Genetic variation of TGF-ΒR2 as a protective genotype for the development of colorectal cancer in men
Noyko Stanilov,Antonia Grigorova,Tsvetelina Velikova,Spaska Angelova Stanilova
Noyko Stanilov,Oncoplastic Unit,University College London Hospital,London NW1 2BU,United Kingdom
Antonia Grigorova,Spaska Angelova Stanilova,Department of Molecular Biology,Immunology and Medical Genetics,Medical Faculty,Trakia University,Stara Zagora 6000,Bulgaria
Tsvetelina Velikova,Department of Clinical Immunology,University Hospital Lozenetz,Sofia 1407,Bulgaria
Tsvetelina Velikova,Medical Faculty,Sofia University St.Kliment Ohridski,Sofia 1407,Bulgaria
Abstract BACKGROUNDThe role of transforming growth factor beta(TGF-β)signaling,including both the cytokine and their receptors,in the etiology of colorectal cancer(CRC)has been of particular interest lately.AIMTo investigate the association between promoter polymorphism in TGF-β receptor 2 TGF-ΒR2G[-875]A with a CRC risk in a cohort of Bulgarian patients using a casecontrol gene association study approach,as well as the protein levels of TGF-β1 in the peripheral blood.METHODSA cohort of 184 CRC patients and 307 sex and age-matched healthy subjects were recruited in the study.A genotyping of the TGF-ΒR2G[-875]A(rs3087465)polymorphism was performed by primer-introduced restriction analysespolymerase chain reaction approaches.RESULTSThe frequency of TGF-ΒR2G[-875]A genotype was decreased in male patients with CRC than in healthy men(31.3% vs 44.8%;P=0.058).Among males,the TGFΒR2G[-509]G genotype was related to a significantly increased risk of CRC development(OR=1.820,95%CI:0.985-3.362,P=0.055)than the GA + AA genotype.Also,TGF-ΒR2[-875]*A-allele itself was rarer in men with CRC than healthy men(19.1% vs 26.9%,P=0.086)and was associated with a protective effect(OR=0.644;95%CI:0.389-1.066;P=0.086).Regarding the genotypes,we found that TGF-β1 serum levels were higher in GG genotype in healthy persons above 50 years than the CRC patients[36.3 ng/mL interquartile range(IQR)19.9-56.5 vs 22.4 ng/mL IQR 14.8-29.7,P=0.014].We found significant differences between higher levels of TGF-β1 serum levels in healthy controls above 50 years(GG genotype)and CRC patients(GG genotype)at the early stage(36.3 ng/mL IQR 19.9-56.5 vs 22.8 ng/mL IQR 14.6-28.6,P=0.037)and advanced CRC(36.3 ng/mL IQR 19.9-56.5 vs 21.6 ng/mL IQR 15.9-33.9,P=0.039).CONCLUSIONIn summary,our results demonstrated that TGF-ΒR2 AG and AA genotypes were associated with a reduced risk of CRC,as well as circulating levels of TGF-β could prevent CRC development in a gender-specific manner.Notably,male carriers of TGF-ΒR2 -875A allele genotypes had a lower risk of CRC development and progression,suggesting that TGF-ΒR2 -875A/G polymorphism significantly affects the protective biological factors that also impact the risk of colon and rectal carcinogenesis.
Key Words:Colorectal carcinoma;Cytokine;TGF-ΒR2 gene;TGF-ΒR2G[-875]A;Single nucleotide polymorphism
INTRODUCTION
The role of transforming growth factor beta(TGF-β)signaling in the colon and rectal cancer etiology has been intensively studied for the last decades.It is thought that disruptions in TGF-β-associated cancer mechanisms are essential in early-stage tumor development.In contrast,activation of TGF-β-signaling can encourage invasion and metastasis of cancer[1].In addition,its involvement in the tumor microenvironment control typically includes inhibition of the tumor-specific immune cells and facilitation of the cancer cells survival.This again emphasizes that the TGF-β signaling in cancer exerts multi-directional functions between cancer cells encouragement and tumor micro-environment inhibition[2].
Furthermore,it was shown that TGF-β-signaling has dual roles in developing and progressing gastrointestinal tumors - as both a tumor promoter and tumor suppressor[3].Although the mechanism by which TGF-β converts its inhibitory into stimulating growth effect is not well known,it is thought that the cytokine enhances many mitogenic growth factors,including TGFα,FGF,and EGF,and oncogenic pathways,such as Ras/MAPK pathway,JNK pathway,and PI3 kinase/Akt pathway,etc[4].Thus,many mechanisms of TGF-β are involved in the proliferation of colorectal cancer(CRC)cells.Nevertheless,TGF-β also promotes angiogenesis and immunosuppression.This is especially valid for CRC.In the last decade,the role of TGF-β1 was recognized in colorectal tumorigenesis.There is an increasing amount of proof that TGF-β-signaling modifications induced by TGF-β1 or SMAD mutations or polymorphisms contribute to the development and progression of CRC[5].
Inflammation also plays a significant role in the support and promotion of CRC growth.Indeed,dysregulated immune response in CRC patients involved different immune cell types,leading to the release of a range of cytokines,chemokines,and growth factors that control both inflammation and carcinogenesis[6].Furthermore,colonic epithelial cells are simultaneously producers and respondents to cytokines[interleukins(IL),and chemokines],signals that modulate the behavior of epithelial cells by influencing their proliferation,migration,and survival[7].In such a way,cytokines carry out the cross-talk between cells of the immune system and the CRC cells,forming networks with anti-tumor(interferon-γ,IL-12,IL-15,IL-17F,and IL-18),pro-tumor(IL-4,IL-6,IL-8,IL-11,IL-17A,IL-22,IL-23,IL-33,tumor necrosis factor,TGF-β,and vascular endothelial growth factor)or bivalent or unclear properties to intestinal cancer(IL-1,IL-9 IL-10,IL-21,and granulocyte-macrophage colonystimulating factor)[8-10].
Amongst these mediators,TGF-β was shown to exert functions such as induction of reactive oxygen species,inflammation-associated epithelial-mesenchymal transition,angiogenesis,and metastasis[7,11].TGF-β1 serves its functions by binding to two receptors-type I and type II receptors,i.e.,TGF-ΒR1andTGF-ΒR2,respectively.Through binding toTGF-ΒR2,downstream signaling involving cell-cycle checkpoint genes[e.g.,CDKN1A(p21),CDKN1B(p27),and CDKN2B(p15)]is initiated.As a result,cell growth is arrested[12].Therefore,TGF-β1 exerts tumor-suppressing effects in the intestines' epithelium by inhibiting cell proliferation and inducing apoptosis.In line with this,many colorectal cancers can avoid the suppressive effects of TGF-β1 that make them resistant to TGF-β-induced growth inhibition[5].
