Ag nanoparticles anchored on MIL-100/nickel foam nanosheets as an electrocatalyst for efficient oxygen evolution reaction performance
Tao Zhao,Dazhong Zhong,Genyan Hao,Guang Liu,Jinping Li,Qiang Zhao
Shanxi Key Laboratory of Gas Energy Efficient and Clean Utilization,College of Chemistry and Chemical Engineering,Taiyuan University of Technology,Taiyuan 030024,China
Keywords:Ag@MIL-100/NF Metal–organic frameworks Catalysis Kinetics Overpotential Electrochemistry
ABSTRACT Metal-organic frameworks (MOFs) exhibit excellent application potential in the field of electrocatalysis.In this study,we first prepare MIL-100 nanosheets on nickel foam (MIL-100/NF) and then successfully anchor Ag nanoparticles (NPs) on the nanosheets (Ag@MIL-100/NF) for oxygen evolution reaction(OER)catalysis.This strategy dramatically improves the conductivity of MIL-100 and the Ag NPs are uniformly dispersed on the nanosheets.The Ag@MIL-100/NF catalyst has excellent electrocatalytic performance and long-term corrosion resistance,with a low overpotential of 207 mV and a long-term stability of at least 100 h at a current density of 50 mA·cm-2.The experimental results demonstrate that this high OER catalytic performance is due to the improved charge transfer after loading Ag NPs,the combination of nanosheets and highly dispersed Ag NPs that expose more active sites and the adjusted chemical valence states of Fe and Ni in MIL-100.This work provides a surface decoration approach for the preparation of excellent catalysts directly used in the OER.
1.Introduction
Metal-organic frameworks(MOFs)are crystalline porous materials constructed by the coordination of metal ions or clusters with organic linking groups [1,2].In recent years,MOFs have been widely used in the oxygen evolution reaction (OER) because of their large specific surface areas,high porosities and adjustable chemical compositions [3–5].However,MOFs are still rarely used directly in electrochemistry.Generally,MOFs are calcined at high temperature to prepare effective OER catalysts due to their poor conductivity and stability [6,7].Unfortunately,MOFs cause structural damage and reduce active sites during high temperature processes,which are not conducive to catalytic reactions [8].Therefore,it is necessary to develop a strategy to use MOFs for effective OER catalysis.
Previous work has proved that the preparation of MOFs on highly conductive nickel foam (NF) is an effective way to obtain catalysts with excellent performance [9].Multimetal MOF materials also have superior electrocatalytic activity than single-metal MOFs.In addition,anchoring metal nanoparticles(NPs)on the surface of MOFs is another effective means to enhance their OER activity [10].To date,precious metal NP-modified catalysts have been used in catalysis and exhibit excellent catalytic performance [11–13].It is noteworthy that Ag can directly change the conductivity of transition metals,thereby improving OER performance [14–16].More importantly,the cost of Ag is relatively low and its reserves are abundant compared with other precious metals.Therefore,anchoring Ag NPs on MOFs is a very promising strategy for preparing high-efficiency catalysts.
Fe-based MOFs have stable structures and higher OER activities than other MOFs and can therefore ensure structural integrity and achieve more efficient catalytic performance after loading NPs[17,18].Among many materials,MIL-100 not only has a flexible structure but also can realize high stability due to the existence of high valence metals[19].In particular,MIL-100 can be prepared using environmentally friendly methods.Because of these advantages,MIL-100 can be used as a suitable OER catalyst.Most reported MIL-100 particles agglomerate very easily and reduce the active sites.Therefore,there is an urgent need to develop new strategies to prevent agglomeration and improve their performance.
Zhao et al.[20] reported that two-dimensional MOF nanosheets arrays were grown on NF and showed excellent catalytic performance towards OER with a small overpotential of 240 mV at 10 mA·cm-2.Therefore,the preparation of MIL-100 with nanosheets on NF plays a key role in electrochemistry because the nanosheets can easily transfer mass and provide a larger surface area to load metal NPs.Moreover,the integration of MOFs and metal NPs further improves the electrocatalytic activity via increasing the charge transfer of MOFs and the synergistic effect between these metal ions.
