Photocatalytic degradation of tetracycline hydrochloride with visible light-responsive bismuth tungstate/conjugated microporous polymer
Fenghongkang Pan,Yimeng Wang,Kaiqing Zhao,Jun Hu, ,Honglai Liu,Ying Hu
1 State Key Laboratory of Chemical Engineering and School of Chemistry &Molecular Engineering,East China University of Science and Technology,Shanghai 200237,China
2 Prinbury Biopharm Co.,Ltd,Shanghai 201203,China
Keywords:Photochemistry Degradation Visible light response Pollution Inorganic/organic heterojunction composite
ABSTRACT Conjugated microporous polymer(CMP)is an emerging organic semiconductor with π-conjugated skeletons,and the bandgap of CMP can be flexibly modulated to harvest visible light.Based on the diversity and adjustability of monomers in CMP,we designed and synthesized donor-accepter (D-A) type BTNCMP through Sonogashira-Hagihara cross-coupling polymerization,further in-situ constructing series of inorganic/organic Z-scheme BW/BTN-n composite in the presence of Bi2WO6.After optimization,the tetracycline hydrochloride (C0=10 mg·L-1) degradation efficiency reached 84% with BW/BTN-2 as catalyst in 90 min under visible light irradiation,the apparent rate constant k1 is 0.017 min-1,which is 1.7 and 5.7 times higher than bare Bi2WO6 and BTN-CMP.X-ray photoelectron spectra and UV–Vis diffuse spectra showed that the enhanced photocatalytic activity originated from the tight heterojunction between Bi2WO6 and BTN-CMP,which can extend the light absorption range and facilitate the separation and transport of photogenerated charges in the interface of heterojunction.The active species trapping experiments and electron spin resonance technique revealed that h+ was the dominant active species during the photodegradation process of tetracycline hydrochloride (TCH).The present study demonstrated the feasibility to construct inorganic/organic composite for the photocatalytic degradation of environmental pollutants.
1.Introduction
Tetracycline hydrochloride (TCH) have been widely used in aquaculture and livestock industries,most of which are poorly metabolized by organisms and excreted in the form of parent chemical or bioactive intermediates,entering environment through wastewater and manure,which endangered ecological environment and public health[1,2].Therefore,it is extremely crucial to remove or decompose TCH to non-toxic small molecules.
Photocatalysis as an efficient oxidation process with clean,nonpolluted and renewable solar as energy,can degrade organic contaminations under mild reaction conditions.Most reported semiconductors with photocatalytic activity are mainly metal oxides(TiO2[3,4],ZnO [5],ZrO2[6],Fe3O4[7],Co3O4[8]),carbon-based materials (rGO [9],MWCNTs [10],g-C3N4[11–13]) and metal–organic frameworks (NH2-UiO-66 [14],MIL-101 [15],ZIF-67 [16]).Recently,conjugated microporous polymers(CMPs),as an emerged organic semiconductor have become promising candidates for effective photodegradation process [17,18].
CMPs,consisting of earth-abundant elements such as C,N and S,can be easily designed and synthesized through flexible modulation of monomers and abundant π-conjugated skeletons,which also demonstrate unique optical and electronical properties in photocatalysis.CMPs have excellent visible light harvesting capacity and photocatalysis activities for photocatalytic H2evolution,water splitting,CO2reduction and degradation of environmental pollutants [19–21].However,the fast recombination of photogenerated electrons-holes caused by high exciton binding energy and low crystallinity hinders its further development.To address this issue,the rational design of Donor-Accepter type CMP with broad spectra and narrow bandgap has been proved an effective strategy to improve the photocatalytic performance [22].Wang et al.synthesized triazine-based CMPs linking the electron-withdrawing(benzothiadiazole)and electron-donating units(thiophene),which demonstrated the highest CO2photoreduction rate to CO(18.2 μmol·h-1) due to the incorporation of electronwithdrawing BT units into the triazine poly-network[23].Stronger electron-withdrawing acceptors in CMP skeleton can stabilize to improve the photocatalytic performance [22].Wanget al.[23] synthesized triazine-based CMPs linking the electronwithdrawing (benzothiadiazole) and electron-donating units(thiophene),which demonstrated the highest CO2photoreduction rate to CO (18.2 μmol·h-1) due to the incorporation of electronwithdrawing BT units into the triazine poly-network.Stronger electron-withdrawing acceptors in CMP skeleton can stabilize LUMO orbitals,lower LUMO energy levels,and reduce optical band gaps.For instance,benzothiadiazole monomers,with stronger electron-withdrawing ability and suitable energy levels,have been used in the preparation of photocatalyst.Vilela et al.synthesized series of surface tuned conjugated poly(benzothiadiazole) networks with BET surface area from 270 m2·g-1to 660·m2·g-1using SiO2NPs as template,CMP-60 with the biggest surface area 660 m2·g-1exhibited the best conversion efficiency (96%) of αterpinene into ascaridole [24].He et al.designed and synthesized two triazine-based CMPs P1 and P2 using a metal-free approach.D-A type P1,linking light absorbing pyrazole-benzothiadiazole-pyr azole and triazine units with an ideal bandgap of about 2.3 eV and demonstrated a higher H2evolution rate of 50 μmol·h-1under visible light irradiation compared with P2,which replaced electronwithdrawing benzothiadiazole by electron-neutral benzene [25].Chen et al.prepared the poly (benzothiadiazole)/TiO2(denoted as BBT/TiO2) composites through in-situ polymerization in the presence of TiO2,the H2evolution rate and ciprofloxacin degradation performance of 13.3% BBT/TiO2were 18.0 and 24.0 times higher than bare BBT due to the formed tight heterojunctions between BBT and TiO2,which dramatically enhanced visible-light induced photocatalytic activity [26].
