Lipid peroxidation and cancer
Daniel Weber,Remo Lanci
1Tianjin TCM University,Tianjin,China.2Sydney Institute of Traditional Chinese Medicine,Sydney,Australia.
Abstract Lipoxidation is formed during the oxidation of lipids and specific proteins, causing an alteration in proteins function.Although this occurs under physiological conditions, in many cases, it has been associated with pathological processes, including cancer.Oxidative DNA damage is a significant contributor to cancer development, and lipoxidation products such as malondialdehyde (MDA) and 4-hydroxy-2-nonenal (4-HNE) play a role in the induction of mutations responsible for DNA modifications.Elevations of reactive aldehydes can be seen in patients with metastatic disease in comparison to those without metastatic disease.Lipoxidation adducts can modify the immune response, consequently causing either positive or negative alterations in cancer progression.When reactive aldehydes are added to tumor cells, they exert similar effects to chemotherapeutic drugs by forming DNA adducts and consequently steer the tumor cells toward apoptosis.This article highlights the role of botanical compounds in lipid metabolism, lipid peroxidation, and cell cycle arrest and discusses the potential role they may have in oncology treatments.
Keywords:lipid peroxidation; cancer
Introduction
Lipid peroxidation is a process where oxidants such as reactive oxygen species (ROS) attack lipids giving rise to the formation of lipoperoxyl radicals (LOO) and lipid hydroperoxides (LOOH).The main targets of lipid peroxidation are phospholipids, glycolipids, and cholesterol.During the process of lipid peroxidation, lipid hydroperoxides decompose to form reactive aldehydes such as malondialdehyde(MDA) and 4-hydroxynoneal (4-HNE).
Lipid-derived aldehydes act as second messengers of free radicals due to their stability and ease in diffusing across cell membranes.These aldehydes are significant as they can successfully bind to DNA,leading to adduct formation and promoting mutagenic effects [1, 2].When lipid peroxidation rates are low, cells can up-regulate their antioxidant defense systems or stimulate antioxidant transcription factors.However, when lipid peroxidation rates are high, the oxidative damage overpowers the cell's regulatory antioxidant mechanisms, inducing cell damage or cell death [1].
There is growing evidence that lipid peroxidation products are implicated in a number of pathological states such as inflammation,atherosclerosis, diabetes, neurodegenerative conditions, and importantly carcinogenesis[2, 3].
Substrates of Lipid Peroxidation
Polyunsaturated Fatty Acids (PUFAS).The major substrates for lipid peroxidation are the Polyunsaturated Fatty Acids (PUFAS).PUFAS can be classified into omega-3 or omega-6 depending on the location of the last double bond.Peroxidation will generate aldehydes and other carbonyl species based on the PUFA initially targeted.
The predominant omega-6 fatty acid- Arachidonic Acid (AA) can be reduced by peroxidation to MDA, 4-HNE, isoprostanes, and other lipid peroxidation end products [1, 3].
Lipid peroxidation and the formation of reactive adducts can dramatically alter the cell integrity by altering the permeability and fluidity of the membrane lipid bilayer [4].
Cardiolipins (CLs).Cardiolipins (CLs) are crucial for maintaining mitochondrial membrane structure and functioning of multiple protein complexes of the electron transport chain (ETC) [5].However,CLs are vulnerable to oxidative stress due to the presence of unsaturated fat acyl chains[6].
Secondary Lipid peroxidation Products
Aldehydes such as malondialdehyde (MDA) and 4-hydroxynoneal(4-HNE) are produced as secondary products of lipid peroxidation.MDA appears to be the most mutagenic,and 4-HNE the most toxic[1].
Malondialdehyde.Malondialdehyde, also known as MDA, is generated by the peroxidation of membrane PUFAS or during prostaglandin production.Due to its stability and membrane permeability, MDA can react with biomolecules such as proteins or DNA and generate MDA-DNA adducts.The reactivity of MDA increases at a low pH, where it becomes beta-hydroxyacrolein.
When cellular repair mechanisms are overwhelmed, MDA-DNA adducts can lead to mutations, strand breaks, cell cycle arrest, and the induction of apoptosis.As mentioned, this type of DNA damage may greatly contribute to carcinogenesis.
4-hydroxy-2-nonenal (HNE).4-hydroxy-2-nonenal (HNE) is the main product of the peroxidation of n-6 PUFAs (linoleic acid and arachidonic acid).4-HNE can be generated from the oxidation of cardiolipin, a mitochondria-specific phospholipid, and through several non-enzymatic oxygen radical-dependant routes[1, 7].
4-HNE has a dual role as a signaling molecule and as a cytotoxic product of lipid peroxidation.As a signaling molecule, 4-HNE stimulates gene expression through the regulation of transcription factors such as nuclear factor erythroid 2-related factor 2 (Nrf2),Nuclear factor kappa B (NF-KB), and Peroxisome proliferator-activated receptor (PPAR).High levels of 4-HNE can react with proteins or DNA, resulting in genotoxic effects [1].