Based on the above considerations,we synthesize MIL-100 nanosheets on NF by a solvothermal method and then Ag@MIL-100/NF is prepared by anchoring Ag NPs on MIL-100/NF for OER catalysis.Compared with a pure MIL-100/NF material,Ag@MIL-100/NF has significant OER catalytic performance with a low overpotential of 207 mV at a current density of 50 mA·cm-2,which shows that anchoring Ag NPs on MIL-100/NF can greatly improve its OER activity.In particular,the electrochemical durability of Ag@MIL-100/NF reaches 100 h at a constant current density of 50 mA·cm-2and the curve has no obvious downward trend,indicating that it has long-term corrosion resistance in a 1 mol·L-1KOH solution.Hence,this is a very promising catalyst for water decomposition.
2.Experimental
2.1.Materials
All chemical reagents used were not further purified.Iron nitrate nonahydrate (Fe(NO3)3·9H2O),Hydrochloric acid (HCl),Sodium borohydride (NaBH4) and Ethanol were provided from Sinopharm.1,3,5-Benzenetricarboxylic acid (H3BTC),Potassium hydroxide (KOH) and Silver nitrate (AgNO3) were purchased from Aladdin Ltd.(Shanghai,China).Nickel foam (NF;thickness:1.5 mm) was provided by Shanxi Taiyuan Power Battery Material Co.,Ltd.
2.2.Preparation of the samples
2.2.1.Synthesis of the MIL-100(NiFe)/NF (Mark as MIL-100/NF)
The bimetallic MIL-100 was generated in situ on the surface of bare NF by simple solvothermal.Before starting the experiment,NF was processed to obtain a clean surface.First,the NF(3 cm × 6 cm) was treated with hydrochloric acid (2.0 mol·L-1HCl)under ultrasonic treatment for 10 min to remove surface oxides,followed by washing above NF with deionized water and ethanol in turn to clean under the ultrasonication for another 10 min to remove the remaining residue.After that,the NF was taken out for drying.Fe(NO3)3·9H2O (1.2 mmol,0.4850 g) and BTC (1.2 mmol,0.2520 g)were dissolved in 72 ml deionized water in a Teflon vessel(100 ml)to form a uniform solution by magnetic stirring at the room temperature.Then,the NF is added to the vessel and the vessel was transferred to a stainless-steel autoclave.The autoclave was heated and maintained at 100 °C for 12 h.After cooling to room temperature,the NF was rinsed with deionized water and the precipitate (MIL-100 powder) was centrifuged three times to dry at 80 °C.
2.2.2.Synthesis Ag@MIL-100/NF
40 mg AgNO3was dissolved in 10 ml deionized water to form a uniform solution,then the MIL-100/NF (1 cm × 3 cm) was added and place at room temperature for 5 h.Next take out the NF and rinse it with deionized water,then put it into the newly prepared NaBH4solution(1 mmol·L–1,10 ml)to reduce it for 0.5 h.Catalysts with different Ag content were also prepared to study OER performance,the preparation method is the same as Ag@MIL-100/NF,except that the amount of AgNO3(10 mg,20 mg,30 mg,50 mg)added is different.In addition,catalysts with different reaction times were prepared to study the performance of OER,except the reaction time (1 h,3 h,7 h) is different.
2.3.Characterization
The X-ray diffraction (XRD),scanning electron microscopy(SEM),energy-dispersive X-ray spectrometry (EDS),highresolution transmission electron microscopy (HRTEM),Brunauer–Emmett-Teller(BET),X-ray photoelectron spectroscopy(XPS)characterization methods used in this experiment are consistent with our previous reports [21].In addition,we also performed some other characterization techniques.The electrocatalyst was analyzed using FT-IR spectrometer with Thermo Electron NEXUS 670.The metal content of the catalyst was determined by an inductively coupled plasma (ICP) spectrometer (0.8 mg catalyst dissolved in 50 ml acid solution).