Many mutations in different genes were associated with CRC development,such as theAPCgene,mutation ofKRASandTP53,deletion on chromosome 18q,germline mutation in DNA mismatch repair genes(MLH1,MSH2,MSH6,orPMS2).Furthermore,mutations in microsatellites of certain tumor-suppressor genes(e.g.,TGF-ΒR2,BAX,E2F4,and IGFR2,have also been identified in colorectal tumors[5].
The polymorphic variant in the promoter region of TGF-ΒR2(rs3087465)due to a G to A transition was reported previously in patients with Lynch syndrome and other cancer types[1,5,13].However,there are still many unanswered questions.For example,TGF-ΒR2 polymorphisms were not explored extensively as a genetic protective factor for CRC.Furthermore,since TGF-β signaling is altered in CRC,targeting this intracellular pathway may represent a potential therapeutic approach.However,it is difficult to efficiently discover and administer therapeutic products for the treatment of cancer.This is because the simple blockage of TGF-β signaling could enhance immunity in the tumor microenvironment but may lead to the development of more aggressive cancer phenotypes[1].
Since TGF-β1 exerts its effects by these receptors and given the importance of the TGF-β1 signaling pathway in CRC development,we could hypothesize that genetic polymorphisms in the TGF-β1 gene and genes for TGF-β receptors may also play a role in CRC susceptibility.Previously,we reported the role of circulating TGF-β1 and the -509C/T functional promoter polymorphism(rs1800469)within the TGF-β1 gene(TGFΒ1)in the susceptibility,progression,and prognosis of CRC among Bulgarian patients in a gender-dependent manner[14].
Therefore,we aimed to investigate the association between another promoter polymorphism(in TGF-ΒR2G[-875]A)with a CRC risk in a cohort of Bulgarian patients using a case-control gene association study approach.We also estimated the role of this polymorphism at different stages of the disease,defined as early and advanced in men and women.Additionally,we were interested in the TGF-β1 in the peripheral blood associated with the different genotypes as a non-invasive marker for CRC development and progression and the relationship between this polymorphism and serum levels of TGF-β1 in patients and healthy people.
MATERIALS AND METHODS
CRC patients
A total of 184 patients with CRC at a mean ± SD age of 65 ± 10 years,obtained in the University Hospital and Trakia Hospital,Stara Zagora,during 2011-2017,were included in the study.The diagnosis was made by employing standard clinical,laboratory,endoscopic,histopathological,and radiological criteria.CRC patients underwent curative surgical resection of the tumors.Previous diagnosis of inflammatory bowel disease or other autoimmune diseases or individual or family history of any known hereditary cancer syndromes were considered exclusion criteria for the patients and controls.Additionally,patients did not receive any neo-adjuvant chemotherapy or radiation therapy prior to surgery.
Tumor,node and metastasis classification was used for tumor grading and staging.Accordingly,CRC patients were divided into two groups:88 patients with early CRC(1ststage + 2ndstage)and 96 patients with advanced CRC(3rd+ 4thstages).The CRC group was composed of 115 males(62.5%)and 69 females(37.5%).There were no significant differences between the mean age of males and females(P=0.65).
A total of 307 healthy volunteers were included in the study,matched with patients by age and gender.The healthy control group consisted of 240 females and 67 males,with a mean ± SD age 42 ± 13 years.
The blood concentrations of TGF-β1 in CRC patients were compared to matched controls over 50 years,where the gender distribution was considered similar(χ2=0.055;P=0.814).
The demographic and clinical characteristics of the CRC populations of patients and control subjects were summarized previously in our paper investigating the TGF-β1 gene promoter-509C/T polymorphism[14](https://doi.org/10.1371/journal.pone.0201775.t001).
Ethical considerations
According to the Helsinki Declaration and local Ethics Committee's ethical guidelines,all participants gave written informed consent for the study.All patients were informed about the purpose of the study.
Specimen collection and preparation
At least 6 mL of peripheral venous blood from the CRC patients and healthy controls were collected in sterile tubes.Plasma samples were obtained and frozen at -80°C before use to determine the protein level of TGF-β1.The genomic DNA from 200 μl peripheral blood was extracted using Gene Matrix Purification Kit(EURx,Poland)following the manufacturer’s instructions and stored at -80°C until use.We measured the DNA samples' concentration and purity spectrophotometrically at 260/280 nm using a GeneQuant 1300 spectrophotometer(GE Healthcare Life Sciences,Switzerland).
Genotyping of TGF-ΒR2G[-875]A promoter polymorphism
We performed the genotyping of theTGF-ΒR2G[-875]A(rs3087465)by primerintroduced restriction analysis-polymerase chain reaction(PCR)assay[15].The primer sequences were the following:forward primer- 5’-GCAAGAAAGGAAATTTGA AAGTTTGT-3’ and reverse primer 5’-TCACCTGAATGCTTGTGCTTTT-3’.The PCR amplification was accomplished as follows:(1)denaturation at 94°C/5 min;(2)30 cycles at 94°C/45 s,57°C/45 s and 72°C/45 s;and(3)final extension cycle at 72°C/7 min).Rsal(10 U/μL)restriction enzyme was used to digest the 124bp PCR products at 37°C overnight.Then,we electrophoresed the final products on 3.5% agarose gel and visualized them directly with ethidium bromide staining.Two fragments of 99bp and 25bp resulted from theTGF-ΒR2[-875]*A allele,whileTGF-ΒR2[-875]*G allele produced a fragment of 124bp.
GeneAmp PCR System 9700(Applied Biosystems)was used to perform all PCR reactions by using Thermo Fisher Scientific(United States)and Metabion GmbH(Germany)PCR reagents and primers.
Measurement of TGF-β1 in the plasma samples
Enzyme-linked immunosorbent assay(ELISA)method was performed to measure the protein level of latent acid-activated TGF-β1 protein in the participants' plasma samples(Quantikine ELISA Kits,R&D systems,Minneapolis,MN,United States).
Latent serum TGF-β1 was firstly activated by acid(1N HCl)and neutralized by 1.2N NaOH/0.5M HEPES,following the manufacturer protocol.The activated Serum samples from the patients and controls were stored in a fridge(2-6°C)for less than 16 h.Then we analyzed them together in the same analytic batch.A 4-point parametric standard curve calculated the results using the manufacturer`s standards within the range from 0-2000 ng/mL and expressed as ng/mL.The minimum detectable levels ranged from 1.7–15.4 ng/mL.
Statistical analysis
Statistical analysis of the data was performed using the Statistical software package(StatSoft v.12.0,Inc.,United States).We calculated the sample size by the GAS Power Calculator,given the significance level α=0.05,prevalence=0.1,the anticipated effect size(Cohen's d=0.5),and desired statistical power level 1-β=0.8,which determined the sample size of casesn=184 and controlsn=307.
χ2test was used to determine the statistical differences in the distribution of genotype and allele frequencies between CRC patients and healthy controls.The Hardy-Weinberg equilibrium was tested by comparing the observed genotype frequencies to the expected frequencies for cases and controls byχ2test.