Low levels of 4-HNE can protect cancer cells from further damage(e.g., UCPs decrease superoxide production).Strategies that focus on manipulating mitochondrial ROS production, lipid peroxidation, and 4-HNE formation might be therapeutic in treating or preventing cancer [7].
Oxidative Stress
The body's cells and systems must be maintained in a state of redox equilibrium to create the right conditions for survival.Excessive ROS production overloads antioxidant defense mechanisms in cells, leading to a weakened balance between antioxidants and pro-oxidants.
The major source of the exogenous reactive oxygen species (ROS)production are the mitochondria, plasma membrane endoplasmic-reticulum, and peroxisomes.Nitric oxide (NO) is also produced by the mitochondrial respiratory chain under hypoxic conditions, resulting in other reactive nitrogen species(RNS).
Large amounts of can directly damage lipids.Two of the most significant ROS that can affect the lipids are the hydroxyl radical(HO*) and the hydroperoxyl radical (HO2).Recent evidence shows that lipid peroxides play a major role as mediators in various conditions, such as neurodegenerative diseases and cancer.Furthermore, lipid peroxidation may be the most important downstream characteristic of ferroptosis[1, 8].
Ferroptosis
Ferroptosis is a non-canonical kind of cell death that is characterized by iron-dependent accumulations of lipid peroxides.The excess iron is produced through the Fenton reaction.However, this isn't the only significant factor in ferroptosis.NADPH oxygenase, P53 as well as GSH depletion are all positive factors that regulate ferroptosis.They enhance ROS production and block the expression of SLC7A11.
GPX4 plays an important role in the development of ferroptosis through inhibiting the production of lipid peroxides.GPX4 converts GSH into an oxidized Glutathione (GSSG) and decreases the cytotoxic lipid oxides into lipid alcohols.A reduction in the GPX4's activity could result in an accumulation of lipid oxides, an indicator for ferroptosis.The collapse of the glutathione (GSH)-glutathione peroxidase 4 (GPX4) antioxidant system may be a key driver of ferroptosis.
Emerging research looking at the relationship between ferroptosis and lipid metabolism in breast cancer has provided insight into how changes in lipid metabolism control the development, metastasis, and treatment resistance of cancer [9].
Ferroptosis inducers
Radiation is commonly used in the treatment of cancers,but resistance can develop and generally involves the activation of DNA repair and apoptosis inhibition.As a result, drugs that sensitize cancer cells to radiation via different cell death pathways are important.
Ferroptosis is a type of nonapoptotic cell death induced by lipid peroxidation, is somewhat responsible for cancer cell death induced by radiation treatment.
The use of ferroptosis inducers increased the antitumor effect of radiation in both a human patient-derived model and murine models of glioma and lung adenocarcinoma[10].
Inflammation and Lipid Peroxidation
The ability of lipids to signal the pro-inflammatory or anti-inflammatory pathways depends on the length of their fatty acid chains, the amount of un-saturations, and the location where the oxidation occurs.
During lipid peroxidation, oxygen molecules are joined to the unsaturated fatty acyl chains of non-polar lipids, increasing their water solubility and enhancing diffusion.This increases the accessibility of COX-2 and 5-LOX to their substrates, stimulating inflammation-related lipid compounds and encouraging interaction between proteins and receptors that recognize lipid oxidation products[11].
The microenvironment of inflamed tissue contains cells which produce mediators such as cytokines and chemokines as well as growth factors, ROS, RNS, remodeling enzymes, and neuropeptides.These mediators orchestrate the recruitment and maintenance of new cells at the inflammatory site.Although this happens mainly within the tissue, it can also have systemic effects on distant organs.
During chronic inflammation, excess DNA-reactive aldehydes are produced, which can destabilize cellular homeostasis and lead to DNA damage and malignancy.Studies show that excessive copper or iron storage causing LPO-derived DNA damage is implicated in disease pathogenesis.Epsilon DNA adducts may be used as potential markers to assess the progression of inflammatory cancer-prone conditions[12].
The cyclooxygenase enzyme produces arachidonate-metabolites in macrophages as well as other cell types.There are two forms of cyclooxygenase currently: COX-1, the constitutive, and COX-2, the inducible.COX-2 is increased in cancer.The lipid oxidation signatures created by stimulation of 12-LOX and 5-LOX activities and COX-2 activities are cancer biomarkers.Inhibitors have anti-tumoral or anti-proliferative properties [13].
Redox Homeostasis/ Balance
The pathology of cancer is defined by a redox imbalance and the shift to oxidative conditions.Lipid peroxidation has been implicated in the progression of many cancers due to the increase in lipid peroxidation-related products seen in these cancers [14].