2.4.Electrochemical measurements
All electrochemical measurements were performed in a standard three-electrode system using a Princeton electrochemical workstation (PARSTAT MC,Princeton,USA).The Ag@MIL-100/NF(MIL-100/NF,NF) as the working electrode (1 cm × 1 cm),a Pt rod and a Hg/HgO were used as auxiliary electrode and reference electrode,respectively.All measurements were performed in 1 mol·L-1KOH electrolyte.Polarization curves were conducted by linear sweep voltammetry (LSV) with IR compensation in a scan rate of 2.0 mV·s-1.The Tafel slope was calculated by the formula:η=a+blgj;where η is the overpotential,b refers to the Tafel slope,j represents the current density [22].Electrochemical impedance spectroscopy (EIS) were obtained in the frequency range from 100 kHz to 0.05 Hz at the potential 1.46 V(vs.RHE).Cyclic voltammogram(CV)measurements were acquired with various scan rates of 20,40,60,80 and 100 mV·s-1in the overpotential range from 0.80 V to 0.90 V (vs.RHE) to obtain the double-layer capacitances(Cdl).The chronopotentiometry was measured at 50 mA·cm-2using an electrochemical workstation system.According to the following equation:ERHE=EHg/HgO+0.098+0.059 pH,the potentials for all OER were calibrated as reversible hydrogen electrode(RHE).The OER overpotential(η)was obtained according to the following formula:η=ERHE–1.23.
3.Results and Discussion
3.1.Materials characterization
The preparation process of Ag@MIL-100/NF is shown in Scheme 1.MIL-100 with a nanosheet morphology was first prepared on NF by a solvothermal process.MIL-100/NF was then immersed in a AgNO3solution at room temperature for 5 h.Finally,Ag NPs were generated on the surface of MIL-100/NF by a NaBH4reducing agent (Ag@MIL-100/NF) and had a uniform appearance(Fig.S1,see Supplementary Material).Different characterization techniques were used to investigate the morphology and structure of the catalyst.
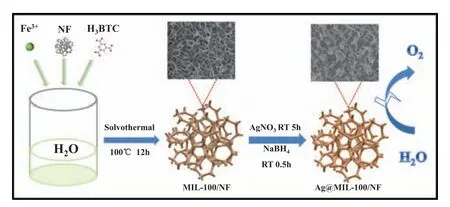
Scheme 1.Schematic of synthesis process for Ag@MIL-100/NF.
The morphology and microstructure of the catalyst were studied by SEM and TEM.Fig.S2 indicates that the blank NF has a smooth surface and large holes.The SEM images show pure MIL-100 with nanosheet arrays grown uniformly on the surface of NF(Fig.S3).Obviously,the similar morphology is maintained in the Ag@MIL-100/NF sample loaded with Ag NPs.(Fig.1(a) and S4).The nanosheet arrays of Ag@MIL-100/NF can fully contact with the electrolyte,thereby accelerating the diffusion of ions and gas for improved OER kinetics.However,because of the smaller size of the Ag NPs,the magnified SEM image also did not show the presence of NPs(Fig.1(b)).Fig.S5 shows that MIL-100 powder is composed of nanoparticles,which is different from the morphology of MIL-100/NF.
TEM clearly reveals that Ag NPs with a good dispersion could be observed on the nanosheets (Fig.1(c) and (d)).As shown in Fig.1(e),the 0.240 nm lattice in HRTEM corresponds to the Ag (1 1 1)crystal plane and the selected area electron diffraction(SAED)pattern can also show the (1 1 1) and (2 2 0) crystal planes of Ag(JCPDS 04–0783) (Fig.1(f)).The element composition in Ag@MIL-100/NF is provided by EDS mapping (Fig.1(g)).Fe,Ni,Ag,C and O are uniformly distributed on the nanosheets and the particle size of Ag NPs is about 10–20 nm.In addition,some smaller Ag NPs can be seen,which may be caused by Ag NPs entering the pores of MIL-100.These results all demonstrate that Ag NPs have been successfully loaded on MIL-100/NF and Ag NPs are effectively dispersed,thereby increasing the utilization rate of the catalyst and ultimately speeding up the reaction.