When the observed frequencies were a smaller group,we used the Fisher exact test and Yates’ correctedP-value(c).The association between TGF-ΒR2 genotypes and risk of CRC was evaluated by calculating the odds ratios(ORs)and 95%CI using the StatPages.net website(http://statpages.org/index.html).
Results were considered significant atP≤ 0.05.
RESULTS
Association of TGF-ΒR2G[-875]A polymorphisms with CRC susceptibility
The genotype distribution for TGF-ΒR2G[-875]A(rs3087465 polymorphism)demonstrated no deviation from Hardy-Weinberg equilibrium in the cases(χ2=0.2,P=0.905)and controls(χ2=0.122,P=0.940).
The different genotype and allele frequencies of the TGF-ΒR2G[-875]A promoter polymorphisms in patients with CRC and controls are presented in Table 1.However,we did not observe differences between cases and controls regarding the distribution of the studied polymorphism(χ2=1.38,P=0.50).When we stratified the data according to the study participants’ sex,we obtain the following results.The frequency of TGF-ΒR2G[-875]A genotype was decreased in male patients with CRC than in healthy men(31.3%vs44.8%;P=0.058).
Moreover,male subjects with the GG genotype exhibited a higher risk of CRC development than the GA + AA genotype(OR=1.820,95%CI:0.985-3.362,P=0.055).In contrast,the GA genotype was associated with a lower risk compared with the GG + AA genotype in men(OR=0.562,95%CI:0.302-1.047,P=0.068).Additionally,the TGF-ΒR2G[-875]A genotype was associated with a reduced risk of CRC development(OR=0.544;95%CI:0.289-1.023;P=0.058)referred to the TGF-ΒR2G[-875]G genotype among men.