Considering all above,it might be a promising and effective strategy to link well-designed CMPs with traditional semiconductors to construct II-type or Z-scheme inorganic/organic heterojunctions and to synergistically enhance the photodegradation performance.Knowing that Bi-based photocatalysts with small band gap (<3 eV) can be easily stimulated under visible light irradiation [27],we selected the simplest Aurivillius oxides Bi2WO6(BW) as inorganic moiety,of which the crystal structure is consisted of alternative sheetand octahedralto promote the delivery efficiency of electrons and holes in different directions[28,29]and a series of BW/BTN-n composites were synthesized in the presence of Bi2WO6in one step through in-situ Sonogashira-Hagihara cross-coupling polycondensation of 4,7-dibromobenzo[c] [1,2,5] thiadiazole (BTD) as acceptor with tri (4-ethynylphenyl)amine(tEPhA)as donor.The photocatalytic activity was performed by TCH photodegradation molecule under visible light illumination (>420 nm).The characterization results of XPS,PL,EIS showed that Bi2WO6and BTN-CMP formed compact heterojunctions,which was beneficial for the separation and transfer of photogenerated charge carriers across the heterojunction interface.
2.Materials and Methods
Bi(NO3)2·5H2O and Na2WO4·2H2O were purchased from Aladdin company;tri(4-ethynylphenyl) amine,4,7-Dibromo-2,1,3-benzo thiadiazole,Pd (PPh3)2Cl2and copper (I) iodide (CuI) were purchased from Tokyo Chemical Industry (TCI);triethylamine,toluene,chloroform,methanol,and acetone were purchased from Adamas-general reagent.All the reagents were analytic grade and used directly without further purification.
2.1.Synthesis of photocatalyst
Bi2WO6was synthesized through hydrothermal method according to the reported method [30].Typically,Bi (NO3)2·5H2O(5 mmol) was dissolved in 40 ml deionized water with drastically stirred for 30 min at room temperature.Then Na2WO4·2H2O(2.5 mmol) was added into Bi(NO3)2·5H2O solution and stirred for 60 min at the same conditions.Then the suspension was transferred to 100 ml Teflon lined stainless steel autoclave and reacted at 180 oC for 12 h.After cooling down to the room temperature,Bi2WO6can be collected by filtration and washed with deionized water and ethanol several times,respectively.Finally dried under vacuum at 80 oC for 24 h.
Photocatalysts composite were synthesized through an in-situ Sonogashira-Hagihara cross-coupling by Pd(II)catalyst in the presence of Bi2WO6[31].By modulating the mass ratio of BTN-CMP to synthesize a series of photocatalysts BW/BTN-n (n=1%,2%,5%,10%,denoted as BW/BTN-n).The route was shown in Fig.1a.For BW/BTN-2,1000 mg Bi2WO6was dispersed in the mixture of 5 ml toluene and 5 ml triethylamine (TEA) with ultrasonic for 5 min and degasification for 60 min under the argon atmosphere.Then 12.82 mg BTD,14.29 mg tEPhA,1.58 mg Pd (PPh3)2Cl2and 0.43 mg CuI were added into the solution.The mixture was heated at 80 oC for 72 h under the argon atmosphere.After cooling down to the room temperature,the targeted sample was collected by filtration with chloroform,deionized water,methanol,and acetone several times to remove the unreacted monomers and residue Pd(II) catalysts,respectively.Further purification was implemented by Soxhlet extraction with methanol at 110 oC for 48 h.Finally,the composite was dried under vacuum at 70 oC for 24 h.
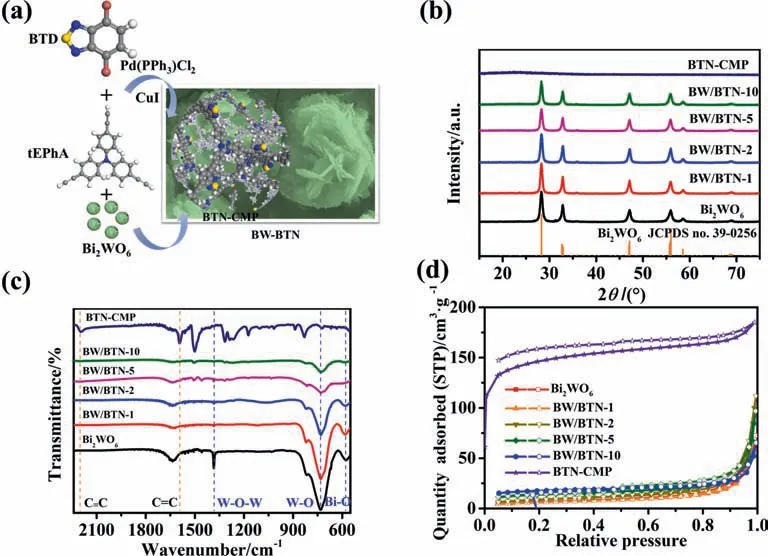
Fig.1.Synthesis schematic illustration of BW/BTN-n composite;(b) XRD patterns;(c)FT-IR spectra;(d)N2 adsorption–desorption isotherms of bare Bi2WO6,BTN-CMP and BW/BTN-n composite.