The crystal structures of MIL-100/NF and Ag@MIL-100/NF were characterized by XRD.For MIL-100/NF,there are no obvious peaks except for the peak of NF (JCPDS 04-0783) at 44.45° (Fig.S6).Therefore,we conducted XRD on the MIL-100 precipitate and it can be seen that the peak position of the MIL-100 powder is consistent with the simulated peak [23],indicating that MIL-100 was successfully prepared on the NF (Fig.S7).Similarly,XRD was performed on Ag@MIL-100/NF,with no obvious characteristic peaks observed (Fig.S8).However,the typical characteristic peak at 2θ=10.97° is observed on Ag@MIL-100 scraped from Ag@MIL-100/NF,corresponding to the (248) crystal plane of the MIL-100 nanosheets.This phenomenon is similar to previous reports [24].And it is also compared with the XRD standard card of LDH (PDF#38-0715),which excludes the possibility that the produced substance is LHD.The diffraction peak of Ag is not observed,which may be caused by the low Ag content(Fig.S9)[25].There is no significant change in the XRD pattern after loading Ag,which proves that the crystal structure of MIL-100 is not damaged during the loading and reduction process (Fig.S10).
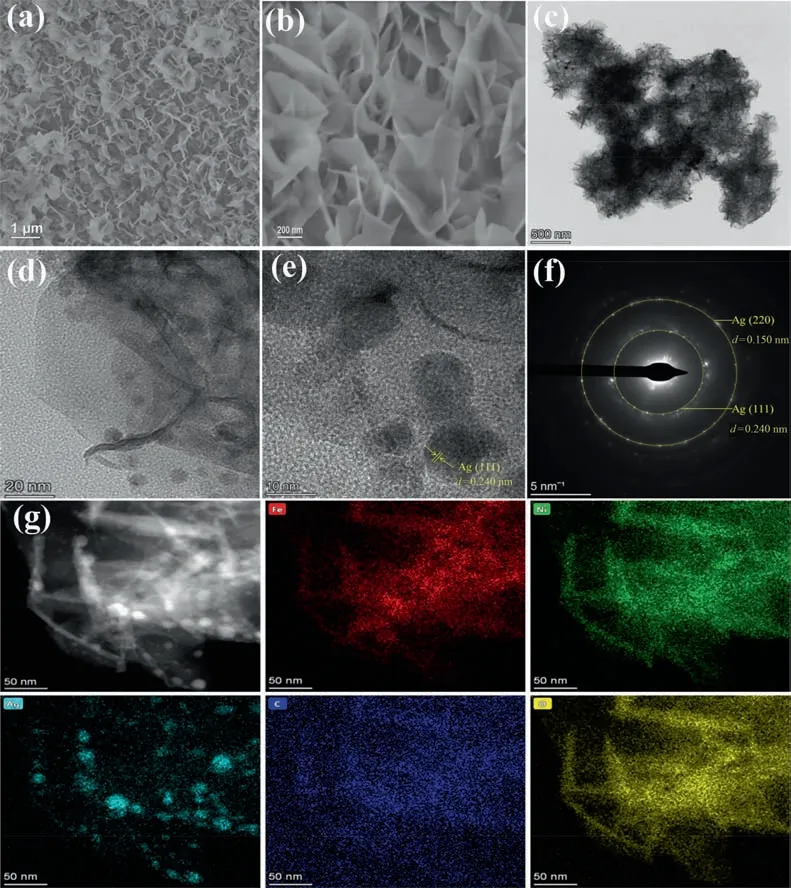
Fig.1.(a)-(b) SEM images,(c)-(d) TEM images,(e) HRTEM image,(f) SAED pattern and (g) EDS elemental mapping images of Ag@MIL-100/NF.