Reactive aldehydes
Cancer cells undergo a constant turnover of lipids which stimulates the cytotoxic effects of lipid peroxidation and the growth-regulating reactions of reactive aldehydes [2].Reactive aldehydes have been identified to play a role in intracellular signaling and gene activation within cancer cells.This is crucial for the development of cancer since malignant cells usually have less antioxidant capabilities compared with healthy cells [15].
The progression of cancer may be affected by lipid peroxidation products.This can happen either directly, through modulation in cancer cells behavior or via modulation in the immune response.The modulation of inflammation, and the immune response, is also a key role of oxidative modified molecules (Lipoxidation adducts).They are capable of inducing adaptive immunity and may be involved in the pathogenesis of many diseases[16].
Dichotomous nature of ROS
ROS has a dichotomous nature depending on the grade of cancer development,for example,early or late stage,based on the differential influence of ROS in tumor cells during progression [17].Moderate ROS levels in the pre-cancerous or early stages of tumor progression induce tumorigenesis, metastasis, and survival [18].
During tumor progression, raised ROS levels outside the toxic threshold precede apoptosis, cell death, and senescence.ROS's abnormal production precedes cancer growth and advancement via numerous signaling pathways (MAPK, MMPs, PI3/Akt/mTOR, PTEN,and VEGF/VEGFR).It has been shown that ROS levels can significantly increase, thereby affecting the progression of cancer,kick-starting apoptosis, and promoting cell death when used in traditional therapies, such as chemotherapy [19].
HNE in the Tumor Microenvironment
HNE triggers apoptosis through JNK activation, increasing the activation of other TGF-B1 pathways in tumor cells.Therefore tumor cells can become resistant to TGF-B1-mediated growth inhibition, thus contributing to inhibition of tumor growth [2].The carcinogenic and mutagenic effects on lipid peroxidation have been implicated in large part by 4-HNE.Strategies that focus on manipulating mitochondrial ROS production, lipid peroxidation, and 4-HNE formation might be therapeutic in treating or preventing cancer.However, a variety of cancers are associated with elevated levels of oxidative stress[7].
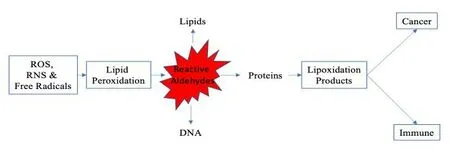
Figure 1.The formation of lipoxidation adducts and the possible effects they may have on the progression to cancer.
ROS, RNS & Free radicals.Oxidants are formed from the mitochondrial respiratory chain, plasma membrane endoplasmic-reticulum, and peroxisomes.
Lipid peroxidation.Oxygen radicals attack lipids giving rise to the formation of lipoperoxyl radicals (LOO) and lipid hydroperoxides(LOOH).The polyunsaturated fatty acids (PUFAs) are the main targets of oxygen radicals.
Reactive aldehydes.Oxidized lipids decomposed to form reactive aldehydes.The predominant omega-6 fatty acid Arachidonic Acid(AA) can be reduced by peroxidation to malondialdehyde (MDA) and 4-hydroxynoneal (4-HNE).These aldehydes can react with amino and thiol groups to form lipid-protein adducts or lipoxidation products.
Lipoxidation products.High levels of 4-hydroxynoneal (4-HNE) can react with proteins or DNA, resulting in genotoxic effects.HNE triggers apoptosis through JNK activation, increasing the activation of other TGF-B1 pathways in tumor cells.Therefore tumor cells can become resistant to TGF-B1-mediated growth inhibition, thus contributing to inhibition of tumor growth.The carcinogenic and mutagenic effects on lipid peroxidation have been implicated in large part by 4-HNE.Malondialdehyde (MDA) can react with biomolecules such as proteins or DNA and generate MDA-DNA adducts.When cellular repair mechanisms are overwhelmed, MDA-DNA adducts can lead to mutations, strand breaks, cell cycle arrest, and the induction of apoptosis.
Lipoxidation and Autoimmune Disease
Patients with active autoimmune diseases have a higher level of lipid peroxidation as measured by HNE and MDA concentrations [20].
Systemic lupus erythematosus (SLE).Systemic lupus erythematosus(SLE) suffers who have active SLE have significantly lower HNE levels than the controls [21].
Studies have shown that MDA may contribute to disease pathogenesis by altering the immunogenicity of self-molecules,eliciting an immune response.High levels of MDA-specific antibodies have been found in the complement-fixing IgG2a and IgG2b subclasses[22].
Children with high disease activity of SLE have also been found to have significantly higher concentrations of the aldehyde modified protein [21].
Autoimmune Disease and cancer risk
Autoimmune rheumatic diseases can raise the risk of cancers, such as lymphoma, lung cancer, and human papillomavirus-associated tumors[23].It is suspected that people with autoimmune diseases have altered immune responses that could make them more susceptible to malignancies.Numerous studies have shown a link concerning gastric cancer and chronic autoimmune gastritis [24].
