Cell-free DNA liquid biopsy for early detection of gastrointestinal cancers:A systematic review
Isabelle Uhe,Monika Elisabeth Hagen,Frédéric Ris,Jeremy Meyer,Christian Toso,Jonathan Douissard
Isabelle Uhe,Monika Elisabeth Hagen,Frédéric Ris,Jeremy Meyer,Christian Toso,Jonathan Douissard,Abdominal Surgery Division,Geneva University Hospitals,Geneva 1211,Switzerland
Abstract BACKGROUNDGastrointestinal tumors are among the most common cancer types,and early detection is paramount to improve their management.Cell-free DNA(cfDNA)liquid biopsy raises significant hopes for non-invasive early detection.AIMTo describe current applications of this technology for gastrointestinal cancer detection and screening.METHODSA systematic review of the literature was performed across the PubMed database.Articles reporting the use of cfDNA liquid biopsy in the screening or diagnosis of gastrointestinal cancers were included in the analysis.RESULTSA total of 263 articles were screened for eligibility,of which 13 articles were included.Studies investigated colorectal cancer(5 studies),pancreatic cancer(2 studies),hepatocellular carcinoma(3 studies),and multi-cancer detection(3 studies),including gastric,oesophageal,or bile duct cancer,representing a total of 4824 patients.Test sensitivities ranged from 71% to 100%,and specificities ranged from 67.4% to 100%.Pre-cancerous lesions detection was less performant with a sensitivity of 16.9% and a 100% specificity in one study.Another study using a large biobank demonstrated a 94.9% sensitivity in detecting cancer up to 4 years before clinical symptoms,with a 61% accuracy in tissue-of-origin identification.CONCLUSIONcfDNA liquid biopsy seems capable of detecting gastrointestinal cancers at an early stage of development in a non-invasive and repeatable manner and screening simultaneously for multiple cancer types in a single blood sample.Further trials in clinically relevant settings are required to determine the exact place of this technology in gastrointestinal cancer screening and diagnosis strategies.
Key Words:Cell-free DNA;Tumor DNA;Liquid biopsy;Next-generation sequencing;Cancer genomics;Pancreatic cancer;Colorectal cancer;Hepatocellular carcinoma;Multicancer detection;Cancer screening;Public health;Precision oncology
INTRODUCTION
Tumors developing from the gastrointestinal tract are among the most common cancer types,colorectal and stomach cancer,counting for 19.5% and 11.1% respectively worldwide in 2020[1].Risk factors notably include smoking,obesity,poor diet,genetic factors,and infections with hepatitis B virus or Helicobacter pylori bacteria[2].Early detection and diagnosis represent a crucial component to allow effective treatment and improve survival.Nowadays,different screening strategies have been developed,such as colonoscopy for colorectal cancer or blood testing for alpha-fetoprotein(AFP)or magnetic resonance imaging in high-risk patients for liver cancer,but other types of tumors often lack screening strategies and non-invasive testing.For instance,so far,no efficient screening methods are available for pancreatic cancer;most patients experience their first symptoms at advanced and metastatic stages,explaining the 5-year survival rate of only 5% to 10%[3].
These past few years,researchers have focused their attention on a new promising diagnostic method,liquid biopsy,which uses biomarkers such as circulating cell tumor,RNA fragments,or cell-free DNA(cfDNA).Unlike tissue samples obtained by invasive methods like needle biopsies or endoscopies,biomarkers can be detected in body fluids,mostly blood[4],and address limitations of tissues biopsies not only in diagnosis and screening,but also in diagnosis and screening the treatment response and follow-up[5-7].Among liquid biopsy options,cfDNA raises the most significant hopes in early cancer detection.Historically,it was first reported in 1948 by Mandelet alamong healthy patients.In 1977,Leonet aldescribed elevated levels of cfDNA in the serum of cancerous patients for the first time[4,8,9].CfDNA is continuously released in the bloodstream through different mechanisms such as apoptosis,necrosis,and active secretion by the tumor cell.When originating from a cancer cell,cfDNA is called circulating tumor DNA(ctDNA)[4].Concentration levels seem to correlate with the cancer stage and size;advanced-stage cancer patients show a higher concentration of cfDNA[8,9].While cfDNA quantification in the bloodstream might indicate the presence or absence of cancer,sequencing and analyzing the mutation patterns of this cfDNA goes one step further:mutational profiling might give the researchers clues on the tumor’s tissue of origin,providing information to target further specific investigations[9].Recent progress in genomic technology also provides highly sensitive detection of low-prevalence mutations,even in high signal-to-noise configurations,thus theoretically enabling very early cancer diagnosis.The ability to run repeatable,non-invasive,multi-cancer early detection tests would bring significant advantages in the global care of frequently hardly reachable cancer locations,such as gastrointestinal cancers.
The present systematic review of the literature aims to describe the current state of developing cfDNA liquid biopsies as a means of early gastrointestinal cancer detection and screening.
MATERIALS AND METHODS
A systematic review of the literature was performed following the PRISMA guidelines[10].All articles written in English from January 2010 to January 2021 were searched on January 19th,2021,through the PubMed database using the following research algorithm:(liquid biopsy OR cfdna)AND(multiple OR gastrointestinal OR colon OR colorectal OR gastric OR oesophag* OR liver OR hepatocellular OR pancreatic)AND(cancer OR tumor OR tumour)AND(screening OR diagnos* OR detect*)AND early AND(blood OR venous OR plasma)NOT review.
After a first selection based on titles for screening,eligible articles were selected based on abstract analysis.Then,full-text analysis of the eligible articles searched for criteria of the finally included articles.Two investigators(I Uhe,J Douissard)independently assessed the articles for eligibility and inclusion.Discordances in study inclusions were solved by re-evaluation between the two reviewers.