The bonding nature of Ag@MIL-100/NF and MIL-100/NF was analyzed by FT-IR spectroscopy.The FT-IR spectra of Ag@MIL-100/NF and MIL-100/NF are shown in Fig.2a and are consistent with the previous literature reports [26].Obviously,a broad peak at 3425 cm-1indicates the existence of O-H vibration,which proves that there are a large number of hydrophilic groups.The two peaks at 1550 and 1355 cm-1are caused by asymmetric and symmetric -COO–,respectively.The vibration of the C-C bond in the benzene ring appears at 1617 and 1434 cm-1.The peak at~500 cm-1is due to the formation of M-O bonds between Fe or Ni and carboxyl groups.This result further proved that MIL-100 was prepared on NF and showed that Ag NP loading on MIL-100 had no significant structural changes.This is consistent with the XRD results.
In order to explore the specific surface area of the prepared catalyst,we conducted N2adsorption–desorption isotherm tests at 77 K(Fig.2(b)).The BET test results indicated that the specific surface area of MIL-100/NF (84.88 m2·cm-2) was higher than that of Ag@MIL-100/NF (66.64 m2·cm-2),which might be caused by the generated Ag NPs entering the pores of MIL-100.The BJH method is used to calculate the pore size distribution,indicating that they can still maintain a similar pore size (mainly microporous)(Fig.S11) [27].The content of each metal in the catalyst was detected by ICP (Fig.S12).It is obvious that the molar ratio of Ni and Fe in the two catalysts is basically the same,which also reflects that the metal in the MOFs will not be lost after loading Ag NPs.The Ag content in Ag@MIL-100/NF is 10 μg·mg-1.Therefore,trace amounts of Ag were successfully loaded onto MIL-100.
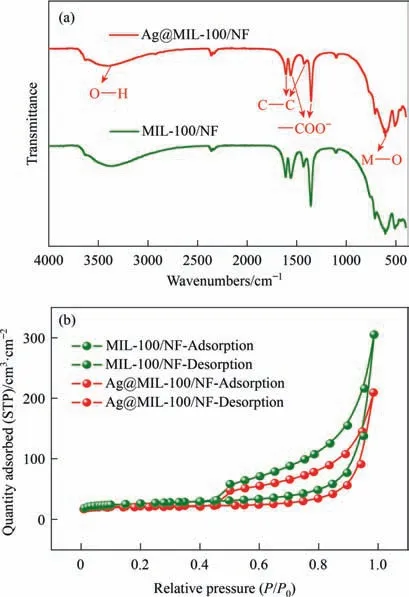
Fig.2.(a) FT-IR spectra and (b) nitrogen adsorption–desorption isotherms of MIL-100/NF and Ag@MIL-100/NF(MIL-100 and Ag@MIL-100 were not scraped from NF).
The electronic structure and elemental valences of the prepared catalyst were analyzed by XPS.The full spectrum shows that Fe,Ni,Ag,C and O are present in Ag@MIL-100/NF,indicating that Ag was also successfully prepared on MIL-100/NF(Fig.3(a)),which is consistent with the EDS mapping results.In the XPS spectrum of Ag@MIL-100/NF,the binding energies of Fe 2p1/2and Fe 2p3/2locate at 725.95 and 713.07 eV,respectively,and there is a satellite peak at 718.05 eV,suggesting Fe element in the form Fe3+(Fig.3(b)).In the region of Ni 2p,the two peak positions at 873.90 and 856.27 eV are attributed to Ni 2p1/2and Ni 2p3/2,respectively.The binding energy at 879.92 and 862.15 eV belongs to the two satellite peaks Ni 2p1/2and Ni 2p3/2,respectively,implying Ni element in the form Ni2+(Fig.3(c)) [28,29].