Trace minerals and Lipid peroxidation in cancer
Liver Cancer.Studies have shown that non-alcoholic fatty liver disease(NAFLD) is linked to a raised risk of liver cancer, primarily hepatocellular carcinoma (HCC).Supplementation with Zinc (Zn) and Selenium(Se) has been shown to decrease lipid oxidation.The serum ALT/AST levels of Zn and Selenium (Se) were lower in the before and after supplementation groups (BS & AS) than in the model group(M).The AS group had significantly lower malondialdehyde (MDA),fasting plasma glucose(FPG), insulin, VEGF, and HOMA-IR levels than the M group.In an experimental model, Zn and Se supplementation following non-alcoholic fatty liver disease progression (NAFLD)reduced risk indicators[25].
Cervical Intraepithelial Neoplasia.In patients with Cervical Intraepithelial Neoplasia (CIN) and cancer, serum levels of Se, Zinc,and lower ratios of copper (Cu)/Zn were higher compared with controls.Serum total antioxidant status (TAS) and GSHPx activity were decreased in the CIN group.
Serum malondialdehyde levels decreased the most in the CIN group compared to the cancer and control group, highlighting the involvement of reactive oxygen species during pre-cancerous phases.The association of lipid peroxidation along with decreases in Se and Zn levels and a weakened serum antioxidant system have been implicated in the pathogenesis of cervical dysplasia[26].
Prostate cancer.Alterations of trace minerals levels may be related to several diseases, including metabolic syndrome, cellular growth disturbance, cellular mutation, and tumorigenesis.Serum blood levels of Zn, Se, and Manganese (Mn) have been shown to be reduced in those with prostate cancer in comparison to the control groups.
A correlation between trace mineral levels and prostate cancer has been suggested.Reduced levels of Zn, Se, and Mn and an increase of Fe and Cu levels can affect the initiation of prostate cancer significantly [27].
Research was conducted to assess the impact of the following minerals (selenium, iron, zinc, copper, and calcium) on the advance of the neoplastic process and the concentrations of chosen biomarkers in prostate cancer cells from oxidative damage.It was found that supplementation with chosen minerals (selenium, iron, zinc, copper,and calcium) influences prostate tumor growth.Lipid peroxidation has been shown to be a successful method for early-stage cancer prevention [28].
Botanical Support
Hormesis and Botanicals
Hormesis is used to describe a biphasic dose-response to a compound characterized by high-dose inhibition and low-dose stimulation, and this knowledge is vital for optimal treatment protocols.Hormesis isa fundamental dose-response concept relationship in medical sciences,nutrition, and toxicology.
A noteworthy dose-response characteristic is that it can occur mainly in tumor cells and is independent of the organs.Research has shown that botanical compounds display this biphasic dose-response,in which the low dose activates signaling pathways, antioxidant enzymes, and cytoprotective proteins.Some of the compounds that have shown this effect are Vitamin C, curcumin, and resveratrol [29].
Vitamin C, which is a well-known antioxidant at low doses, also displays pro-oxidant effects in cancer and selectively destroys cancer cells at high doses.Researchers have found that the high doses of Vitamin C generates ROS, which then influences cytochrome c release in mitochondria and lastly precedes to apoptosis[30].
Curcumin has been shown in many cancer cells, such as breast,prostate, and colon, to behave hermetically, at low doses, antioxidant actions, and then at high doses, it induces autophagy and cell death[31].
Clinical trials will ultimately be needed to verify these effects in humans.For example, curcumin is currently subject to more than 200 clinical trials, which will further deepen our understanding of it.A challenge to get this effect in humans is the absorption of these compounds orally to reach the amounts needed in the body to have the pro-oxidant effects.Intravenously or nano-type delivery models will need to be studied to confer these important effects.
ROS and Lipid Peroxidation Inhibitors
Resveratrol
The age of oncologic patients is decreasing, and the use of natural compounds is currently being discussed as a preventative measure.Resveratrol, a polyphenol extracted from Polygonum cuspidatum and grapes, has therapeutic anti-inflammatory, antioxidant, and anti-cancer effects.
Certain changes occur in the metabolism of lipids in tumor cells.Resveratrol alters cancer's metabolism by affecting cell proliferation,signaling, and invasion, as well as the storage of energy [32].In a Breast cancer in vivo model, resveratrol was shown to decrease lipid peroxidation, significantly inhibit 5-LOX activity, and prevent DNA damage[33].
Genistein
The principal anticancer mechanisms of genistein include chemoprevention effects and inhibition of carcinogenesis effects.The chemoprevention effects include decreases of COX-2 and Oxidative stress.Inhibition of carcinogenesis effects includes increases in apoptosis, regulation of epigenetic changes, G2/M cell cycle arrest,autophagic cancer cell death and decreased invasion, cancer cell proliferation, tumor angiogenesis, metastasis, and activation of survival [34].
Cancer and heart disease have been shown to be associated with oxidative damage.