All relevant articles reporting human studies investigating cfDNA liquid biopsy as a screening method or diagnosis method for newly discovered untreated primary gastrointestinal cancers were included.Studies investigating multiple cancer screening,including gastrointestinal but not limited to them,were also included.Excluded articles were studies investigating cfDNA as a follow-up method after cancer treatment,minimal residual disease detection,studies investigating cfDNA as a prognosis method only,reviews,meta-analyses,theoretical papers,and biological studies not reporting clinical outcomes.Studies reporting cancer patients who were already treated,surgically or medically,have also been excluded.To improve the present review’s clinical relevance,only the total number of participants in the papers’ validations cohorts were considered.If available,test performances were reported in terms of sensitivity(Se),specificity(Sp),positive and negative predictive values,or area under the curve(AUC).
Literature search and studies characteristics
A total of 263 articles were identified through the PubMed search.Two articles were not written in English,11 were not original publications,and 119 did not involve cfDNA.Thirty-five articles did not mention gastrointestinal cancer,and 44 did not investigate cfDNA as a screening or diagnosis method,leaving 52 articles.After fulltext reading,thirteen studies were ultimately included for analysis,representing a total of 4824 patients(Table 1,Figure 1).The largest study included blood samples from 1194 participants[11],while the smallest study included samples of 130 participants[12].Six studies took place in China[11,13-17],three in the United States[9,18,19],and four in Europe[12,20-22].Five were multicentric[9,11,16,18,19],four monocentric[13,14,17,22]and four studies did not mention the information.Five studies focused on colorectal cancer(CRC)[9,12,17,20,22],three on various cancer types[14,19,21]of which two included gastric cancers[14,19],three on hepatocellular carcinoma(HCC)[11,15,16]and two on pancreatic ductal adenocarcinoma(PDAC)[13,18].All studies compared cancer and non-cancer individuals.Five of them also included in their analysis a group of patients with pre-cancerous lesions,such as colorectal adenoma or hyperplasia,liver cirrhosis,or chronic hepatitis B virus infection[11,12,15,16,22](Table 2).
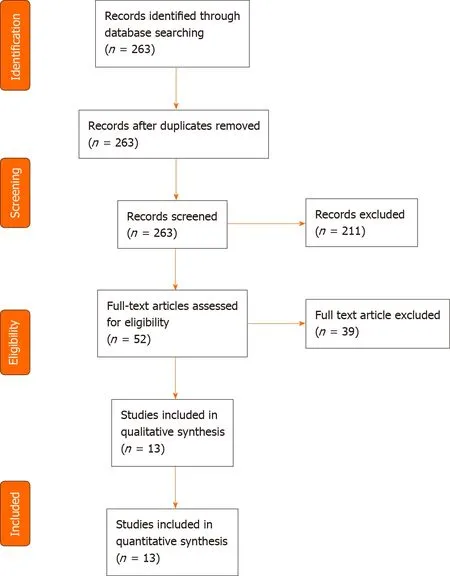
Figure 1 PRISMA flow diagram summarizing the search strategy.
Risk of bias of included studies
The risk of bias of included studies was determined using the ROBINS-I tool(2016)[23].Except for one study with an overall low risk of bias[16],all included studies were at moderate risk(Table 3).
Extraction and sequencing methods
All studies collected cfDNA from plasma samples.Kits used for cfDNA extraction from plasma samples can be found in Table 4.The QIAamp circulating nucleic acid kit was the most employed,a spin column-based kit(n=7/13).A large majority of studies used next-generation sequencing(NGS)(n=9/13),two used real-time polymerase chain reaction(RT-PCR),one digital droplet PCR,and one multiplexmethylation-specific PCR.Various mutational patterns and genomic profiling strategies were investigated(Table 4).Most studies focused on methylation variations(n=7/13),while others investigated specific mutation locations such asKRASandBRAFor more complex mutational patterns.
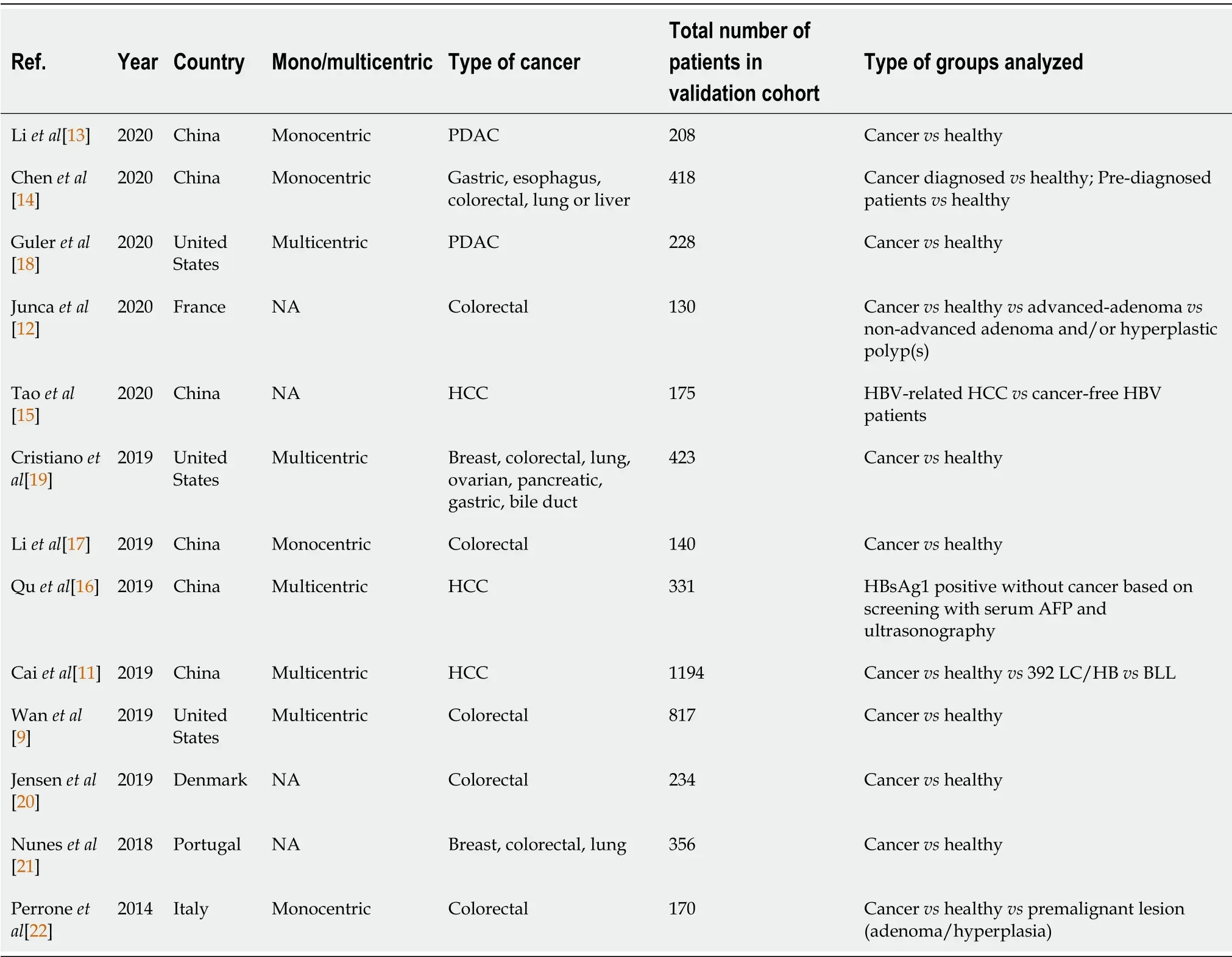
Table 1 Characteristics of included studies
Tests performance
Overall test performances for each cancer subgroup are described in Table 5.