However,TGF-ΒR2[-875]*A-allele itself was rarer in men with CRC than healthy men(19.1%vs26.9%,P=0.086)and was associated with a protective effect(OR=0.644;95%CI:0.389-1.066;P=0.086).
The observed genotype distribution of TGF-ΒR1 rs4743325 polymorphism in men was similar among women with CRC and healthy women but did not reach statistical significance.
Association of TGF-ΒR2G[-875]A polymorphism with the stage of CRC
After stratification of CRC patients into those with early CRC(I stage + II stage)and advanced CRC(III stage + IV stage),we did not find differences in genotype distribution among patients with earlyvsadvanced stage(Table 2).
Besides,no differences between genotype distribution among patients with early CRC and healthy controls were observed(Table 3).
When we compared advanced CRC cases with healthy controls,we found that male carriers of the A allele(GA/GA + AA genotypes)had a significantly decreased risk of advanced CRC(OR=0.459,95%CI:0.217-0.969,P=0.039;OR=0.466,95%CI:0.226-0.961,P=0.037,respectively)(Table 4).
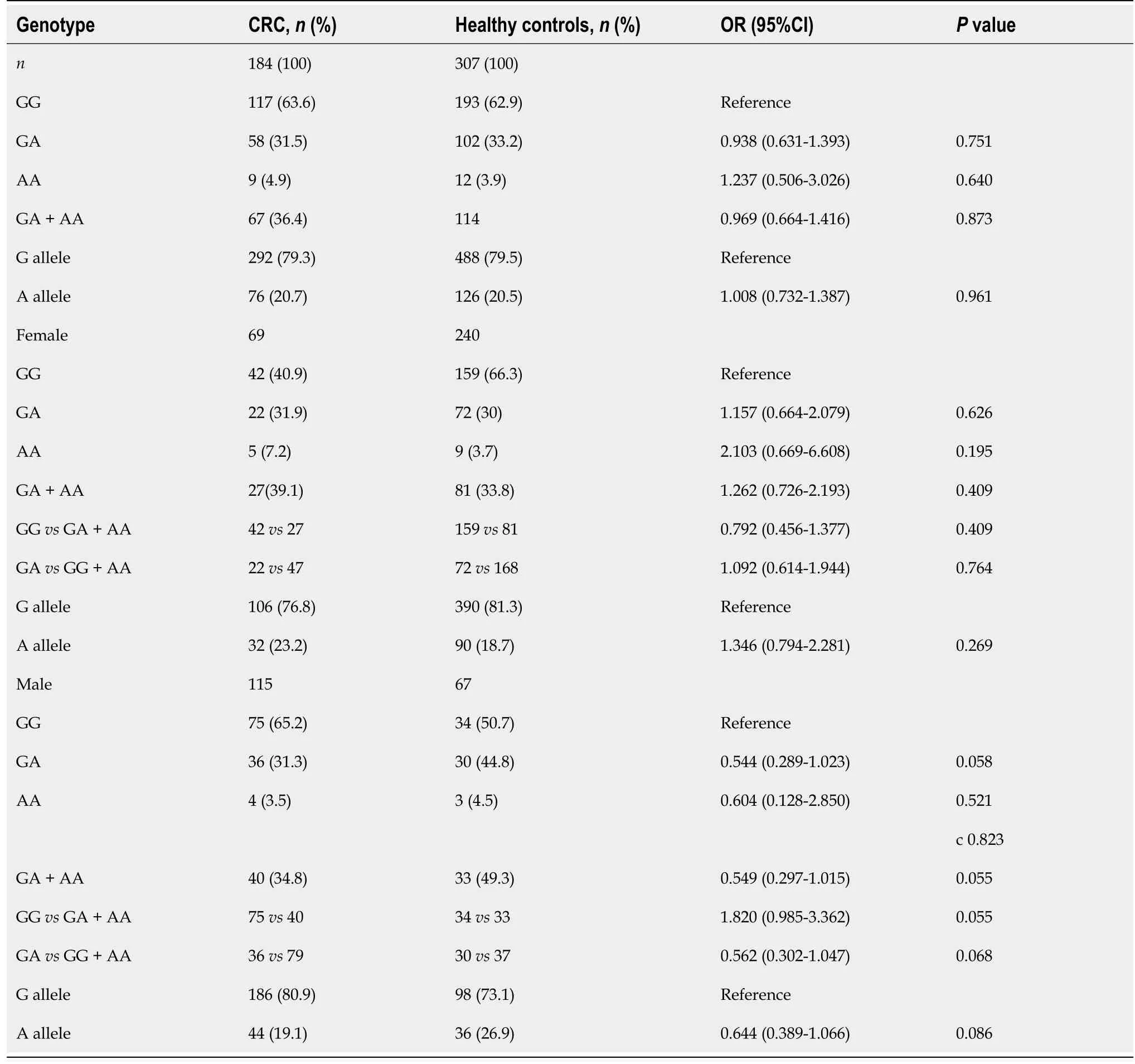
Table 1 Difference in genotype distribution and allele frequencies of TGFBR2 rs3087465 polymorphism between the case(colorectal cancer patients)and control(healthy persons)groups,females and males,respectively
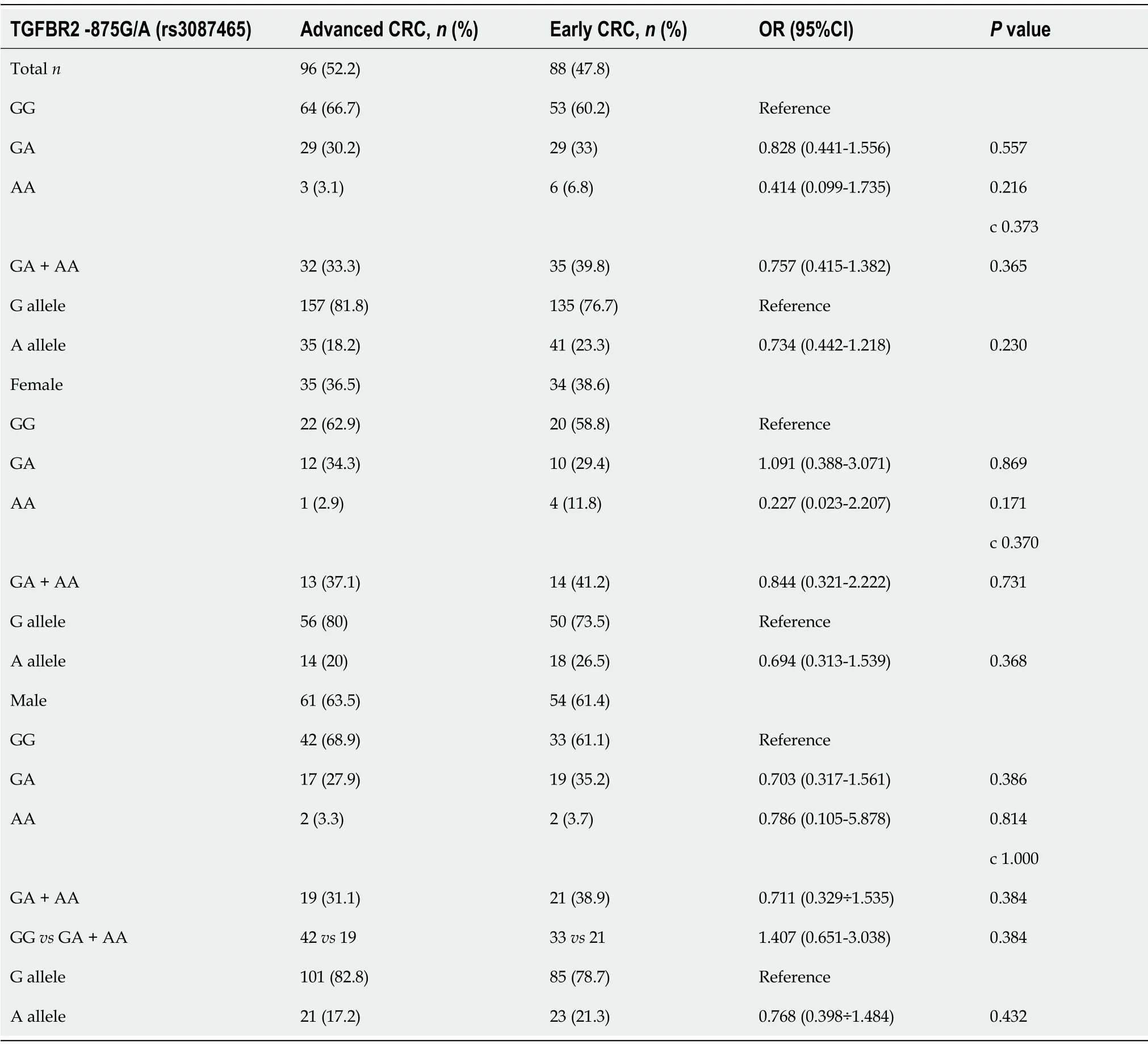
Table 2 Genotype distributions and allele frequencies of TGFBR2 rs3087465 polymorphism in the case group divided into advanced and early colorectal cancer groups(and females and males,respectively)
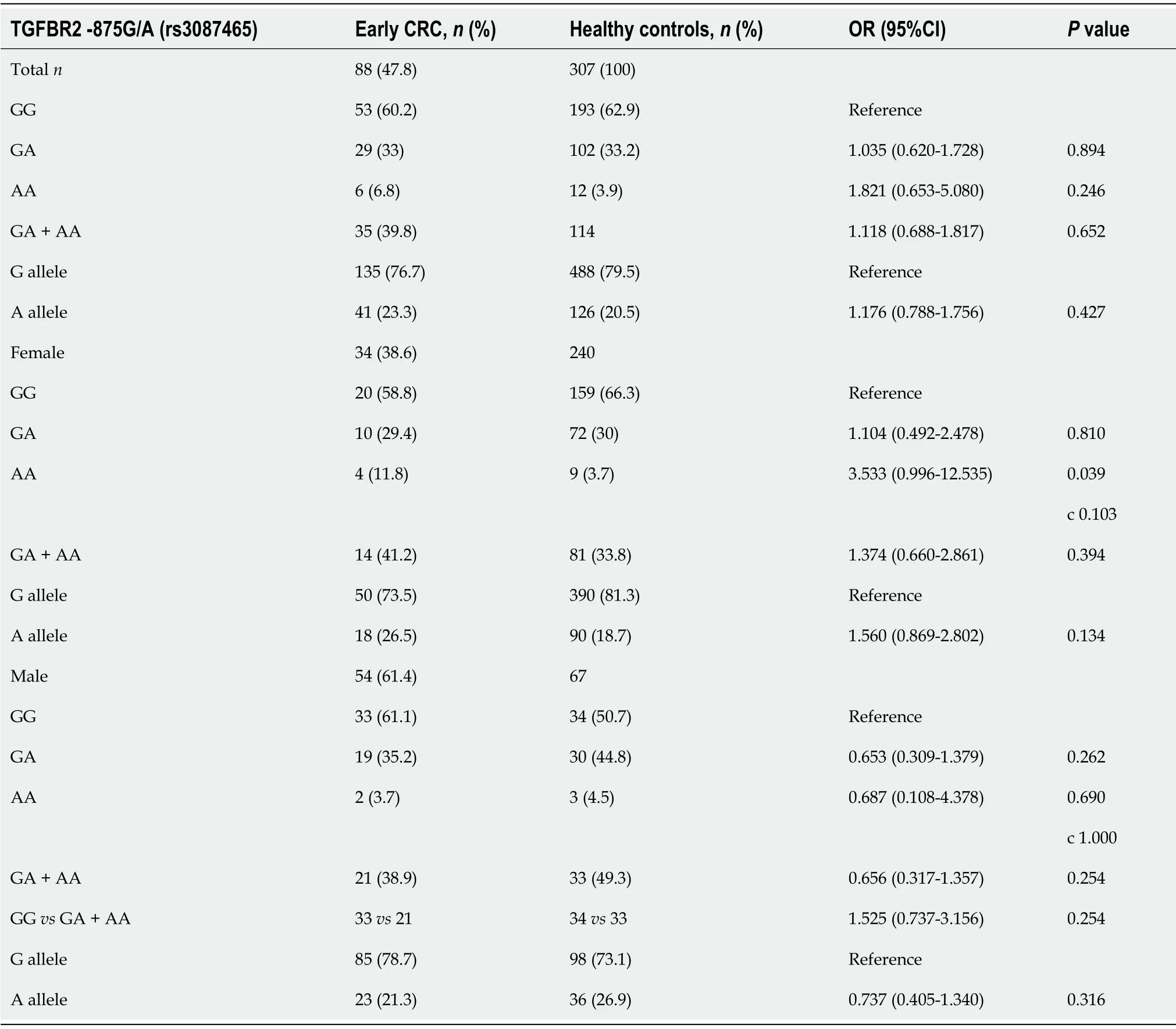
Table 3 TGFBR2G[-875]A rs3087465 polymorphism distribution in early CRC cases compared to healthy controls