The bare BTN-CMP was synthesized as same as the composite photocatalysts without the addition of Bi2WO6.The physical mixture of Bi2WO6and 2%(mass)BTN-CMP was denoted as BW-BTN-2.
2.2.Characterization
Fourier transform infrared (FT-IR) spectra were recorded about the as-prepared samples of chemical functional groups on a spectrometer using the KBr as support.The crystal structures of asprepared samples were obtained on X-ray diffractometer (D/Max2550 VB/PC) with Cu Kα radiation source,X-ray tube voltage and current were set at 40 kV and 200 mA,respectively.The transmission electron microscopy (TEM,JEM-1400) and scanning electron microscopy (SEM,S-3400N) were carried out to acquire the morphology of as-prepared samples.The specific Brunauer-Emmett-Teller (BET) surface area of Bi2WO6,CMP and BW/BTN-n were obtained on Tristar II 3020 by Nitrogen adsorption–desorption isotherms methods,samples need to be dried for 5 h at 100 oC before the test under the atmosphere of nitrogen.To verify surface elements and chemical environment of as-prepared samples,X-ray photoelectron spectrometer (ESCALAB 250Xi) was carried out.The UV–Vis diffuse reflectance spectra (DRS) of Bi2WO6,CMP and Bi2WO6/CMP-n were investigated using a Scan UV–vis spectrophotometer (SHIMADZU,UV-2450) and using BaSO4as a reflectance sample.The photoluminescence spectrums obtained from FLUOROMAX-4 spectrofluorometer.The active free radical species were revealed by electron spin resonance (ESR,JEOL JESFA200).
2.3.Preparation and electrochemical test of catalyst slurry
5 mg catalyst was mixed with 30 μl 5% Nafion solution,333 μl ethanol and 666 μl deionized water.Afterwards,the mixture dispersed evenly in an ultrasonic bath for 60 min.13 μl slurry was dropped on the surface of glassy carbon electrode (area 0.07065 cm2) and dried at 80 oC for 15 min.After cooling down to the room temperature,the transient photocurrent density,and the electrochemical impedance spectrum (EIS) were implemented on a CHI 760E workstation,which is a standard three-electrodes with glassy carbon electrode as work electrode,Ag/AgCl as reference electrode and platinum wire as counter electrode,and 0.1 mol L-1Na2SO4solution as supporting electrolyte.
2.4.Photocatalytic testing
Adsorption and photocatalysis performances were conducted in a circulator bath at 25 oC.Photocatalytic activity was evaluated by the degradation of tetracycline hydrochloride (TCH) and rhodamine(RhB).Typically,taking the degradation of TCH as example,a 300 W xenon lamp(CEL-HXF300,CEAULIGHT)with a cutoff filter(λ >420 nm) was chosen as light source.20 mg as-prepared photocatalyst was dispersed in 100 ml 10 mg·L-1TCH solution,the dispersion was magnetic stirred for 60 min to reach adsorption–desorption equilibrium under the dark before the visible light irradiation.Then 3 ml reaction suspension was taken out and filtered with 0.22 μm nylon filter membrane at certain intervals.The absorbance of collected supernatant were analyzed on an ultraviolet–visible spectrophotometer at the characteristic adsorption peak 357 nm of TC (UV-2250,SHIMADZU Corporation,Japan).The degradation efficiency was calculated as follows:
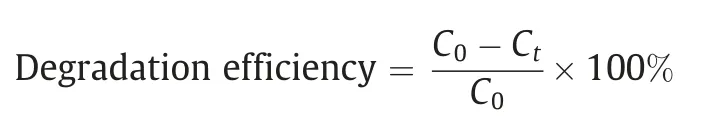
where C0is the initial concentration of TCH,Ctis the concentration of TCH at t mins.
After the degradation experiment was completed,the catalyst was collected by filtration and washed with deionized water,dried at 80 oC overnight.The collected catalyst was used for next degradation of TCH in the same conditions.
3.Results and Discussion
Power X-ray diffraction was conducted to get the crystal phase information of catalysts (Fig.1b).No diffraction peaks could be found in bare BTN-CMP,indicating its amorphous structure.The obvious diffraction peaks at 28.3o,32.8o,47.1o,56.0o,58.5o,68.8o were indexed to the (1 3 1),(2 0 0),(2 0 2),(1 3 3),(2 6 2) and(4 0 0) facets of the orthorhombic Bi2WO6(JCPDS NO.39-0256),respectively [32].With the increasing loading mass of BTN-CMP,the characteristic diffraction peaks intensity of Bi2WO6had a slight decline,while BW/BTN-n composites exhibited the same diffraction peaks with bare Bi2WO6,indicating the excellent structure preservation of Bi2WO6towards BTN-CMP generation during the in-situ polymerization process.The bare BTN-CMP exhibited typical -C≡C-stretching mode at 2196·cm-1and signals at 1317,1499 and 1595·cm-1were corresponded with the aromatic skeletons stretching vibration and C=C stretching modes in the FT-IR spectra(Fig.1c),[18,33–34].The signals at 589,730 and 1382·cm-1were assigned to Bi-O,W-O and W-O-W stretching modes in Bi2WO6,respectively(Fig.S1)[35–37].The co-existence of characteristic peaks of BTN-CMP and Bi2WO6in BW/BTN-n composites clearly demonstrated the successful synthesis.