Soy phytoestrogens (genistein and daidzein) reduce plasma F2-isoprostane concentrations (lipid peroxidation products) and raise the levels of low-density lipoprotein oxidation resistance.Phytoestrogen’s potential antioxidants effects contribute to the therapeutic efficacy in oxidative-related diseases[35].
In a doxorubicin-induced cardiotoxicity model, genistein significantly decreased redox markers such as LPO, ROS, and HNE levels.Genistein also reduced IL-6, IL-8, and TNF-α expression and controlled antioxidant response by increasing Nrf-2, Heme oxygenase-1, NAD(P)H:quinone acceptor oxidoreductase 1 protein expressions[36].
Protopanaxadiol
Protopanaxadiols (PPD) is classed as an Ginsenoside from ginseng and can include the ginsenosides Rb1, Rb2, Rc, and Rd.Protopanaxatriols,the other major ginsenosides group, can include Re, Rf, and Rg1.
Protopanaxadiols were shown to reduce lipid peroxides and MDA plasma counts in liver inflammation.Protopanaxadiol such as R1 and R2 were shown to regulate the expression of cyclooxygenase(COX)-2,IjB-a, phopho-ERK 1/2, and phopho-SAPK/JNK levels and was significant in blocking apoptotic signals (caspase-8, -9) in the liver[37].
The PPD group of ginsenoside from Ginseng was found to possess antioxidant, anti-proliferative, and antigenotoxic effects.PPD reduced DNA damage from oxidative stress caused by HNE [38].
In a colorectal cancer cell model, PPD significantly altered gene expression, increased Sestrin2 expression, activated AMPK, and induced autophagy.The results suggest that the ginseng metabolite activates the stress-sensing kinases GCN2 and PERK to induce Sestrin2 expression, promoting AMPK activation, autophagy, and metabolic health [39].
Si-Miao-San
Si-Miao-San (SMS) is a formula of four herbs: Cortex Phellodendri,Radix Achyranthis Bidentatae, Semen Coicis, and Rhizoma Atractylodis.Rhizoma Coptidis, due to its potent anti-inflammatory activity, has gradually replaced Radix Achyranthis Bidentatae as one favored component of the modified SMS formulation.
SMS and mSMS are suggested to be significant antioxidants and anti-inflammatory agents through DPPH radical scavenging,tyrosinase, and LPO inhibition assays[40].
Si-Miao-San (SMS) has been shown to decrease the production of COX-2, IL-6, PGE2, and TNF-α and reduce the production of anti-collagen type II antibodies IgG1 and IgG2a.Importantly, while SMS significantly reduced levels of MPO and MDA, the levels of SOD and CAT increased.SMS also up-regulated the levels of Nrf2, NQO1,HO-1, and PTEN [41].
Phellodendri Cortex (PC) may induce high superoxide scavenging activity [42].PC significantly delayed the progression of prostate cancer tumors using a xenograft model [43].
Berberine, an isolate from both Cortex Phellodendri and Rhizoma Coptidis, has a wide range of pharmacological effects, for instance,free radical scavenging anti-carcinogenic and anti-apoptotic actions.Berberine has been shown to correct numerous markers in oxidative stress and has reduced raised enzymes, aspartate aminotransferase,alanine aminotransferase, and alkaline phosphatase during liver injury studies [44].
Modified Si-Miao-San (mSMS)
An over-production of mediators such as nitric oxide, prostaglandins and reactive oxygen species (ROS), and pro-inflammatory cytokines like TNF-a, IL-1, and IL-6 is a hallmark of inflammation.Research has shown that mSMS extract inhibits the production of inflammatory mediators like NO, TNF-a, and IL-6 through modulating extracellular signal-regulated proteins kinase(ERK) and nuclear transcription factor(NF-KB).
mSMS has been found to significantly inhibit NO production.mSMS is a potent inhibitor of inflammation, hindering the release of inflammatory mediators from macrophages and reducing the overexpression of relative genes.It inhibited inflammatory response in macrophages, and this activity should be relative with its blockade of ERK/NF-KB mediated-inflammatory pathway(IBED) [45].
Taraxacum officinale
In 3T3-L1 preadipocytes, Taraxacum officinale was able to hinder adipocyte differentiation and lipogenesis, resulting in a reduction in triglycerides, LDL-C, and total cholesterol and a rise in HDL-C levels[46].
Aqueous extract of Taraxacum officinale was shown to be a radical scavenger through its inhibitory activities on the creation of MDA produced through the oxidation process and displayed a strong inhibitory action in lipid peroxidation.The levels of GSH, which plays a vital role in scavenging ROS, were also increased in the study [47].
An ethanolic Taraxacum officinale extract reduced malondialdehyde, total oxidative status, oxidative stress index subject to the dose.The extract also decreased serum levels of total nitrites and nitrates and 3-nitrotyrosine, signifying a significant inhibition on nitric oxide production [48].
Zingiber officinale
Zingiber officinale inhibits high acetylcholinesterase activity, thereby reducing lipid peroxidation.Research has shown a significant inhibitory effect on lipid peroxidation compared to placebo [49].