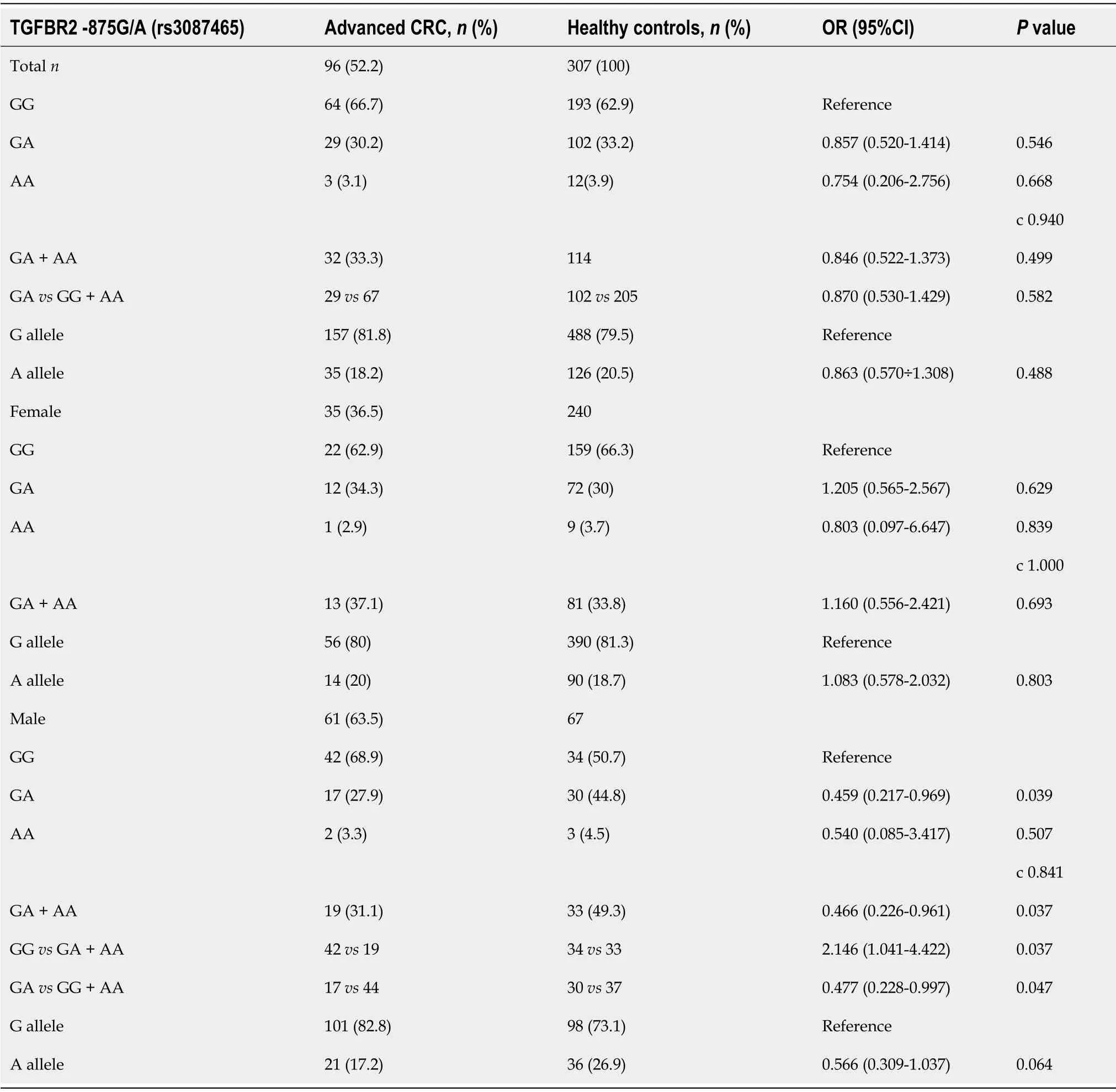
Table 4 TGFBR2G[-875]A rs3087465 polymorphism distribution in advanced CRC cases compared to healthy controls
Additionally,TGF-ΒR2[-875]*A-allele alone was also a protective factor for advanced CRC in men compared to healthy men(17.2%vs26.9%;OR=0.566,95%CI:0.309÷1.037,P=0.064).
The frequency of the TGF-ΒR2G[-875]G genotypes was overrepresented among male patients with advanced CRCvshealthy men compared to GA + AA genotype(OR=2.146,95%CI:1.041-4.422,P=0.037).On the contrary,the GA genotype in men was protective regarding advanced CRC compared to the GG + AA genotype(OR=0.477,95%CI:0.228-0.997,P=0.047).
Analyses in female patients with early or advanced CRC did not show a significant association of genotypes with the cancer stage.
Serum levels of TGF-β1 in relation to the TGF-ΒR2 genotypes
As we previously established,the mean serum levels of TGF-β1 were significantly lower in CRC patients than healthy persons above 50 years(24.72 ± 10.77 ng/mLvs34.54 ± 27.06 ng/mL;P=0.005–t-test)(23.1 ng/mL IQR 18.2-29.3vs24.9 ng/mL IQR 13.5-50.9;P=0.436-U-test)[14].
When we further stratified the CRC patients and healthy controls above 50 by sex,we found a significant increase in TGF-β1 in healthy men above 50 years compared to male patients with CRC(51.9 ng/mL IQR 42.4-65.3vs23.2 ng/mL IQR 18.4-28.1,P=0.000377)and healthy women(51.9 ng/mL IQR 42.4-65.3vs16.9 ng/mL IQR 10.9-31.1,P=0.000482)(Figure 1).
We obtained similar results when we include the healthy controls above 50 years genotyped for TGF-ΒR2 -875G/A(n=48)(not shown).