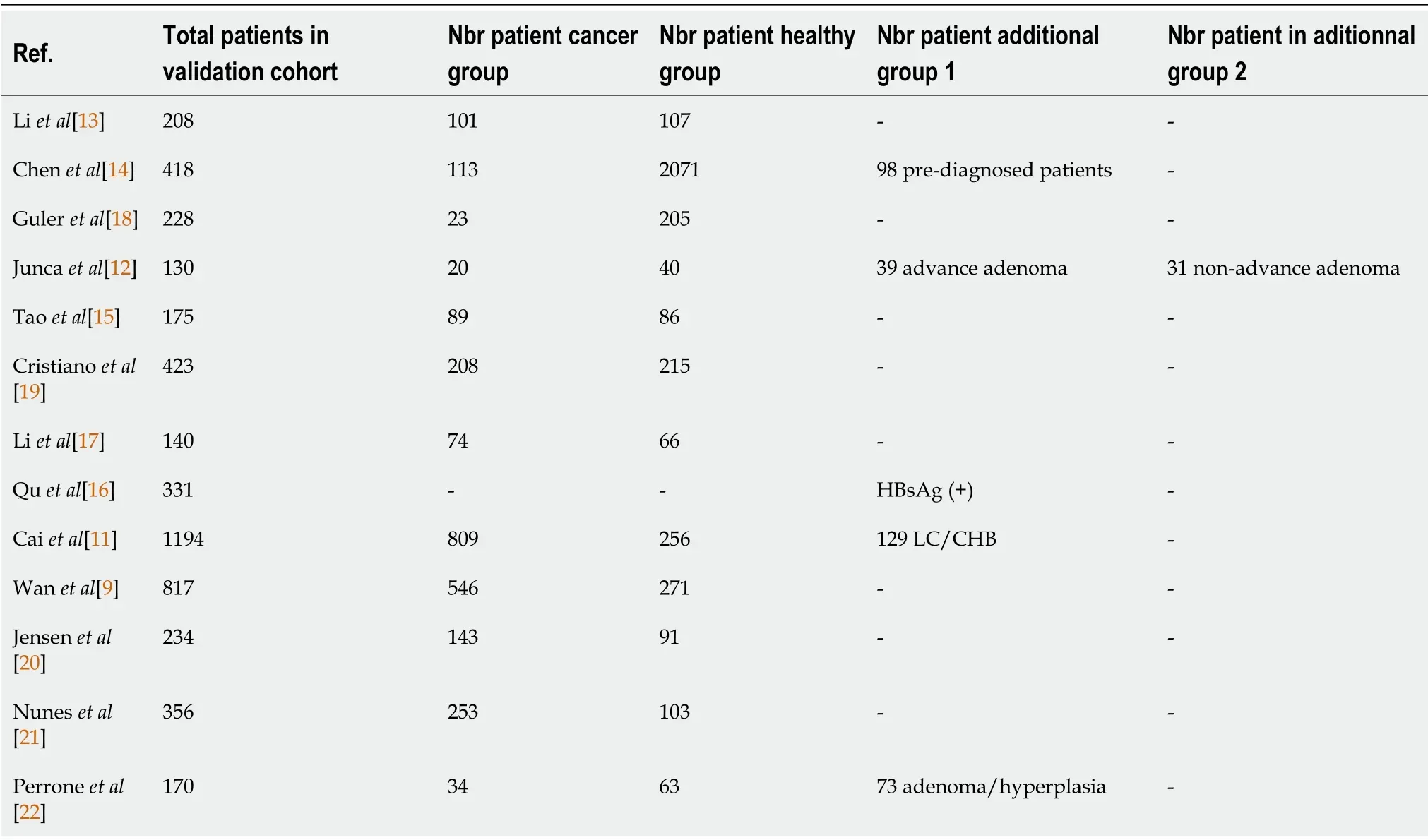
Table 2 Number of patients in each group
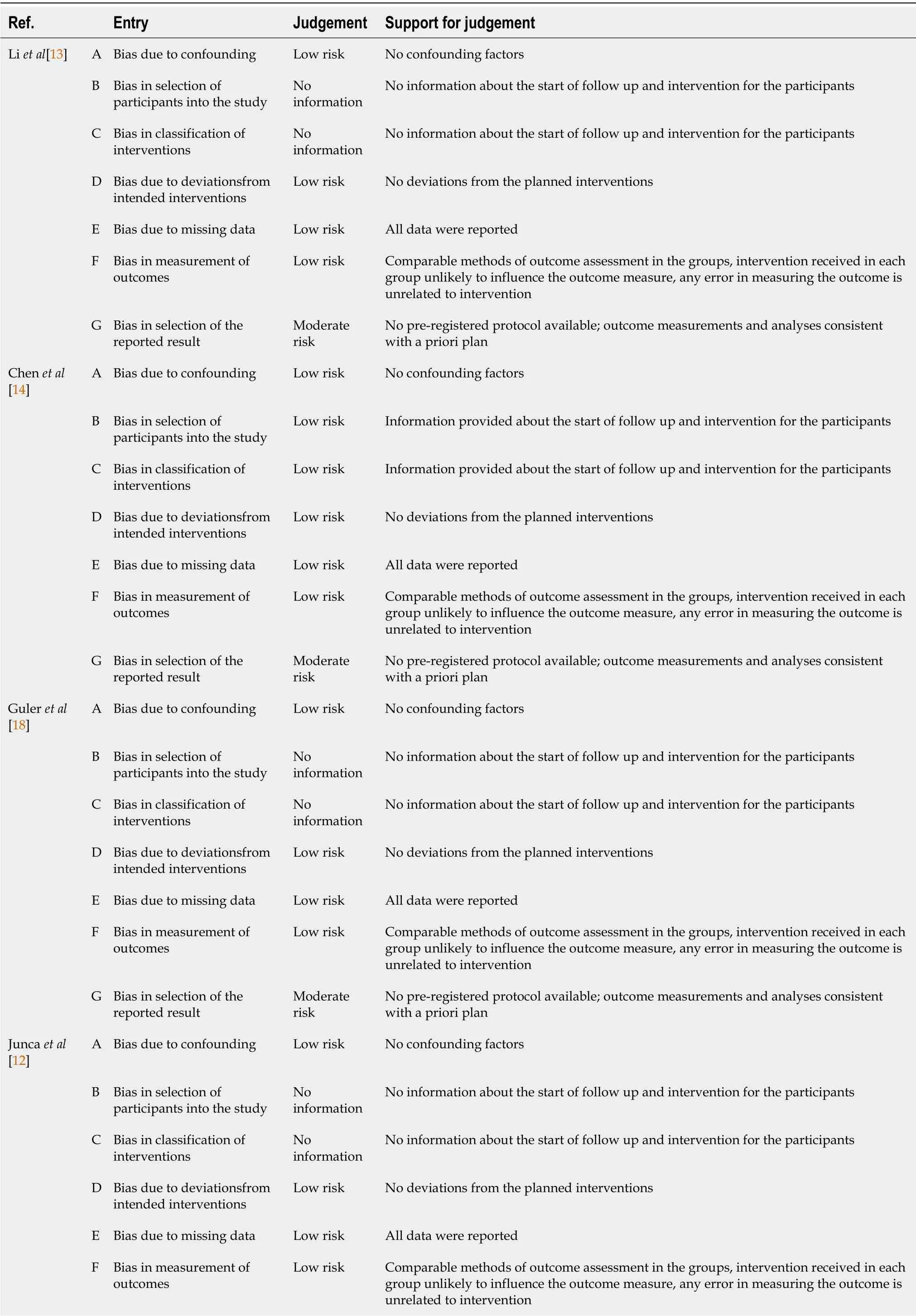
Table 3 Risk of bias of included studies,determined using the ROBINS-I tool(2016)
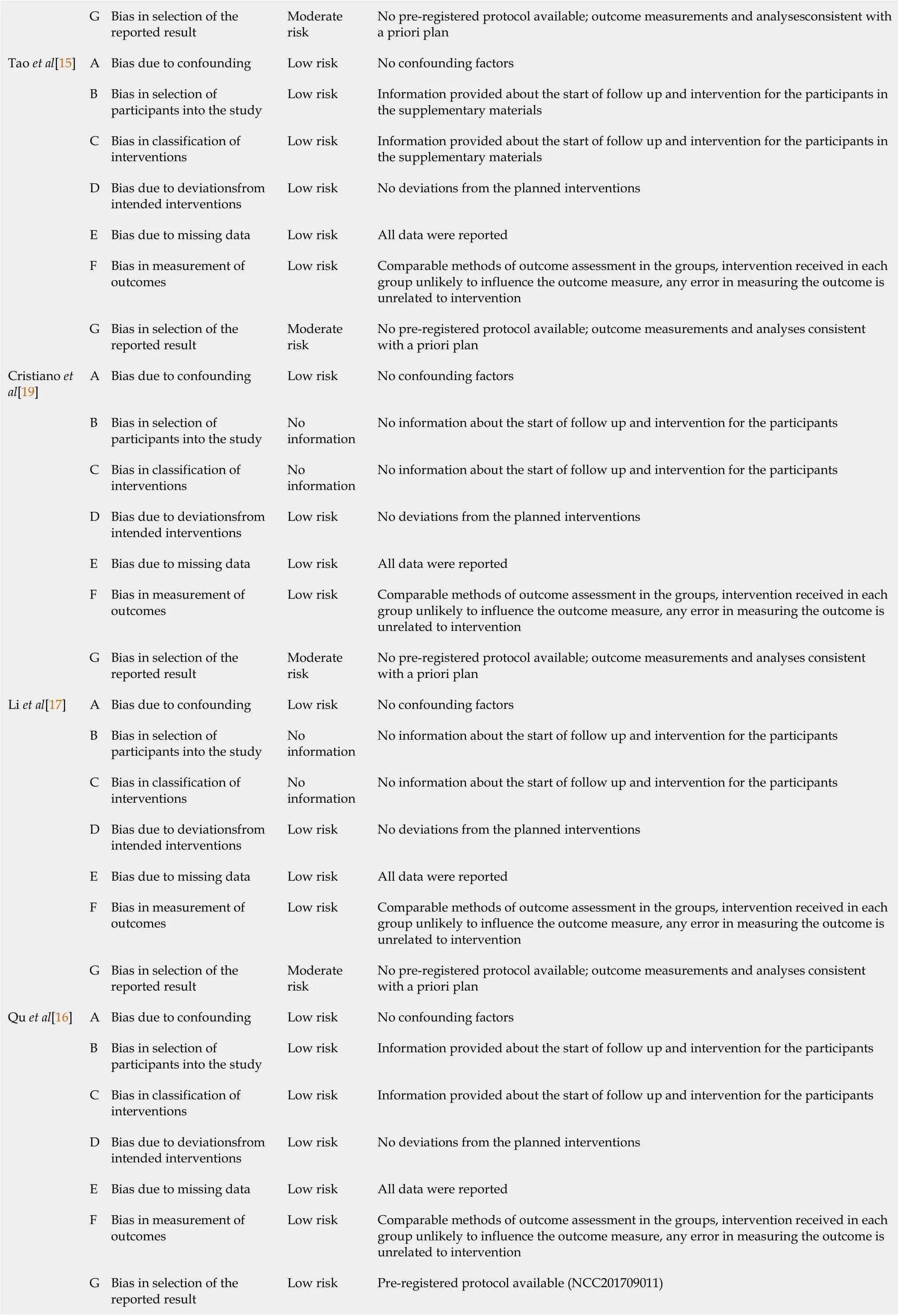
G Bias in selection of the reported result Moderate risk No pre-registered protocol available;outcome measurements and analysesconsistent with a priori plan Tao et al[15]A Bias due to confounding Low risk No confounding factors B Bias in selection of participants into the study Low risk Information provided about the start of follow up and intervention for the participants in the supplementary materials C Bias in classification of interventions Low risk Information provided about the start of follow up and intervention for the participants in the supplementary materials D Bias due to deviationsfrom intended interventions Low risk No deviations from the planned interventions E Bias due to missing data Low risk All data were reported F Bias in measurement of outcomes Low risk Comparable methods of outcome assessment in the groups,intervention received in each group unlikely to influence the outcome measure,any error in measuring the outcome is unrelated to intervention G Bias in selection of the reported result Moderate risk No pre-registered protocol available;outcome measurements and analyses consistent with a priori plan Cristiano et al[19]A Bias due to confounding Low risk No confounding factors B Bias in selection of participants into the study No information No information about the start of follow up and intervention for the participants C Bias in classification of interventions No information No information about the start of follow up and intervention for the participants D Bias due to deviationsfrom intended interventions