For the spectrum of Ag 3d,the peaks at 373.88 and 367.84 eV are attributed to 3d3/2and 3d5/2,respectively,which was in conformity with the standard Ag0spectra and confirmed the metallic state of Ag NPs on MIL-100/NF (Fig.3(d)) [30].The spectrum of C 1s can be fitted to three peaks at 287.84,285.57 and 284.70 eV corresponding to O-C=O,C=C and C-C,respectively (Fig.3(e)) [31].The spectrum of O 1s shows that there are three different peaks at 532.15,531.29 and 530.64 eV,which are associated with adsorbed water,O=C-C and O-M,respectively (Fig.3(f)) [32].
In the XPS spectrum of MIL-100/NF,Fe 2p1/2and Fe2p3/2peak positions associated with 725.65 and 712.60 eV,respectively,and there is a satellite peak at 716.52 eV (Fig.S13(b)).The Ni 2p spectrum is shown in Fig.S13(c).The two peaks at 873.55 and 855.95 eV are attributed to Ni 2p1/2and Ni 2p3/2,respectively,and there are two satellite peaks at 879.83 and 861.86 eV.It is noteworthy that the binding energy of Fe 2p and Ni 2p in the Ag@MIL-100/NF is shifted positively compared with MIL-100/NF,indicating that the electronic structure of Fe and Ni can be adjusted to a high valence state after adding Ag NPs[33].The peak positions of C 1s and O 1s in MIL-100/NF are similar to Ag@MIL-100/NF(Figs.S13(d) and (e)).
The above results indicate that the loading of Ag NPs on MIL-100/NF will not change the original structure of MOFs.This conclusion verifies the XRD and FT-IR results.Therefore,anchoring Ag NPs on the surface of MIL-100/NF can adjust the chemical state of Fe and Ni in the structure of MOFs,which is beneficial to improving the OER catalytic performance.
3.2.Electrochemical performance
The electrocatalytic OER performances of Ag@MIL-100/NF,MIL-100/NF and blank NF were evaluated in a 1 mol·L-1KOH solution using a standard three-electrode system with IR compensation[34].We pretreated the catalyst with multicycle CVs to obtain stable polarization curves.In addition,the polarization curves of Ag@MIL-100/NF at different sweep speeds were also tested,which can verify that the sweep speeds of 2 mV·s-1used in our experiment has almost no effect on the experimental results (Fig.S14).The LSV curves of these samples show that Ag@MIL-100/NF only requires an overpotential of 207 mV at 50 mA·cm-2,which is significantly less than MIL-100/NF (259 mV) and blank NF (370 mV)(Fig.4(a)).This indicates that the OER catalytic performance of Ag@MIL-100/NF was significantly improved by introducing Ag NPs onto the surface of MIL-100/NF.The partially enlarged LSV curves clearly show that the oxidation peak of Ni2+/Fe2+to Ni3+/Fe3+has been crossed when the current density exceeds 42 mA·cm-2.Therefore,it is appropriate to compare the overpotential at 50 mA·cm-2(Fig.S15).
In addition,to obtain the best catalytic performance of Ag@MIL-100/NF,we studied the influence of AgNO3concentration and reaction time.As shown in Figs.S16 and S17,the best catalytic performance can be obtained when the AgNO3was added at 40 mg and the reaction time was 5 h.More importantly,the performance of Ag@MIL-100/NF is better than other reported transition metal catalysts (Table S2) and therefore has excellent potential to replace precious metal catalysts.