Regarding the genotypes,we found that TGF-β1 serum levels were higher in GG genotype in healthy persons above 50 years than the CRC patients(36.3 ng/mL IQR 19.9-56.5vs22.4 ng/mL IQR 14.8-29.7,P=0.0143).On the contrary,TGF-β1 Levels were higher in CRC patients with GA + AA genotype than healthy persons above 50 years who possessed the same genotype.However,this observation did not reach significance(24.04 ng/mL IQR 19.2-28.2vs16.3 ng/mL IQR 10.05-42.3,P=0.171)(Figure 2A).
However,TGF-β1 serum levels were comparable in CRC patients with both GG and GA + AA genotypes(22.4 ng/mL IQR 14.8-29.7vs24.04 ng/mL IQR 19.2-28.2,P=0.397).TGF-β1 serum levels were enhanced in healthy controls with GG genotypes in comparison with GA + AA genotype(36.3 ng/mL IQR 19.9-56.5,vs16.3 ng/mL IQR 10.05-42.3,P=0.057).
When subdivided groups by gender,we found a significant difference between higher TGF-β1 serum levels in female CRC patients with GA + AA genotype compared to healthy women above 50 years with the same genotype(27.2 ng/mL IQR 18.5-31.6vs14.3 ng/mL IQR 8.4-25.9,P=0.04)(Figure 2B).However,TGF-β1 serum levels were significantly higher in healthy men above 50 years with the GG genotypethan the male CRC patients(50.9 ng/mL IQR 42.4-74vs22.6 ng/mL IQR 15.9-28.4,P=0.00022)(Figure 2C).
When we compared TGF-β1 levels among early and advanced CRC cases,and healthy controls,we did not find differences in the levels(Figure 3).
Putting together all data on TGF-β1 serum levels in regards to early CRC(n=39)and advanced CRC(n=34)and healthy people above 50 years(n=48),and their genotypes,we found significant differences between higher levels of TGF-β1 serum levels in healthy controls above 50 years(GG genotype)and CRC patients(GG genotype)at the early stage(36.3 ng/mL IQR 19.9-56.5vs22.8 ng/mL IQR 14.6-28.6,P=0.037)and advanced CRC(36.3 ng/mL IQR 19.9-56.5vs21.6 ng/mL IQR 15.9-33.9,P=0.039)(Figure 4A).Additionally,TGF-β1 serum levels were increased in healthy persons above 50 years with homozygous TGF-ΒR2G[-875]G genotype compared to GA + AA genotype(36.3 ng/mL IQR 19.9-56.5vs16.3 ng/mL IQR 10-42.3,P=0.058)(Figure 4A).
Depending on the sex of the patients and healthy controls above 50 years,the highest levels of the cytokine occurred in healthy males above 50 years in comparison with CRC patients at early or advanced stages of the disease(51.9 ng/mL IQR 42.4-65.3vs23.3 ng/mL IQR 16.5-27.1,P=0.00078;and 51.9 ng/mL IQR 42.4-65.3vs22.9 ng/mL IQR 18.7-30.5,P=0.00039,respectively)(Figure 4B).
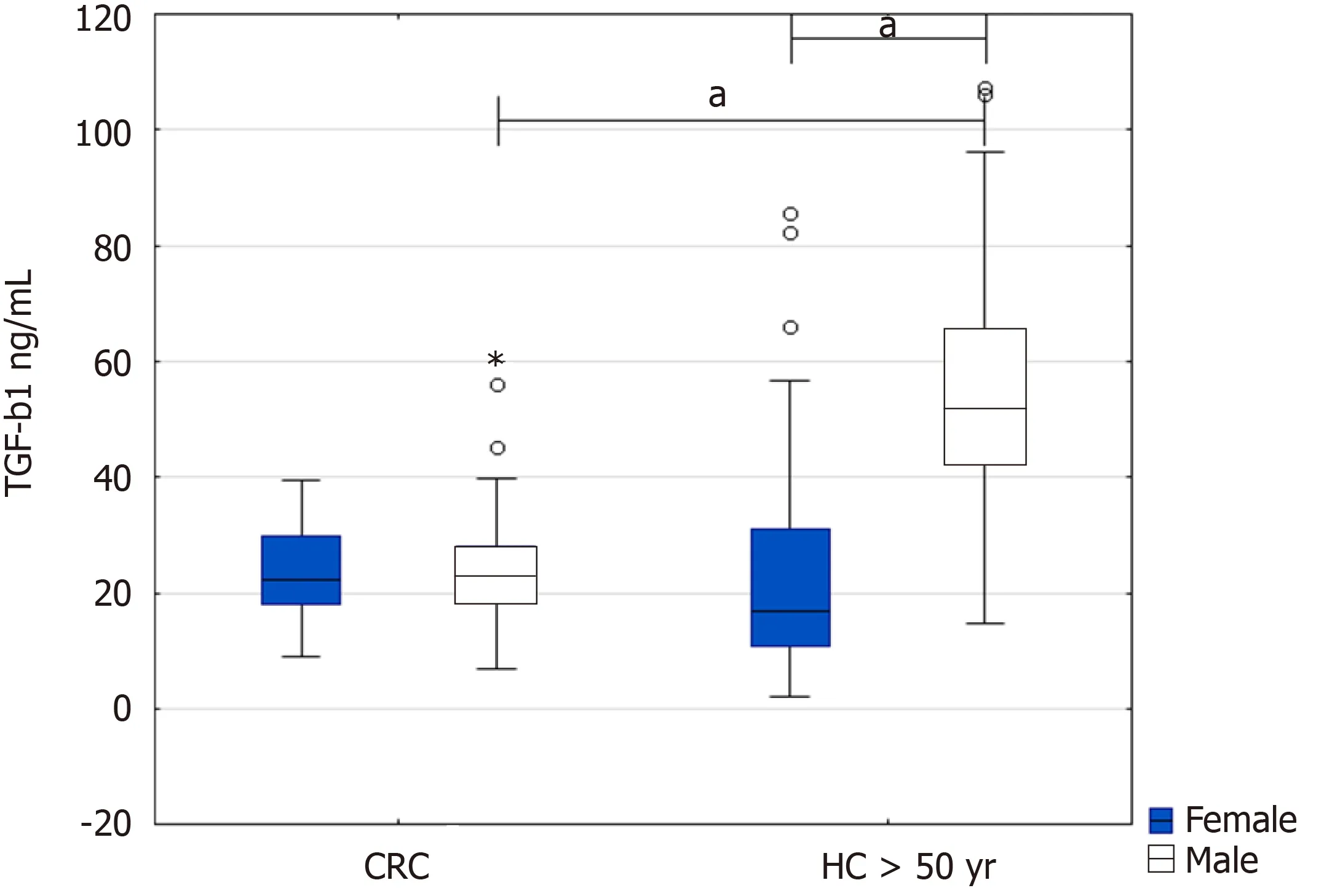
Figure 1 Serum levels of transforming growth factor beta 1 in colorectal cancer patients compared to healthy controls above 50 years.Results are presented as median and interquartile range.aP <0.001,*extremes,?outliers.