Low risk No deviations from the planned interventions E Bias due to missing data Low risk All data were reported F Bias in measurement of outcomes Low risk Comparable methods of outcome assessment in the groups,intervention received in each group unlikely to influence the outcome measure,any error in measuring the outcome is unrelated to intervention G Bias in selection of the reported result Moderate risk No pre-registered protocol available;outcome measurements and analyses consistent with a priori plan Li et al[17]A Bias due to confounding Low risk No confounding factors B Bias in selection of participants into the study No information No information about the start of follow up and intervention for the participants C Bias in classification of interventions No information No information about the start of follow up and intervention for the participants D Bias due to deviationsfrom intended interventions Low risk No deviations from the planned interventions E Bias due to missing data Low risk All data were reported F Bias in measurement of outcomes Low risk Comparable methods of outcome assessment in the groups,intervention received in each group unlikely to influence the outcome measure,any error in measuring the outcome is unrelated to intervention G Bias in selection of the reported result Moderate risk No pre-registered protocol available;outcome measurements and analyses consistent with a priori plan Qu et al[16]A Bias due to confounding Low risk No confounding factors B Bias in selection of participants into the study Low risk Information provided about the start of follow up and intervention for the participants C Bias in classification of interventions Low risk Information provided about the start of follow up and intervention for the participants D