To investigate the porosity of as-prepared materials,N2adsorption–desorption experiments were conducted at 77 K.From Fig.1d,BTN-CMP presented typically I-type adsorption isotherm[38]with the surface area about 476.19 m2·g-1with abundant micropores(Fig.S2).A series of BW/BTN-n composite demonstrated IV-type adsorption isotherms,indicating the existence of mesoporous structure,which is attributed to the slow formation rate between BTN-CMP and sheets of flower-like Bi2WO6during in-situ synthesis process.In Table 1,Brunauer-Emmett-Teller (BET) surface area were determined to be 23.83,21.35,31.35,46.11,56.18 m2·g-1for Bi2WO6,BW/BTN-1,BW/BTN-2,BW/BTN-5 and BW/BTN-10,respectively.The increased surface area of BW/BTN-n composite played an important role in providing more adsorbed and catalytic active sites during the photodegradation process.
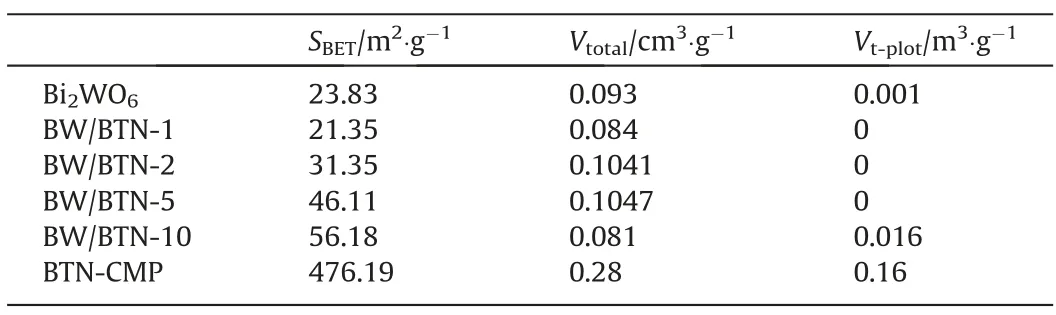
Table 1 BET specific surface area,pore volume,micropore volumes of as-prepared samples
As shown in Fig.2,BTN-CMP was highly hydrophobic with contact angle of 111.21o,indicating that it would easily aggregate in aqueous solution.Thus,it was crucial to integrate the hydrophilic Bi2WO6to improve hydrophilicity of BTN-CMP.In comparation with BTN-CMP,the contact angle of BW/BTN-2 significantly reduced to 32.37o,which endows the photocatalyst with better dispersity in the TCH aqueous solution.
Scanning electron microscope (SEM) and transmission electron microscopy (TEM) were employed to characterize the morphologies of Bi2WO6,BTN-CMP and BW/BTN-2.Bare Bi2WO6exhibited flower-like morphology with average particle size about 2–3 μm(Fig.3a)and many thin sheets can be clearly observed at the edgesof flower-like Bi2WO6,which was formed during hydrothermal process (Fig.3d) while bare BTN-CMP was consisted of irregular and smooth spheres about 2 μm (Fig.3b,e).Interestingly,after in-situ copolymerization on the surface of Bi2WO6,the flowerlike morphology was well preserved in BW/BTN-2 composite,while the shape of BTN-CMP was completely changed and tend to form rod-like microstructure (Fig.3c,f),which was attributed to the confined effect of layers structure of Bi2WO6during in-situ growth of BTN-CMP [26].As shown in EDS mapping (Fig.3g–j),S element evenly dispersed on the surface of bare Bi2WO6,indicating that BTN-CMP could be tightly generated on the layers of Bi2WO6in in-situ synthesis process.Those results showed that BTN-CMP and Bi2WO6could form a compact heterojunction,which was favorable for the adsorption and photodegradation of pollutants.
X-ray electron spectroscopy (XPS) was conducted to reveal the surface composition and chemical interaction of Bi2WO6,BTN-CMP and BW/BTN-2.The survey scan XPS spectra (Fig.4a) showed that BW/BTN-2 contained Bi,W,O,C,N and S elements.The high resolution XPS spectra of Bi and W were shown in Fig.4b and c,the peaks at 158.96 and 164.27 eV were assigned to the Bi 4f7/2and Bi 4f5/2orbitals of Bi3+in bare Bi2WO6[39],while the characteristic peak of Bi3+had a red-shift about 0.23 eV in BW/BTN-2.The peaks at 35.21 and 37.37 eV were ascribed to W 4f7/2and W 4f5/2orbits of W6+in bare Bi2WO6[40],which were 0.17 eV lower than BW/BTN-2.According to the XPS spectra of Bi and W elements in bare Bi2-WO6and BW/BTN-2,it was clear that the peak location of Bi and W both exhibited a red-shift,indicating the higher valence state of W and Bi in the composite.These results clearly showed that nanofiber BTN-CMP and Bi2WO6intimately contacted and formed a heterojunction with strong interaction between Bi2WO6and BTN-CMP,thus,electrons in Bi2WO6could be delocalized through the attraction of diazo sulfide,a strong electron-withdrawing group on the BTN-CMP skeleton.Therefore,electrons could be easily transferred and separated from Bi2WO6to BTN-CMP through the interface of heterojunction.
