamin C are present in theca interna, granulosa cells of the mature graafian follicle, and luteinizing granulosa cells[107], due to the high reducing potential of the ascorbate to protect the oocyte from damage caused by ROS[108]. Graafian follicle and corpus luteum are actively involved in synthesizing steroid hormones, i.e.,estrogen and progesterone. Hence, the accumulation of L-ascorbate in these two sites is helpful[109] since the hydroxylation requires ascorbate in the steroidogenesis pathway[110]. Few studies have found that collagen is necessary for developing ovarian follicles;it is also essential to repair the ruptured Graafian follicle after the ovulation and the luteinization of the ovulated follicle. Hence,the role of L-ascorbate in the synthesis of collagen in the ovary is observed[102,111].
Henmi et al show a positive impact of vitamin C administration in treating infertile women with luteal phase hormone secretion defects,showing an increased progesterone and estrogen hormone[112] and decreased incidence of preeclampsia in high-risk preeclamptic women administered with vitamin C[113]. On the other hand, there is no significant improvement in the oxidative stress markers when vitamin C is administered to the endometriotic patient, but there was a remarkable increase in vitamin C levels in follicular fluid and serum[114]. Another study showed there was no significant improvement in the incidence of preeclampsia in pregnant women subjected to vitamin C supplementation[115]. The impact of vitamin C on female reproduction is illustrated in Figure 2. Doubleblinded randomized control trials performed on 620 infertile patients undergoing in vitro fertilization (IVF)-embryo transfer with supplementation of vitamin C showed no significant improvement in the pregnancy and implantation outcomes[116]. A randomized placebo-controlled trial conducted on pregnant women with a high risk & low risk for preeclampsia with vitamin C & E supplementation is measured for ascorbic acid levels, 8-epi-prostaglandin F2α, leptin,and plasminogen activator inhibitor-1/2 and pregnancy outcomes.This study showed a decrease incidence of preclampsia with antioxidant vitamin supplementation[117]. A prospective study was conducted on 76 infertile women undergoing artificial reproductive techniques of which 38 are smokers and 38 non-smokers with &without vitamin C administration, and reported that ascorbic acid levels in follicles were significantly higher in women with vitamin C supplementation than in the control group, which enhanced the outcomes in assisted reproduction treatment with vitamin C administration, and that vitamin supplementation also had a greater impact on the number of pregnancies in the non-smokers' group than smokers group[118]. A randomized control trial study conducted on 120 women undergoing IVF for treatment of infertility showed vitamin C supplementation improved term pregnancy and decreased the incidence of spotting and miscarriages[119]. Vitamin C and E supplementation effectively reduced the oxidative stress indices like MDA, ROS and improved the dyspareunia, dysmenorrhea, and chronic pelvic pains in endometriosis women[120].
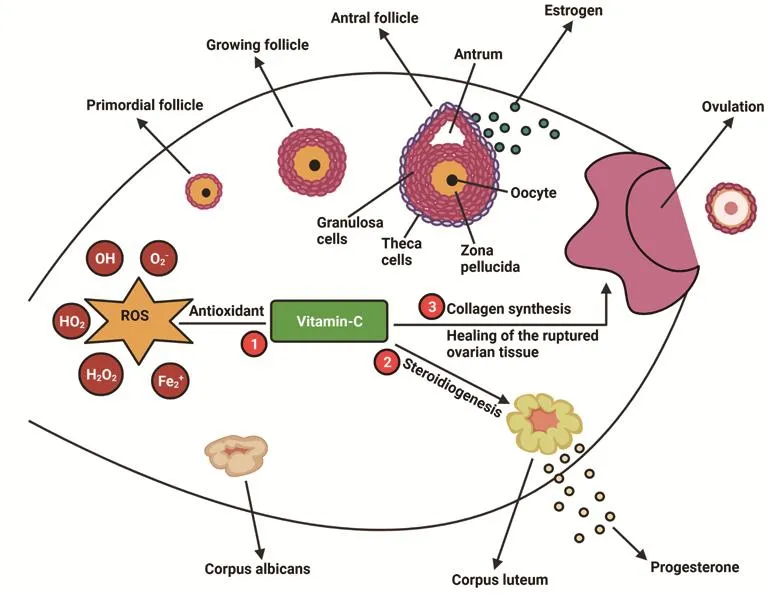
Figure 2. The impact of vitamin C on female reproduction. 1. Vitamin C antioxidant neutralizes the reactive oxygen species (ROS) in ovary and thus protects the follicle from oxidative damage. 2.Vitamin C is utilized by the granulosa cells and corpus luteum for steroidogenesis. 3. Vitamin C is involved in the modification of pro-collagen to collagen and hence heals ruptured ovary and helps in tissue remodeling.
6. Conclusions and future prospect
As discussed, there is a significant impact of oxidative stress on the reproductive life of and women. An overabundance of reactive oxygen molecules such as superoxide, hydrogen peroxide, and hydroxyl ions negatively impacts health by interrupting many natural physiological and biochemical mechanisms in the body. Furthermore,oxidative stress initiates reproductive disorders like endometriosis,preeclampsia, PCOS, and infertility. To minimize these deleterious effects, the body either must reduce the accumulation of ROS or enhance the antioxidant pool to counteract the effect of ROS.One can prevent ROS overproduction by following a healthy lifestyle devoid of drinking and smoking habits, the major external contributors to excessive ROS production. Healthy food habits and intake of antioxidants through diet increase the body's antioxidant pool and hence are favorable to eliminate the reactive molecules.
The role of oxidative stress in some disorders like PCOS and idiopathic infertility is still unclear, and future studies have to be encouraged in this area to enhance the current knowledge and provide an effective treatment option. Studies show a positive impact of supplementation of vitamin C antioxidants in single or in combination helps to reduce the complications of oxidative stressinduced gynaecological disorders. However, few studies report there is no significant improvement in gynaecological conditions after the supplementation of vitamin C. Future studies are important to focus on the therapeutic use and the mechanistic approach of antioxidants in treating or controlling oxidative stress-induced female reproductive disorders.
Conflict of interest statement
The authors declared no conflict of interest.
Funding
The study received no extramural funding.
Authors’ contributions
Kalaivani Manokaran and Pavithra Bhat conceived the study and conducted the literature search. Deepak Nayak, Ravisankar Baskaran, and Karkala Sreedhara Ranganath Pai reviewed the concept and design of the study. Kalaivani Manokaran and Pavithra Bhat drafted the manuscript. Prabu Paramasivam, Shiek Fareeth Ahmed, Keerthi Priya, and Karkala Sreedhara Ranganath Pai reviewed the manuscript. Vignesh Balaji E helped in figure drafts.All authors read and approved the final manuscript.
 Asian Pacific Journal of Reproduction2022年3期
Asian Pacific Journal of Reproduction2022年3期
- Asian Pacific Journal of Reproduction的其它文章
- Sj?gren’s syndrome and reproductive outcomes
- Effect of cholesterol-loaded cyclodextrin enriched extenders on the quality of prefrozen and frozen buffalo semen
- Antioxidant potential of pentoxifylline on spermatozoa of small ruminants
- Placental pathologies and fetal outcome in pregnant women with COVID-19: A retrospective study
- Sperm DNA fragmentation does not affect the clinical outcomes in the cumulative transfers of an ICSI cycle along with blastocyst transfers in couples with normozoospermic male patients
- Investigation of FOXP3 (rs3761548) polymorphism with the risk of preeclampsia and recurrent spontaneous abortion: A systemic review and meta-analysis