Bias due to deviationsfrom intended interventions Low risk No deviations from the planned interventions E Bias due to missing data Low risk All data were reported F Bias in measurement of outcomes Low risk Comparable methods of outcome assessment in the groups,intervention received in each group unlikely to influence the outcome measure,any error in measuring the outcome is unrelated to intervention G Bias in selection of the reported result Low risk Pre-registered protocol available(NCC201709011)
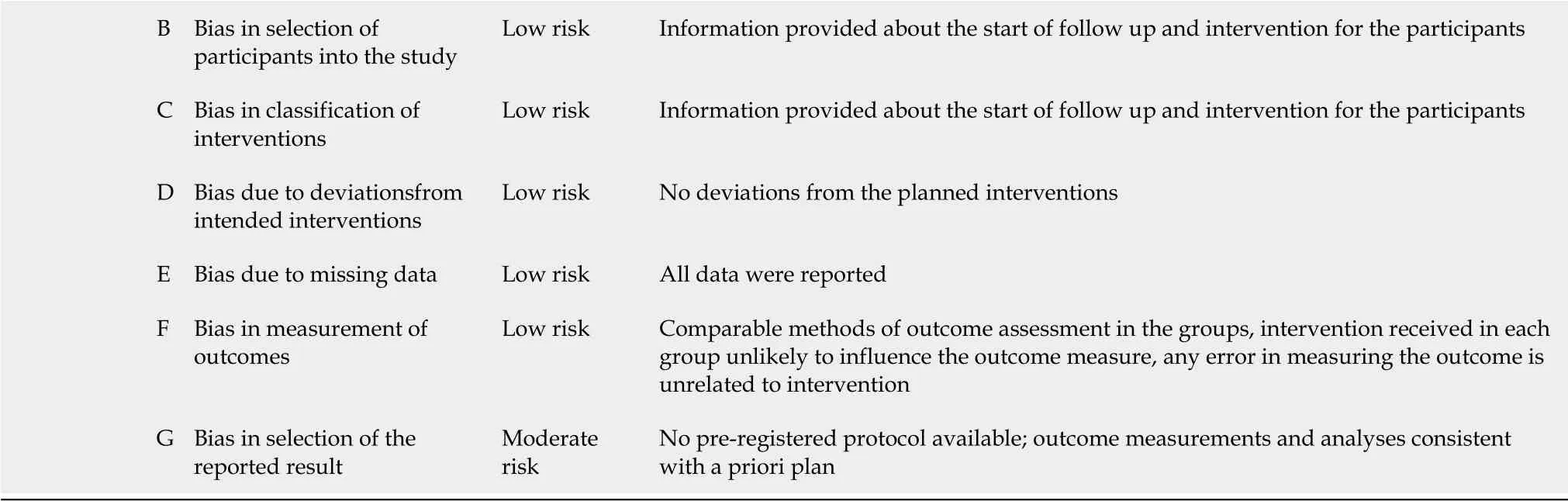
B Bias in selection of participants into the study Low risk Information provided about the start of follow up and intervention for the participants C Bias in classification of interventions Low risk Information provided about the start of follow up and intervention for the participants D Bias due to deviationsfrom intended interventions Low risk No deviations from the planned interventions E Bias due to missing data Low risk All data were reported F Bias in measurement of outcomes Low risk Comparable methods of outcome assessment in the groups,intervention received in each group unlikely to influence the outcome measure,any error in measuring the outcome is unrelated to intervention G Bias in selection of the reported result Moderate risk No pre-registered protocol available;outcome measurements and analyses consistent with a priori plan
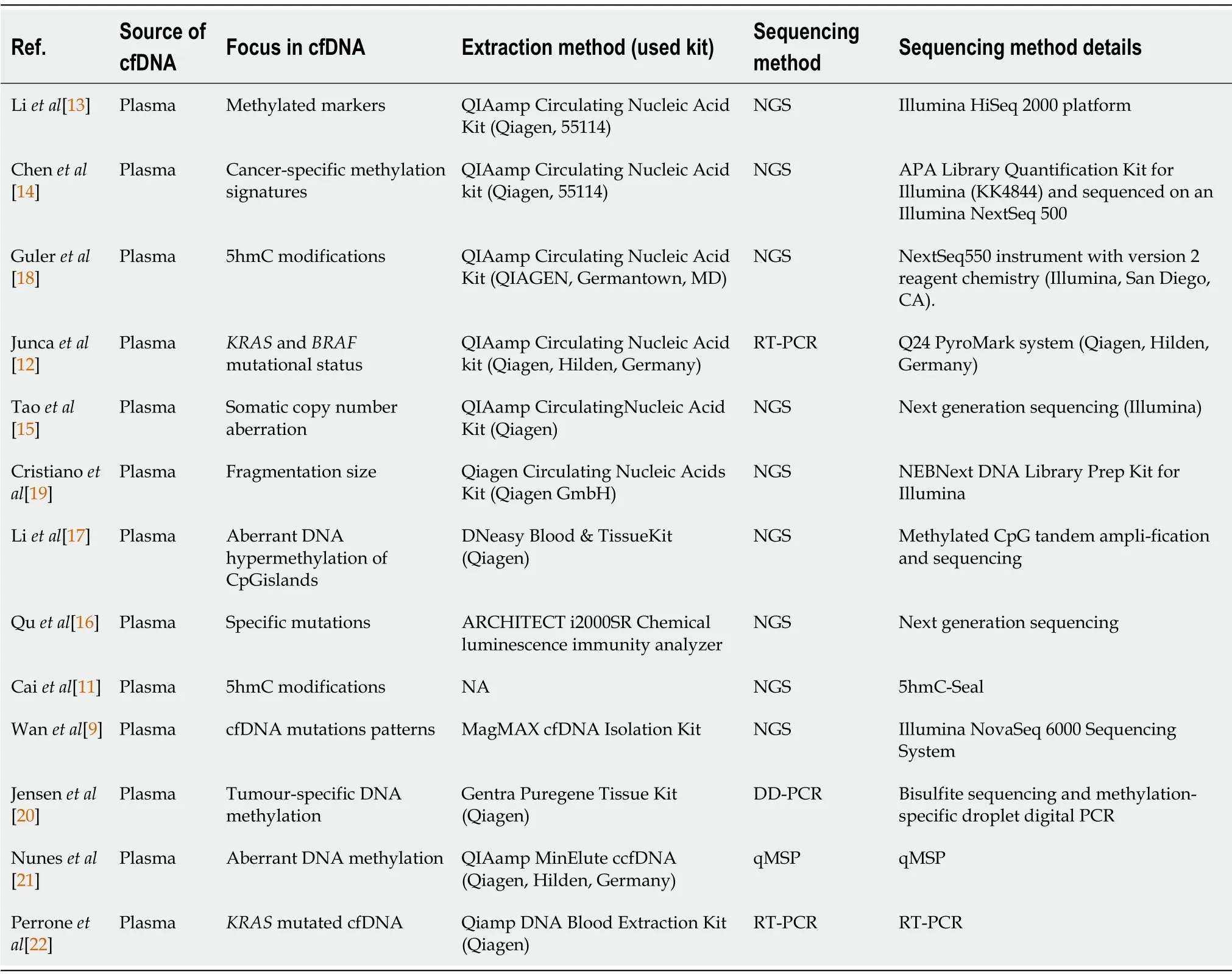
Table 4 Details of extraction and sequencing methods used in each of the included studies
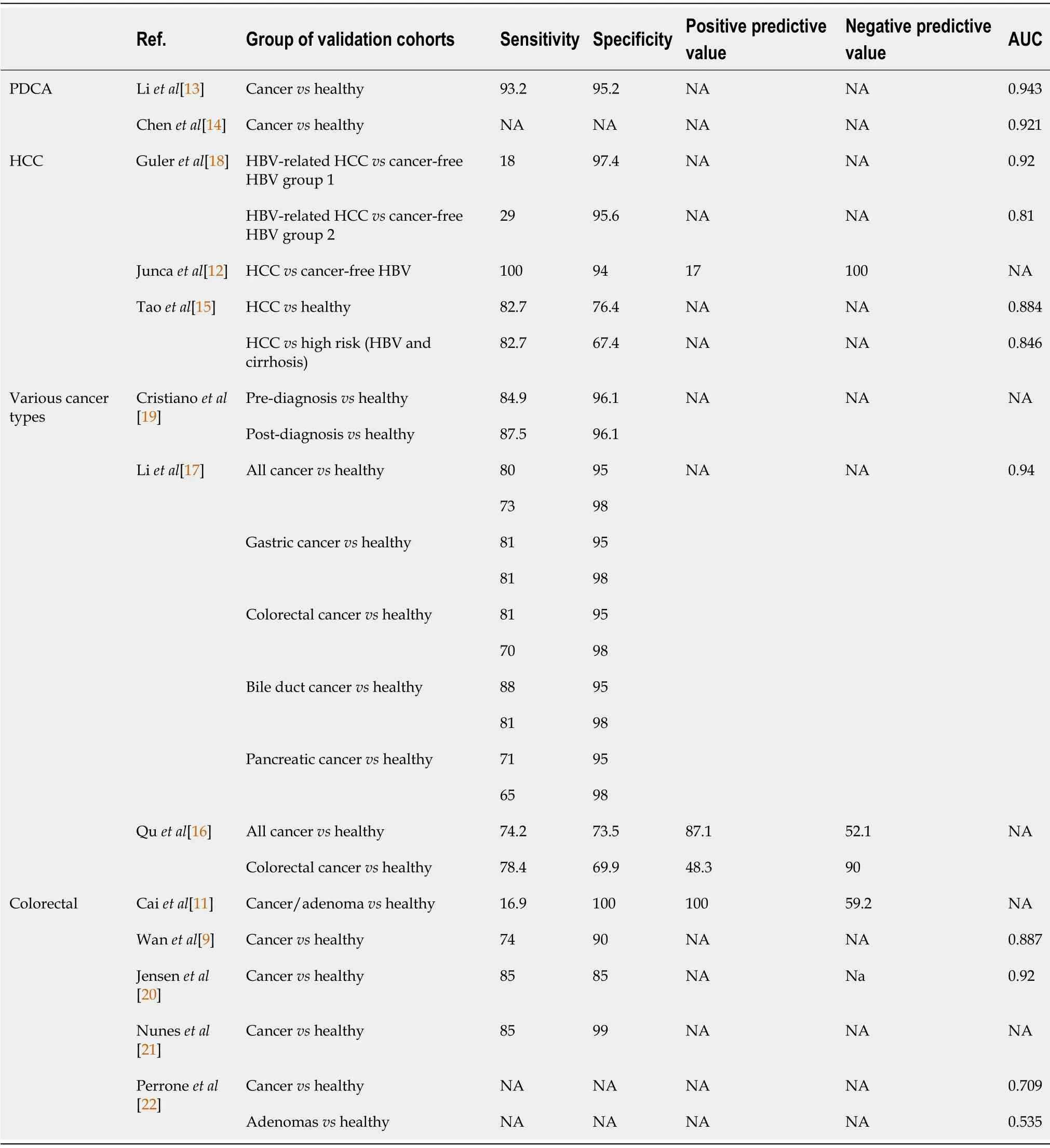
Table 5 Sensibility and sensitivity of included studies
RESULTS
CRC