The optical absorption ability of as-prepared Bi2WO6,BTN-CMP and BW/BTN-2 composite were investigated using a UV–Vis spectrometer.The bandgap(Eg)of photocatalysts were calculated using the formula of Eg=1240/λ,where λ represents the absorption edge.As shown in Fig.5a,the absorption edge of bare Bi2WO6was about 445 nm,corresponding to the Egof 2.78 eV,while bare BTN-CMP possessed stronger optical absorption ability and the absorption edge extended to 788 nm,corresponding to the Egof 1.57 eV.For the series of BW/BTN-n composites,with the increasing loading mass of CMP,the absorption edges gradually red-shifted and the optical absorption capacity was enhanced in the range of 400–600 nm.Photoluminescence (PL) spectra reflected the separation,migration,and recombination rate of photogenerated electrons and holes.Weaker PL emission indicated lower recombination rate[41].In Fig.5b,under the excitation wavelength at 350 nm,for Bi2-WO6,there was a broad blue-green emission peak in the range of 425–500 nm,which was due to the transition of the Bi 6s and O 2p hybrid orbitals in the valence band ofto the W 5d orbitals of conduction band.Compared with bare Bi2WO6,the PL intensity of Bi2WO6was 2.25 times higher than BW/BTN-2,BW/BTN-2 composite possessed depressed PL emission,indicating a small loading mass of BTN-CMP (2%) could dramatically reduce the recombination of photogenerated charges for Bi2WO6[42].Obviously,BTNCMP possess minimum PL intensity,further demonstrating the superiority of linking D-A type CMP with traditional inorganic semiconductors in photocatalysis.The results of photochemical tests all showed that incorporating BTN-CMP with Bi2WO6could effectively reduce bandgap of photocatalysts,improve the utilization of photons and facilitate the separation of photogenerated electrons and holes.
To evaluate the photocatalytic activity of as-prepared samples,we took tetracycline hydrochloride (TCH) as target pollutant to implement the photocatalytic tests under the Xenon lamp irradiation with a 420 nm cutoff filter.As shown in Fig.6a,although BTNCMP demonstrated the biggest surface area (476.19 m2·g-1) and the smallest band gap(1.57 eV),it could merely adsorb 8%of total TCH (C0=10 mg·L-1) in 60 min and the photodegradation efficiency reached 30% in 90 min due to the hydrophobic structure of BTN-CMP,while bare Bi2WO6could degrade 70% of total TCH under the same conditions.For series of BW/BTN-n composites,the same TCH adsorption capacity of 10% were achieved with distinct degradation efficiency of 66%,84%,34% and 37% in 90 min,respectively.Clearly,the degradation efficiency of TCH was increased initially and then decreased with the increasing loading mass of BTN-CMP,indicating that there was a synergetic effect between Bi2WO6and BTN-CMP in the photodegradation process.In addition,the degradation efficiency of BW-BTN-2 composite obtained by physically mixing Bi2WO6and BTN-CMP only reached 67%in 90 min for TCH,which is much lower than that obtained by hydrothermal method,indicating the importance of heterojunction between BTN-CMP and Bi2WO6for the enhanced photocatalytic degradation performance.
Kinetic fitting was further performed for photocatalytic degradation process of TCH according with pseudo-first order kinetics.The slope of the curves represented the reaction rate constant of the photodegradation (Fig.6b).BW/BTN-2 had the highest degradation rate constants of 0.017·min-1,which was 5.7 and 1.7 times higher than bare BTN-CMP(0.003)and Bi2WO6(0.01),respectively.The BTN-CMP loading mass of 2% could effectively enhance the photocatalytic activity of Bi2WO6,which was attributed to the enhanced utilization of photons by extending the absorbance range of Bi2WO6due to the BTN-CMP integration,on the other hand,more active species was exposed during the degradation process of TCH since that the tightly formed BW/BTN-2 heterojunction effectively impressed the recombination of e--h+pairs under visible light irradiation.
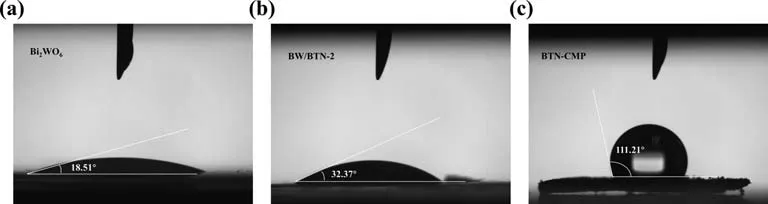
Fig.2.The contact angles of Bi2WO6 (a),BTN-CMP (b) and BW/BTN-2 composite (c).

Fig.3.SEM images of as-prepared samples of(a)bare Bi2WO6;(b)bare BTN-CMP;(c)BW/BTN-2.TEM images of(d)bare Bi2WO6;(e)bare BTN-CMP;(f)BW/BTN-2.(g–j)EDS mapping images of BW/BTN-2.