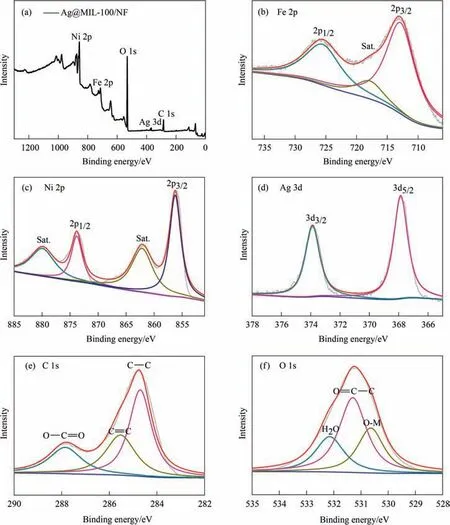
Fig.3.(a) Full XPS spectra and (b)-(f) XPS spectra of Fe 2p,Ni 2p,Ag 3d,C 1s,and O 1s for Ag@MIL-100/NF.
The kinetics of these catalysts in electrochemical reactions were evaluated by the Tafel slope and EIS.The Tafel slope is obtained by fitting the polarization curve.Fig.4(b)indicates that Ag@MIL-100/NF (75.9 mV·dec-1) has a lower value than MIL-100/NF(81.0 mV·dec-1) and blank NF (115.9 mV·dec-1).The Tafel slope value of Ag@MIL-100/NF is the smallest,indicating high charge transfer and an excellent OER kinetic process.Thus,it can be clearly verified that this material has excellent catalytic performance [35].
EIS is also an important tool to further understanding the electron transport kinetics and interfacial reaction in the OER process.According to the Nyquist plots of EIS(Fig.4(c)),we use EIS data to fit the equivalent circuit and obtain the fitting result (Fig.4(c)inset).The Rsvalues of Ag@MIL-100/NF,MIL-100/NF and NF are almost the same,which indicates that the electrolyte has basically the same effect on each catalyst(Rsrepresents the resistance of the electrolyte).The Rctvalues of Ag@MIL-100/NF,MIL-100/NF and NF are 0.297,0.660 and 5.102 Ω,respectively (Table S1).Clearly,the charge transfer resistance of Ag@MIL-100/NF is smaller than MIL-100/NF and blank NF,indicating that the charge transfer process is very fast after introducing Ag NPs,so it has fast reaction kinetics in the OER process.
Previous reports indicate that the introduction of metal NPs on MOFs can expose a large number of active sites [10,36].To prove this hypothesis,we further tested the Cdlof Ag@MIL-100/NF,MIL-100/NF and blank NF to evaluate their catalytic performance(Fig.S18).The Cdlvalue is positively correlated with the electrochemically active surface area.As shown in Fig.4(d),the calculated Cdlvalue of Ag@MIL-100/NF is 1.60 mF cm-2,which is greater than MIL-100/NF (1.34 mF·cm-2) and blank NF (1.02 mF·cm-2).This result evidences that the introduction of Ag NPs increases the active area and can provide more active sites,which accelerates the OER [37].These results indicate that Ag@MIL-100/NF has significant OER catalytic performance,which is mainly due to the increased charge transfer rate and exposure of more active sites.
Long-term stability is an important indicator to evaluate the practicality of the catalyst.Here,a multi-step chronopotentiometry curve was produced with a current density of 20 mA·cm-2increased to 100 mA·cm-2(20 mA·cm-2increase every 2 h)(Fig.4(e)).The overpotential remains constant immediately under changing current density,which shows that Ag@MIL-100/NF can quickly diffuse the oxygen and has excellent mass transfer capabilities [38,39].
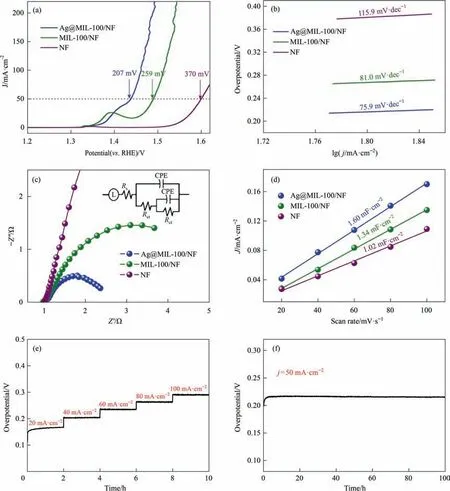
Fig.4.(a) Polarization curves,(b) corresponding Tafel plots,(c) Nyquist EIS plots and (d) Cdl values of Ag@MIL-100/NF,MIL-100/NF and blank NF;(e) Multi-step chronopotentiometry curve at current densities of 20–100 mA·cm-2 and (f) chronopotentiometry curve of Ag@MIL-100/NF at 50 mA·cm-2 in 1 mol·L-1 KOH.