Evening primrose oil
Evening primrose oil affects the glutathione antioxidant-dependent defense system by reducing glutathione peroxidase and increasing glutathione reductase and transferase, thereby targeting lipid peroxidation parameters such as malondialdehyde which was significantly reduced [50].
Eugenia jambolana
Eugenia jambolana improves HMG-CoA reductase(hydroxy-3-methylglutaryl-coenzyme A reductase) activity which maintains lipid homeostasis.The active compounds of Eugenia jambolana include flavonoids, triterpenoids, and saponins, and glycosides [51].
Eugenia jambolana pulp extract was shown to inhibit lipid oxidation induced from iron in numerous organs, such as the liver and its mitochondria,brain,and the testes,in a study using a DPPH radical scavenging assay.The extract had high antioxidant activity at very low levels[52].
ROS and Ferroptosis Inducers
Artemisinin
The anticancer activities of Artemisinin (ARS) have been shown in both in-vivo and in-vitro research.Similar to other natural compounds,ARS acts through various pathways against hematological malignancies.
Artemisinin induces the oxidative stress response and proliferation inhibition in multiple myeloma, lymphoma, and leukemia cells.ARS also stimulates signal transducers like MYC or NF-kB and induces multiple classes of cell death such as ferroptosis and apoptosis,ARS-type drugs induce oxidation and can lead to DNA damage and breaking of double-strands.Thus, many studies have shown the additive or synergistic effects of ARS-type drugs with standard chemotherapies[53].
Resveratrol
In malignant melanoma cells, resveratrol induced ROS via the p38-p53 pathway, which then produced Endoplasmic reticulum (ER)stress and dysfunction of the mitochondria and led to the apoptosis of the melanoma cells [54].
In a highly aggressive malignant tumor cell line, resveratrol increased the production of ROS and oxidative damage in the tumor cells.The decreased levels of Superoxide dismutase 2, catalase, and sulfotransferase, were most likely accountable for the ROS levels in the resveratrol-sensitive tumor cells [55].
Resveratrol treated Ovarian Cancer Stem Cells produce an excessive amount of intracellular ROS, damaging the self-renewal ability and cell death in both dependant and independent ROS pathways [56].
6-Gingerol
6-Gingerol is a major phenol phytochemical component in ginger that may be beneficial in the treatment of tumors and the reduction of inflammation.
The pharmacological actions of 6-gingerol are mediated through the essential pathways of cell signaling, including Bax/Bcl2, Nrf2,p38/MAPK, ERK1/2, TNF-a, SAPK/JNK, ROS/NF-kB/COX-2,caspases-3,-9, and p53 [57].
Supplementation of 6-Gingerol was found to reduce the expression of proteins nuclear receptor coactivator 4 (NCOA4).In addition,6-Gingerol highly increased iron, ROS, and autophagosomes, which significantly decreased the survival and proliferation rate for A549 cells and led to a significant reduction in tumor volume and weight[58].
Omega-3 PUFA alone and with vitamin E
Omega-3 PUFA alone and with vitamin E can increase lipid peroxidation,which is linked with an increase in oxidative stress[59].
Research has shown that n-3 and n-6 PUFAs accumulate in lipid droplets (LD) of acidic cancer cells and undergo peroxidation to induce ferroptosis.Diglyceride acyltransferase (DGAT) inhibitors may benefit by preventing the formation of lipid droplets and promoting ferroptosis.
PUFA’s have been found to preferentially induce ferroptotic cell death under acidosis in proportion to the number of double bonds,suggesting that LDs might represent a strategy to divert the excess of captured PUFAs from lipid peroxidation [60].
Fish oil has numerous cancer modulating properties, including the reduction in raft-associated signal transduction, stimulating the BAD/AKT survival pathway, and decreasing oxidative-stress-induced endothelial ca2+influx.Also, fish oil reduces inflammation due to the effects of E-resolvins (RvE1 & RvE2), D-resolvins (RvD1 & RvD2), and protectin PD1 on COX and LOX pathways[61].
Evening primrose oil
In a topical two-stage skin carcinogenesis model, Evening primrose oil inhibited the promotion phase of skin papilloma creation through increasing lipid peroxide formation.In part by preventing the binding of benzo(a)pyren to the DNA of the skin cell without having an effect on skin cell proliferation [62].
Conclusion
In summary, Lipid peroxidation is an important mechanism involved in pathological processes, including cancer.Its ability to modify the immune response can be a negative detriment to cancer patients.Further research is needed to find an adequate amount of evidence for the use of botanicals in the development of oncology strategies targeting lipid peroxidation for the benefit of improving oncology care.