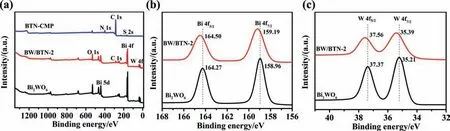
Fig.4.XPS spectra of bare Bi2WO6,BTN-CMP and BW/BTN-2 samples.(a) Survey scan;(b) high resolution Bi 4f XPS spectra and (c) high resolution W 4f XPS spectra.
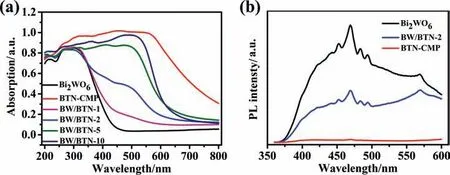
Fig.5.(a) UV–Vis diffuse reflectance spectra of as-prepared samples;(b) photoluminescence spectra of bare Bi2WO6,BTN-CMP and BW/BTN-2.
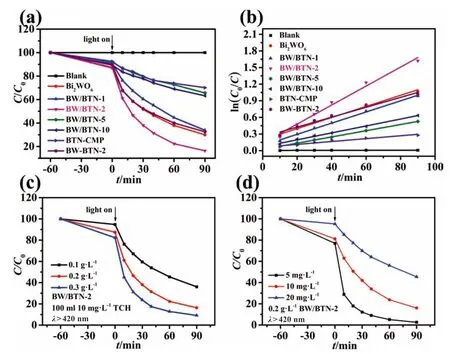
Fig.6.(a)Photocatalytic degradation of TCH by different photocatalysts under visible light irradiation(photocatalysts:20 mg,TCH(10 mg·L-1,100 ml));(b)the pseudo-first order kinetic fit for the degradation of TCH;(c)the effect of different photocatalyst dosage by BW/BTN-2(photocatalyst:0.1,0.2,0.3 g·L-1,TCH(10 mg·L-1,100 ml));(d)the effect of different initial TCH concentration by BW/BTN-2 (photocatalyst:0.2 g·L-1,TCH (5,10,20 mg·L-1,100 ml)).
In addition,the influence of photocatalyst dosages during the photodegradation process of TCH was further explored.The higher dosage of BW/BTN-2 composite leaded to the better photocatalytic activity.Providing more contacting opportunities with TCH molecules,the highest degradation efficiency of 92%was achieved with the dosage of 0.3 g·L-1(Fig.6c).The influence of different concentration of TCH(Fig.6d)was also investigated.The degradation efficiencies were 97%,85% and 55% for TCH solution in 90 min with initial concentration of 5,10 and 20 mg·L-1,respectively.Excessive TCH may prevent partial photons from reaching the surface of photocatalyst and thus conducted relatively low photocatalytic activity.Moreover,the stability of photocatalysts was tested by the cyclic TCH photodegradation with the dosage of 0.2 g·L-1and initial TCH concentration of 10 mg·L-1(Fig.S4).After three cycles,the adsorption capacity kept unchanged,while the photodegradation efficiency showed a small decline,from 85% to 68% within the same 90 min visible light irradiation.The reason may ascribe to the formation of some stable TC intermediates,blocking the photons transfer during photodegradation process.
Apart from TCH,textile dyes are another type of organic pollutant,and the annual global consumption is about 70 tons.In the dyeing process,about 1%–20% of textile wastewater is generated and aggravates water pollution [43].In addition,most dyes are non-biodegradable,and many nitrogen-containing dyes (such as Rhodamine B (RhB)) inevitably produced potential carcinogenic aromatic amine after natural anaerobic degradation [44,45].Thus,we further evaluated the photocatalytic activity of BW/BTN-2 composite towards RhB degradation.Blank experiment indicated that the self-photodegradation of RhB (C0=10 mg·L-1) was 8% in 90 min under visible light irradiation.In the presence of photocatalysts,the degradation efficiency of RhB was dramatically enhanced,which reached 71% and 86% for bare Bi2WO6and BTNCMP,respectively.Furthermore,RhB was completely photodegraded by BW/BTN-2 composite (Fig.7),indicating that the incorporation of BTN-CMP and Bi2WO6to construct inorganic/organic BW/BTN-n heterojunction could be an effective strategy for the decomposition of various environmental pollutants.
What’s more,Electrochemical impedance spectroscopy (EIS)experiments of Bi2WO6,BTN-CMP and BW/BTN-2 were conducted to study the transfer process of photogenerated electrons.BW/BTN-2 with inorganic/organic BW/BTN-n heterojunction had the smallest radius of arc under visible light irradiation,corresponding to the fastest charges transfer during photocatalytic process compared with Bi2WO6and BTN-CMP (Fig.8a),thus demonstrating the fastest reaction kinetics (Fig.6b).
Fig.8b showed the transient photocurrent response of Bi2WO6,BTN-CMP and BW/BTN-2.Photocatalyst could produce and transfer photogenerated electrons to the interface of electrode and to produce photocurrent under visible light irradiation.Obviously,BW/BTN-2 had the maximum photocurrent density as 0.41 μA·cm-2,which was 1.1 and 6.8 times higher than BTN-CMP and Bi2WO6,respectively,indicating that BW/BTN-2 possessed optimal separation efficiency of photogenerated e--h+pairs.The photoelectrochemical characterization results of EIS measurement was consistent with the photocurrent analysis,elucidating that the combination of BTN-CMP with Bi2WO6was a valid strategy to improve photocatalytic activity.