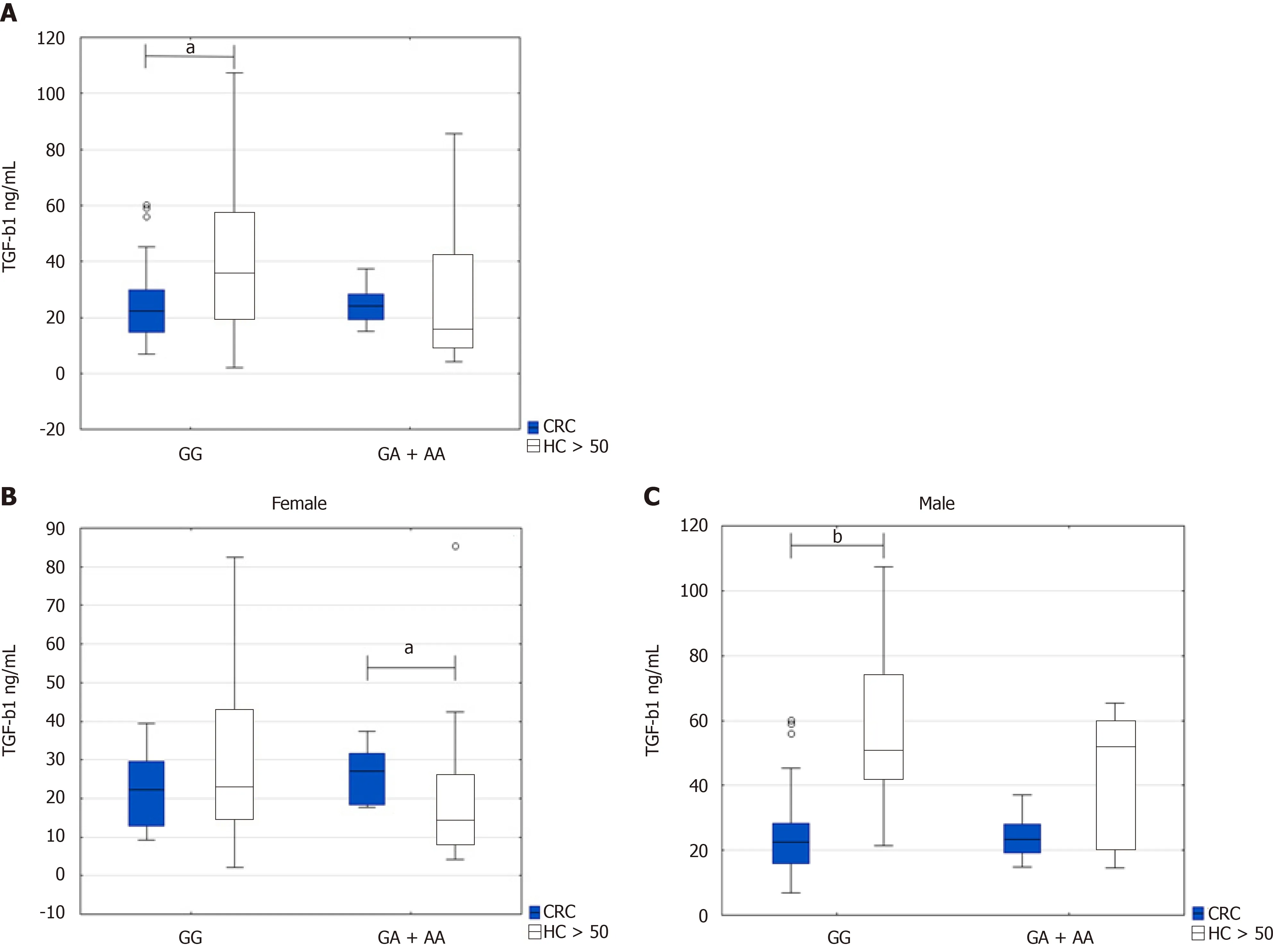
Figure 2 Transforming growth factor beta serum levels in colorectal cancer patients and healthy controls above 50 years.A:Depending on the TGF-ΒR2 -875G/A genotype;B:Depending on the female CRC patients and controls;C:Depending on the male CRC patients and controls.Results are presented as median and interquartile range.aP<0.05,bP <0.001,?outliers.
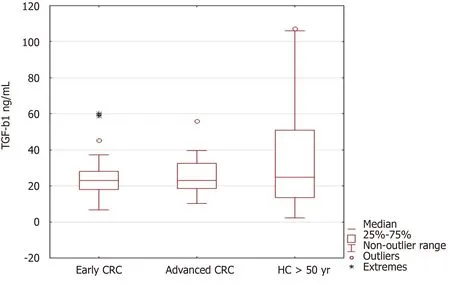
Figure 3 Transforming growth factor beta 1 serum levels in early and advanced colorectal cancer and healthy persons above 50 yearsgenotyped for the TGF-ΒR2 -875G/A.Results are presented as median and interquartile range.*Extremes,?outliers.