Clinically relevant sensitivities and specificities to detect colorectal adenocarcinoma were achieved in three studies[9,20,21],Liet al[17]and Jensenet al[20]focusing on tumor-specific methylations.In contrast,Wanet al[9]investigated complex cfDNA mutational patterns using a machine-learning-based model.Sensitivities ranged from 74% to 85%,while specificities ranged from 85% to 99%.In a fourth study,Perroneet al[22]reported an AUC of 0.709 when discriminating CRC from healthy patients.However,for premalignant lesions,the performance was lower,with an AUC of 0.535[22].Similarly,investigating adenomas and adenocarcinomas through cfDNAKRASandBRAFmutations,Juncaet al[12]found a mean sensitivity of 16.9% for a 100% specificity reflecting a still lower sensitivity in premalignant lesions detection but allowing a high level of precision.
Pancreatic cancer
Examining methylation patterns in cfDNA,Liet al[13]described eight methylation markers in patients suffering from PDAC;SIX3,TRIM73,MAPT,FAM150A,EPB41L3,MIR663,LOC100130148,and LOC100128977.These markers identified PDAC patients efficiently,with a sensitivity of 93.2% and a specificity of 95.2%(AUC=0.943).By investigating 5-hydroxymethylcytosine(5hmC)changes in circulating cfDNA,Guleret al[18]achieved similar performance with an AUC of 0.921.
Hepatocellular carcinoma
Caiet al[11]found promising results using a mutational pattern of 32 gene markers to discriminate HCC patients from healthy individuals,with a sensitivity and specificity of 82.7% and 76.4%,respectively.Furthermore,when comparing HCC patients with cancer-free high-risk patients(chronic hepatitis B or liver cirrhosis),the model performed similarly with an 82.7% sensitivity and 67.4% specificity[11].