Moreover,electron spin resonance (ESR) technique was conducted to confirm the generated active radical during the photodegradation.DMPO (100 mmol·L-1) was used as the scavengers for·OH andTEMPO (100 mmol·L-1) as the scavengers for h+.and·OH could not be detected in the dark,the characteristic signals of theDMPO-·OH and TEMPO-h+adducts could be obviously observed under visible light irradiation (Fig.9a–c),demonstrating that the generation ofand·OH during photodegradation process,and all signals became stronger with prolonging the irradiation time from 5 min to 10 min.In addition,the active radical trapping test was further performed to reveal the main active species during the photocatalytic process(Fig.9d).The isopropanol (IPA),EDTA-2Na and argon were used as the scavengers for·OH,h+andrespectively.Photocatalytic activity was reduced to 43% with the addition of EDTA-2Na(1 mmol·L-1) into the reaction system,which increased to 75%with argon supplement,while only a slight decrease was observed with IPA addition (2 mmol·L-1).Therefore,h+should be the dominant active species during the photodegradation process.Both ESR technique and active radical trapping test have demonstrated that h+was the main active species,furthermore,and·OH also participated in the photodegradation process of TCH.

Fig.7.(a)The changes in UV–Vis absorption spectra of RhB photodegradation by BW/BTN-2 composite;(b)photocatalytic degradation of RhB by bare Bi2WO6,BTN-CMP and BW/BTN-2 composite under visible light irradiation.
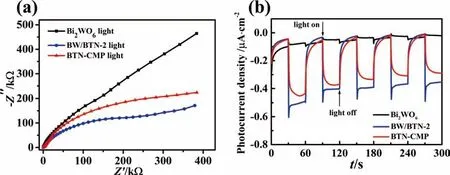
Fig.8.(a) Electrochemical impedance spectroscopy and (b) transient photocurrent response of Bi2WO6,BTN-CMP and BW/BTN-2 composite under visible light irradiation.
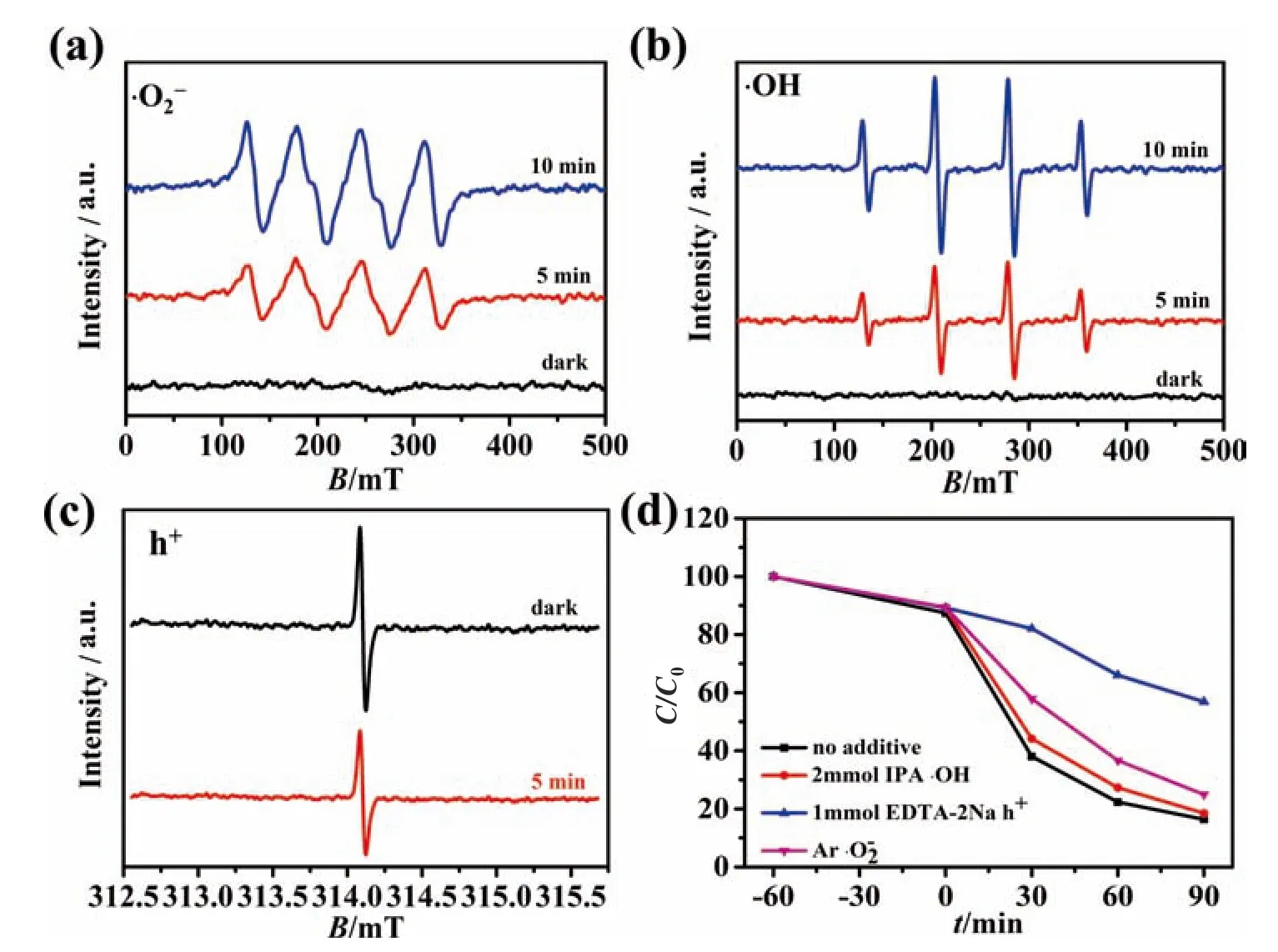
Fig.9.Electron spin resonance spectra of free species and holes trapped by DMPOand TEMPO(h+)in BW/BTN-2,(a)in methanol dispersion for trapping (b)in aqueous dispersion for trapping ·OH;(c)in aqueous for trapping h·under visible light irradiation.(d)Active species trapping experiments during the photodegradation of TC by BW/BTN-2 composite under visible light irradiation.