In addition,Fig.4(f)reveals that Ag@MIL-100/NF has a stability of at least 100 h and the curve is basically unchanged at a constant current density of 50 mA·cm-2,indicating that it has strong corrosion resistance and long-term stability in the 1 mol·L-1KOH solution.Next,we performed XRD analysis of Ag@MIL-100/NF after the stability test (Fig.S19) and it can be clearly seen that some new peak positions have appeared,which can be attributed to the formation of hydroxide [40,41].However,the characteristic peaks of MIL-100 are still retained,which also indicate that Ag@MIL-100/NF can still maintain its structure after long-term durability testing.
We conducted SEM on Ag@MIL-100/NF and it is clear that it still maintains the original morphology,which also shows that the catalyst can withstand a long test (Fig.S20).And EDS element test showed that Ag still exists (Fig.S21).In addition,from the FT-IR spectrum (Fig.S22),a new peak appears at~827 cm-1,which is caused by M-OH generated in the OER process,indicating that a small amount of hydroxide is generated[41].The XPS also analyzes each element in Ag@MIL-100/NF(Fig.S23).Compared with before the test,the peak position of Fe 2p,Ni 2p and C 1s did not change significantly.However,K 2s appeared in the XPS of Ag 3d,which was due to the catalyst test in the KOH solution.And Ag still maintains the original valence state,which indicates that Ag is very stable during the OER process (Fig.S23(d)).In the XPS of O 1s(Fig.S23(f)),the peak at 531.53 eV is caused by the generated hydroxyl oxygen [42].This result is consistent with XRD and FTIR analysis.These strongly indicate that the prepared catalyst has extremely high stability and the hydroxide formed in the OER may play a key role in catalysis.
4.Conclusions
We have successfully anchored Ag NPs on MIL-100/NF with a nanosheet structure for OER electrocatalysis.The Ag@MIL-100/NF has significant catalytic performance and a lower overpotential of 207 mV at 50 mA·cm-2.Moreover,it also exhibits excellent electrochemical stability with a long-term stability of at least 100 h at a current density of 50 mA·cm-2and can maintain structural integrity.Characterization methods and electrochemical tests prove that the introduction of Ag NPs on MIL-100/NF can enhance the reaction kinetics and generate abundant active sites,and the Ag NPs could tune the electronic environment of Ni and Fe in MIL-100,which is very beneficial to improving the catalytic performance of the OER.Excellent performance,long-term stability,simple preparation and low cost make it have the potential for largescale use.This simple and efficient strategy provides inspiration for the design and synthesis of new catalysts.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (21878202,21975175),and the Natural Science Foundation of Shanxi Province(201801D121052).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.11.011.
 Chinese Journal of Chemical Engineering2022年1期
Chinese Journal of Chemical Engineering2022年1期
- Chinese Journal of Chemical Engineering的其它文章
- Struggling as in past, write a glorious future together-CJChE’s 40th anniversary
- Reduced graphene oxide modified melamine sponges filling withparaffin for efficient solar-thermal conversion and heat management
- Photocatalytic degradation of tetracycline hydrochloride with visible light-responsive bismuth tungstate/conjugated microporous polymer
- Performance improvement of ultra-low Pt proton exchange membrane fuel cell by catalyst layer structure optimization
- Anodic process of stibnite in slurry electrolysis:The direct collision oxidation
- Catalytic mechanism of manganese ions and visible light on chalcopyrite bioleaching in the presence of Acidithiobacillus ferrooxidans