Comparing HCC patients with cancer-free asymptomatic HBV patients based on cfDNA mutational pattern of specific locations,Quet al[16]achieved a sensitivity and specificity of 100% and 94%,respectively.Further,using somatic copy number aberration in cfDNA as an alternative to methylation or specific mutations analysis,Taoet al[15]investigated the possibility of discriminating HBV-related HCC from cancer-free chronic HBV patients.Their predictive model performed appropriately,showing a high level of precision in two validation cohorts,with an AUC of 0.92 and 0.81.
Multi-cancer detection
Nuneset al[21]investigated the possibility to diagnose lung,breast,and colorectal cancer patients simultaneously from healthy individuals by detecting aberrant methylations on specific locations.They achieved an overall specificity of 73.5% and a sensitivity of 74.2%.For colorectal cancer,specificity was 69.9%,and sensitivity was 78.4%[21].
With a comparable strategy targeting five cancers(gastric,oesophageal,lung,liver,and colorectal),Chenet al[14]demonstrated the potential ability of cfDNA liquid biopsy to achieve multicancer detection several years before the actual diagnosis.Based on blood samples from a large biobank,they analyzed samples from 3 groups.The post-diagnosis group included patients with a newly discovered and untreatedmalignancy at the time of sampling.The pre-diagnosis group included patients with no known malignancy at the sampling time but who developed cancer within four years after sampling(pre-diagnosis).Finally,the control group included healthy individuals who were still free of malignant disease four years after sampling.Their model achieved an overall detection specificity of 96% when comparing healthy individuals to pre-diagnosis and post-diagnosis groups.Overall sensitivity was 87.5% for the post-diagnosis group,ranging from 75% in colorectal cancer to 96% in lung cancer.It reached 94.9% in the pre-diagnosis group,ranging from 91% in oesophageal cancer to 100% in liver cancer[14].