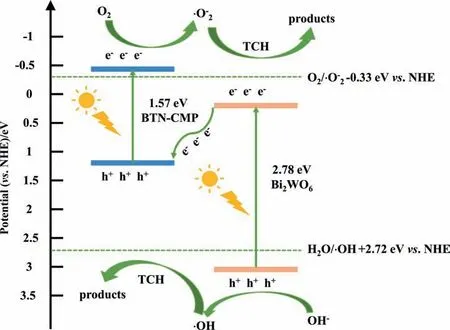
Fig.10.Schematic diagram for the separation and transport of photogenerated electron-hole pairs in BW/BTN-2 composite under visible light irradiation.
Based on the above analysis,the possible photodegradation mechanism of TCH could be proposed (Fig.10).Both BTN-CMP and Bi2WO6were excited to generate electrons and holes at conduction band(CB)and valence band(VB)under visible light irradiation,respectively.Combining the valence band XPS measurement(Fig.S3),UV–Vis DRS technique and ESR experiments,the conduction band (CB) of Bi2WO6and BTN-CMP were calculated to be 0.24 eV and -0.43 eV,respectively [46],and the valence band(VB) as 3.02 eV and 1.14 eV.Electrons in the CB of Bi2WO6and holes in the VB of BTN-CMP were all not strong enough to generateand·OH,thus a Z-scheme electron transfer mechanism was proposed.Electrons in the CB of CMP could remain and react with the adsorbed O2to generate=-0.33 V vs.NHE) [47]which further reacted with H2O to generate·OH,meanwhile,h+in the VB of Bi2WO6could oxidize H2O to·OH (·OH/H2O=2.72 V vs.NHE) [48].Electrons in the CB of Bi2WO6could transfer and combine with h+in the VB of BTN-CMP,which was beneficial for more h+in the VB of the Bi2WO6and e-in the VB of BTN-CMP to participate in the photodegradation process of TCH.Therefore,the enhanced photocatalytic performance is attributed to the effective restrain of photogenerated e--h+pairs recombination due to the Z-scheme BW/BTN-2 heterojunction.
4.Conclusions
In summary,we have successfully synthesized Z-scheme series of BW/BTN-n composite with in-situ Sonogashira-Hagihara crosscoupling copolymerization in the presence of Bi2WO6.Compare with pure Bi2WO6and BTN-CMP,BW/BTN-2 composite exhibits the highest TCH photodegradation efficiency as 84% at 90 min under visible light irradiation.The enhanced photocatalytic activity can be attributed to the π-conjugation polymer backbone of BTN-CMP with unique separation and transport ability of photogenerated charges.Incorporating BTN-CMP on the surface of Bi2-WO6and constructing intimate heterojunction is beneficial to overcome the intrinsic drawback of pure Bi2WO6,and the proposed degradation mechanism elucidates that construction of Z-scheme heterojunction reduces the combination rate of e--h+pairs and enhances the photocatalytic performance.The design and fabrication of CMPs with inorganic semiconductor can be a promising strategy to construct organic/inorganic composites with broadened light response and matched band edges heterojunction,which can be further applied in photodegradation of various environmental contaminations.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (91834301 and 21878076) and the Science and Technology Commission of Shanghai Municipality(19160712100).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.08.030.
 Chinese Journal of Chemical Engineering2022年1期
Chinese Journal of Chemical Engineering2022年1期
- Chinese Journal of Chemical Engineering的其它文章
- Struggling as in past, write a glorious future together-CJChE’s 40th anniversary
- Reduced graphene oxide modified melamine sponges filling withparaffin for efficient solar-thermal conversion and heat management
- Ag nanoparticles anchored on MIL-100/nickel foam nanosheets as an electrocatalyst for efficient oxygen evolution reaction performance
- Performance improvement of ultra-low Pt proton exchange membrane fuel cell by catalyst layer structure optimization
- Anodic process of stibnite in slurry electrolysis:The direct collision oxidation
- Catalytic mechanism of manganese ions and visible light on chalcopyrite bioleaching in the presence of Acidithiobacillus ferrooxidans