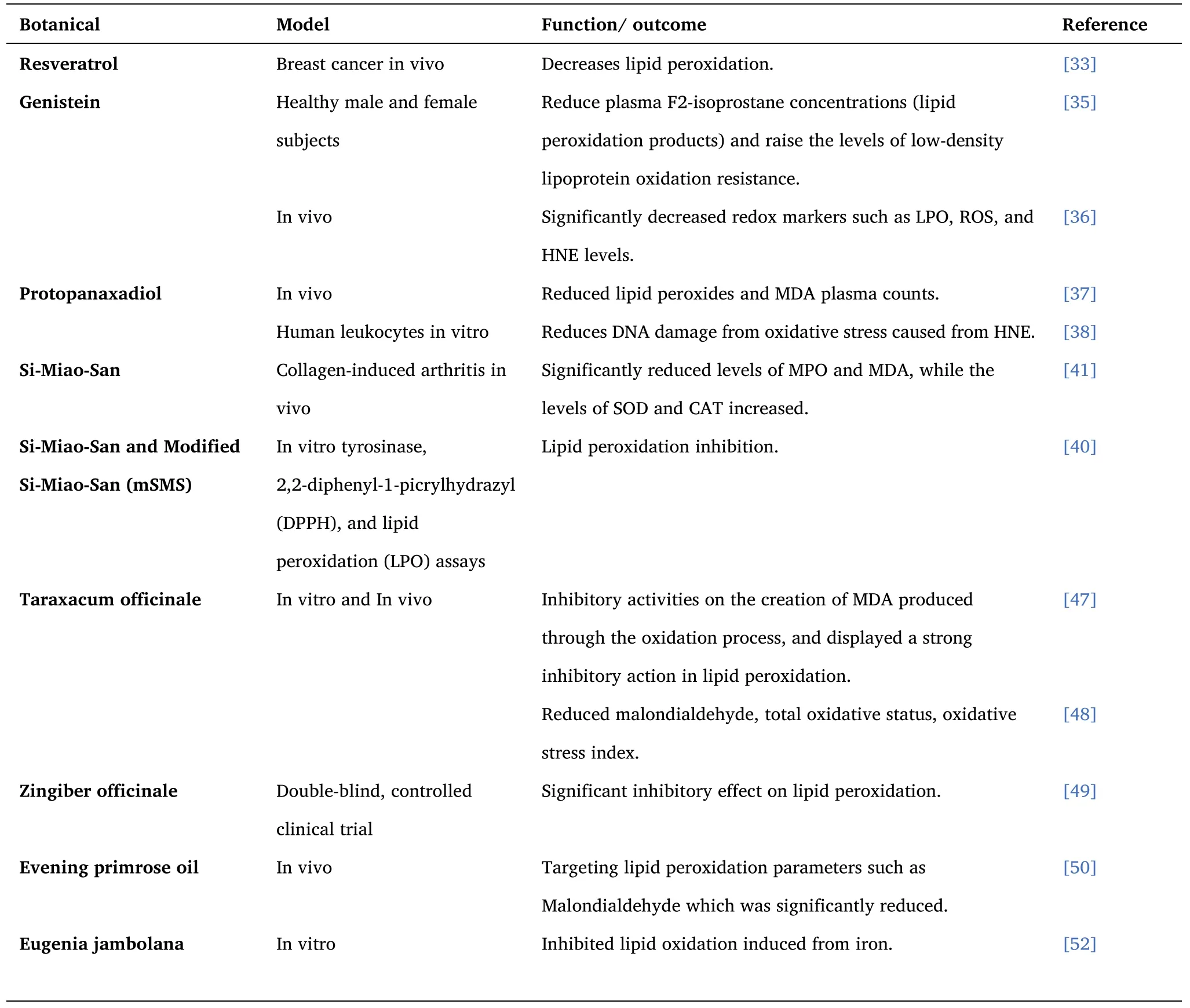
Table 1 ROS and Lipid Peroxidation Inhibitor - Botanicals
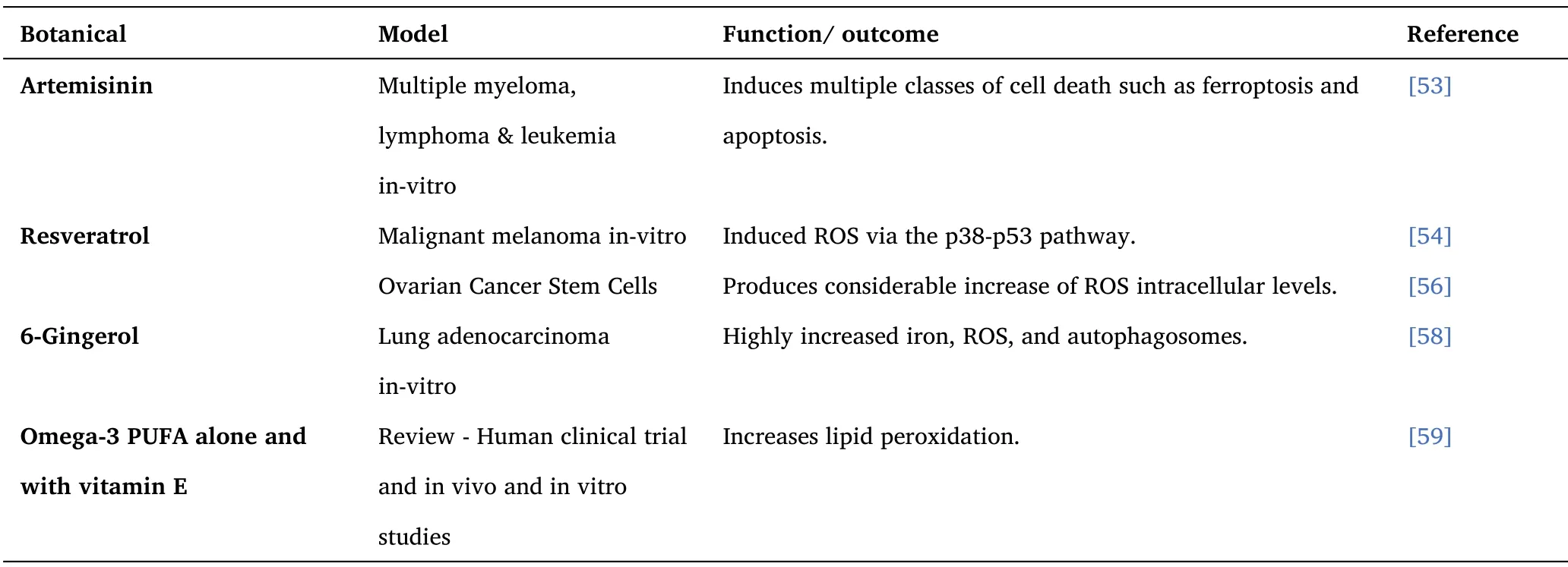
Table 2 ROS and Ferroptosis Inducers - Botanicals
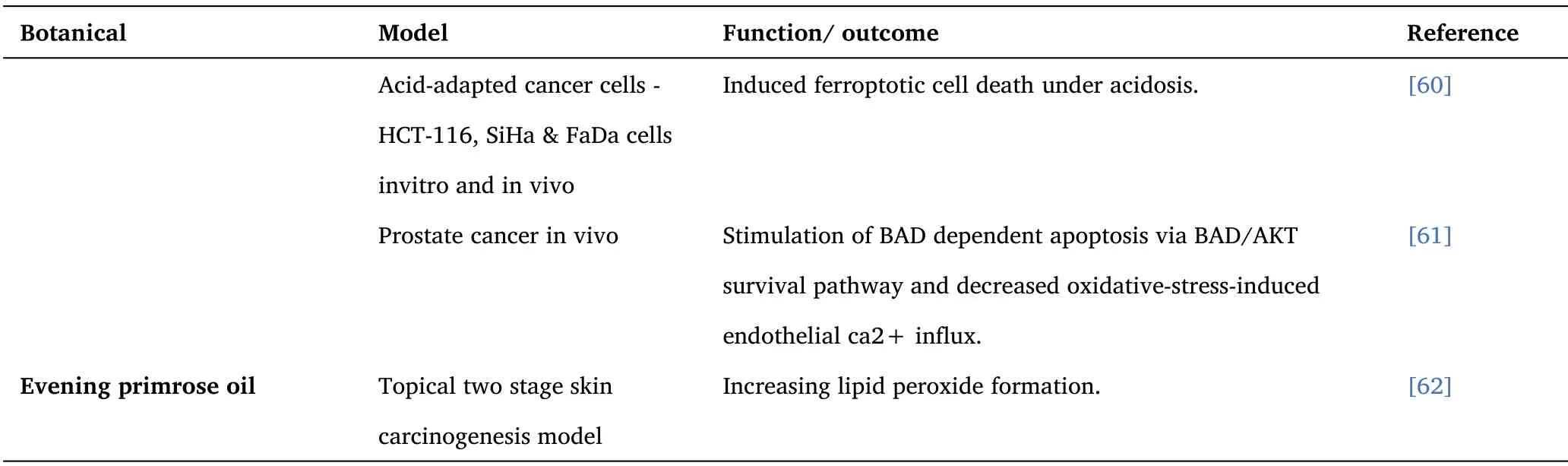
Table 2 ROS and Ferroptosis Inducers - Botanicals(Continued)
- Cancer Advances的其它文章
- Fibrous histiocytoma of the spermatic cord 1 case and review of the literature
- Study on the Correlation between Syndrome Differentiation of Malignant Pleural Effusion Treated by External Treatment of Traditional Chinese Medicine and Immunohistochemistry of Biopsy Tissue Based on Medical Video-assisted Thoracoscope
- Reporting Quality Assessment of systematic reviews on Quality of Life in Patients with Enterostomy in China
- "Endogenous wind"Theory theory in the prevention and treatment of anti-tumor treatment-related adverse reaction related research progress