In contrast to these two studies focused on cfDNA methylations,Cristianoet al[19]explored a multi-cancer detection model analyzing cfDNA fragmentation patterns,including gastric,bile duct,colorectal and pancreatic cancers.Their model reached an overall detection sensitivity of 80% for a specificity of 95%,or a sensitivity of 73% for a specificity of 98%,and a global AUC of 0.94.Furthermore,enhanced by a machinelearning algorithm,they were able to identify the tissue of origin of cancer samples with a 61% accuracy[19].Detailed performances per cancer type of this model can be found in Table 3.
DISCUSSION
Liquid biopsy appears as a promising non-invasive method for the initial screening and diagnosis of various gastrointestinal cancers.High levels of sensitivity and specificity described in the included studies seem within acceptable ranges for eventual clinical use.In the case of HCC,cfDNA tests demonstrated better detection performances when compared to the standard surveillance of high-risk patients combining AFP dosage and ultra-sound monitoring.It also appears to be a viable solution regarding the challenge of pancreatic cancer screening;due to the paucity of symptoms in the early phases and the absence of acceptable screening strategies even for high-risk groups,this type of cancer remains frequently detected at metastatic or locally advanced and unresectable stages.Conversely,colorectal cancer is one of the few cancers with a standardized and efficient large-scale screening strategy based on the colonoscopy and the fecal occult blood test.Still,there is room for improved and more cost-effective strategies.Of note,cfDNA liquid biopsy’s ability to detect several cancer types simultaneously appears as a potential paradigm shift in global cancer care,and studies investigating such application achieved a high level of performance.Further,as demonstrated by Chenet al[13],this technology bears the potential to predict cancer several years before the onset of clinical symptoms and identify or direct investigations towards specific tissues of origin.
The central role of early cancer detection in improving oncologic and public-health outcomes is well established.However,it is a challenge for liquid biopsy since smaller and earlier-stage tumors tend to release lower levels of ctDNA[24].The signal-to-noise ratio of ctDNA is thus meager compared to non-cancer-derived cfDNA,with a detection percentage ranging from 0 to 11.7%[25,26].The extraction method plays a critical role in improving detection performance.Different procedures have been developed,the more widespread being column-based,polymer-based,phenolchloroform,or magnet-based[9,27].These methods are efficient and allow to reach a high DNA concentration but remain expensive and time-consuming[9,27].In this context,some authors proposed plasma processing methods without the need for DNA extraction.Breitbachet al[28]notably used quantitative RT-PCR to measure cfDNA concentration in plasma.Not only did the method showed great feasibility with higher levels of cfDNA found among cancer patients,but it also proved to be more time effective and more efficient than the eluate of the QIAamp DNA Blood Mini Kit,for example,with levels of cfDNA in unpurified plasma 2.79 fold higher[28].
Regarding the sequencing method,some authors focused their attention on specific mutations while others analyzed the whole genome searching for non-specific mutational patterns,most of them using NGS methods.Different factors can explain the apparent predominance of NGS over other PCR methods such as RT-PCR in the published studies.Although more technically demanding and expensive,NGS is a hypothesis-free approach that carries a higher discovery power of new mutational patterns,in addition to a higher sensitivity to rare variants[29,30].Further,its superior multiplex capabilities tend to improve the workflow when studying a large number of locations and samples.These high throughput and detection sensitivity capabilities might be valuable in a screening configuration for early cancer detection,which deals with lower levels of mutation than advanced stage cancers and aims at testing a high volume of patients.
As the field is at an early stage of clinical exploration,there is still a high variability in trial designs and reporting methods,thus undermining the global quality of tests’ performance analysis.Of note,biocomputational trials based on biobank samples often report higher levels of sensitivity and specificity but are less likely to translate into clinically relevant performances as prospective trials would.Applicability to real-life clinical applications is thus the most awaited step to achieve for the scientific validation of this technology,and upcoming clinical trials will need to address many questions,such as the appropriate balance between sensitivity and specificity in a screening purpose,the timing of screening tests,patient selection,socio-economic parameters and dealing with the uncertainty around tissues of origin in positive tests.
CONCLUSION
Liquid biopsy cfDNA represents an efficient,non-invasive,and promising method for detecting various gastrointestinal cancers at an early stage of development.These tools could improve the global prognosis of cancers currently diagnosed at an advanced stage due to the lack of effective screening and diagnostic methods,such as pancreatic cancer.Allowing early detection of several types of cancers and reducing the burden of multiple screening tests,cfDNA liquid biopsies could change the course of gastrointestinal cancers care for a significant number of patients and induce a paradigm shift in cancer-related public health policies,provided that they can demonstrate their clinical relevance in future studies.
ARTICLE HIGHLIGHTS
Research background
Liquid biopsy cell-free DNA(cfDNA)represents a promising non-invasive method for detecting various gastrointestinal cancers at an early stage of development.
Research motivation
Various and recent literature is available on this topic,with exponentially growing interest.
Research objectives
To review the current state of development of cfDNA liquid biopsy in the field of gastrointestinal cancer early detection.
Research methods
A systematic review of the literature according to the PRISMA guidelines.
Research results
The current literature suggests a high-performance profile for this technology and the potential to improve the global course of gastrointestinal cancers currently diagnosed at an advanced stage,such as pancreatic cancer.
Research conclusions
cfDNA liquid biopsy showed high potential in the diagnosis of early gastrointestinal cancers and simultaneous screening of multiple cancer types.
Research perspectives
Further trials in clinically relevant settings are required to determine the exact place of this technology in future diagnosis strategies.
 World Journal of Gastrointestinal Oncology2021年11期
World Journal of Gastrointestinal Oncology2021年11期
- World Journal of Gastrointestinal Oncology的其它文章
- Hepatocellular carcinoma biomarkers,an imminent need
- Anatomical vs nonanatomical liver resection for solitary hepatocellular carcinoma:A systematic review and meta-analysis
- Atezolizumab plus bevacizumab versus sorafenib or atezolizumab alone for unresectable hepatocellular carcinoma:A systematic review
- Colorectal cancer in Arab world:A systematic review
- Induction chemotherapy with albumin-bound paclitaxel plus lobaplatin followed by concurrent radiochemotherapy for locally advanced esophageal cancer
- Genetic variation of TGF-ΒR2 as a protective genotype for the development of colorectal cancer in men