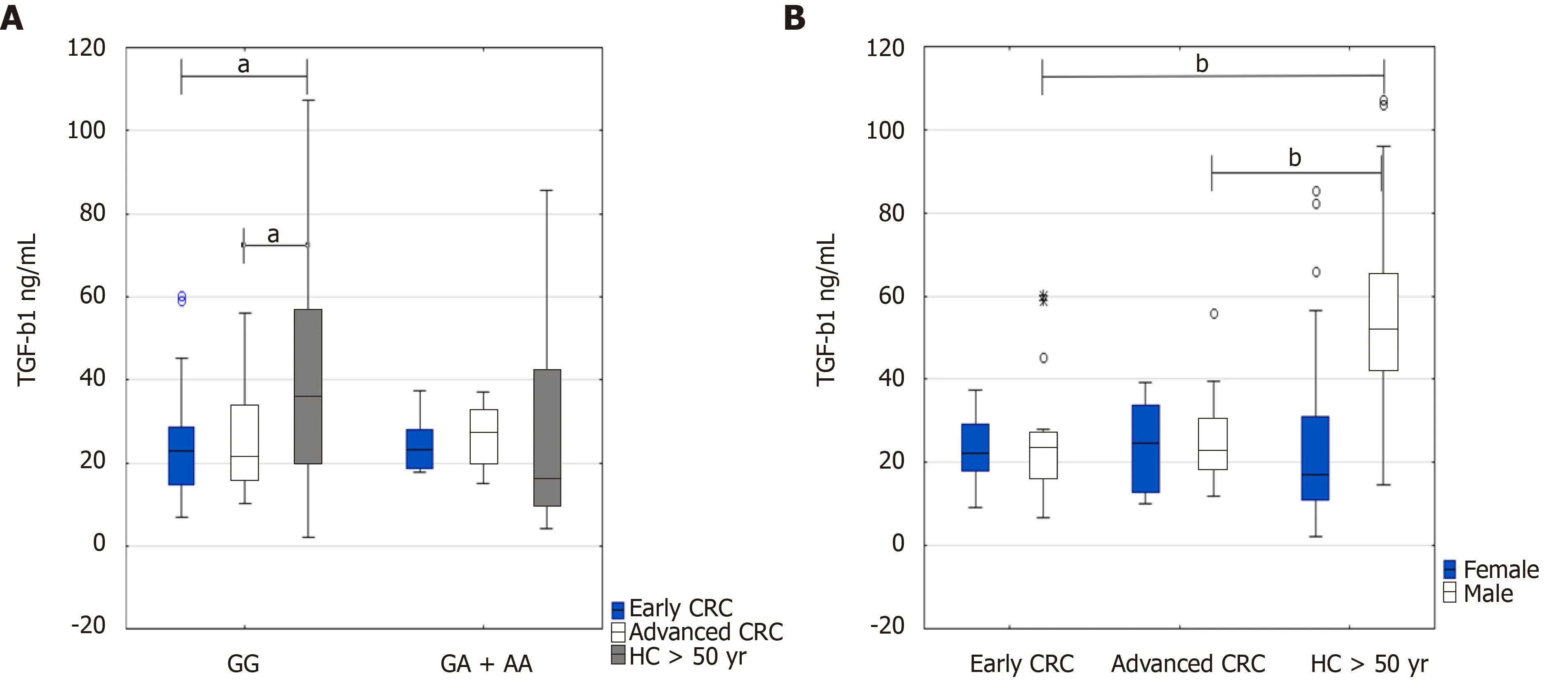
Figure 4 Transforming growth factor beta 1 serum levels in early and advanced colorectal cancer and healthy persons above 50 years.A:Regarding genotype;B:Regarding sex of the patients.Results are presented as median and interquartile range.aP <0.05,bP <0.001,*extremes,?outliers.
No differences in TGF-β1 serum levels in female CRC patients and healthy persons were observed.
DISCUSSION
CRC is globally the second- to third largest cancer-associated cause of death[16].
Although recent improvements in diagnostics and surgical treatment options were demonstrated,as well as the CRC mortality rate is gradually decreasing in some western countries.Still,the prognosis is unfavorable in metastasized cases[17].
TGF-β signaling may further contribute to these unfavorable outcomes.In line with this,a more profound understanding of the role of TGF-β and its receptors for tumor cells and the CRC tumor environment is crucial.Moreover,this can widen the therapeutic strategies,especially in novel target therapy development[16].
It was shown that TGF-β possesses a double role in the development and progression of the various gastrointestinal tumor,acting as both a tumor suppressor and tumor promoter[3].
However,beyond the recognized role of TGF-β in colorectal tumorigenesis,there is growing evidence that mutations or polymorphisms of TGF-β receptors could also contribute to CRC development[5].It was also demonstrated that inactivated or absent TGF-ΒR2 might be a factor causative for CRC transformation[18,19].
This gene inactivation due to mutation of TGF-ΒR2 occurs in about 30% of CRC and >90% in microsatellite unstable CRC[20-22].Thus,primary attention is paid to accumulated mutations of TGF-ΒR2 in the context of microsatellite instability in CRC.However,despite frameshift mutations in theTGF-ΒR2gene,the mutated gene still can produce a functional protein[23,24].
More research is devoted to a polymorphic allele of the type I receptor.Different polymorphisms of TGF-ΒR2 donate to CRC development are still unclear[5].Previously,we found decreased TGF-β1 in serum samples of male CRC patients significantly associated with CC genotype,indicating that -509C/T functional promoter polymorphism(rs1800469)within the TGF-β1 gene(TGF-Β1)increased the cancer risk,particularly for advanced stages[14].A noteworthy finding of the study of Xuet al[25]was that TGF-Β1 -509C/T and TGF-ΒR2 -875A/G gene polymorphisms acted synergistically on the decreased risk of gastric cancer.The risk of gastric cancer was notably lower among subjects who carried both the TGF-Β1 -509C and TGF-ΒR2 -875A allele genotypes.We also demonstrated that a combination of TGF-Β1T[-509]T with the TGF-ΒR2G[-875]A genotype might be a protective factor against relapsing-remitting multiple sclerosis development in men[15].
Additionally,TGF-ΒR2 polymorphisms were observed in various conditions and diseases,such as hypospadias[26],thyroid carcinoma[27],cardiovascular diseases[28],etc.
In the present case-control study,we evaluated the distribution of the allele and genotype frequencies of TGF-ΒR2 -875 G/A polymorphism in Bulgarian CRC patients and assessed CRC development risk and the association of serum TGF-β protein expression in a gender-dependent manner.
Our findings from this case-control study suggested that the highest risk for developing colorectal neoplasia was found for the GG genotype.The increased risk for CRC development was associated with male CRC patients homozygous for GG genotype,whereas the lowest risk–with GA genotype.To the best of our knowledge,no other studies for this polymorphism and CRC association from the literature are available.
A study that investigated the TGF-ΒR2 -875 GG genotype found a significantly decreased risk of gastric cancer development in the Chinese carriers of the A allele(AA/AG genotypes)(OR=0.58;95%CI:0.62-0.91;P<0.001)[25].Additionally,a combination of the TGF-Β1 -509 C and TGF-ΒR2 -875 A alleles were further related to a decreased risk of gastric cancer development(OR=0.42;95%CI:0.32-0.57,P<0.001).The authors also speculated that these findings elucidated the possible biological mechanisms and the underline tumor heterogeneity.
In our study,TGF-ΒR2[-875]*A-allele itself was associated with a protective effect regarding CRC development.When we further stratified the healthy controls by age,we found a significant increase in serum TGF-β1 in healthy men above 50 years compared to male patients with CRC,especially those carrying the GG genotype.Still,the highest serum TGF-β levels were observed in healthy men with GA + AA genotype.
After stratifying CRC patients into those with early and advanced,we found that male carriers of the A allele(GA/GA + AA genotypes)had a significantly decreased risk of advanced CRC.
The reasons for these disparities in the distribution of genetic polymorphisms in populations stratified by gender are not fully understood.However,recently developed gene-sequencing technologies have elucidated some of the possible mechanisms.It is well-known that TGF-β acts as a tumor promoter in the advanced stages of CRC carcinogenesis.Higher expression of TGF-β was related to recurrence and decreased survival rate of CRC patients[29,30].Furthermore,prolonged expression of TGF-β in the intestines stimulates the neoplastic transformation,invasion,and metastasis[31,32].
On the contrary,TGF-β usually inhibits tumor progression in premalignant epithelial cells.However,in TGF-β pathway dysregulation,signal reprogramming occurs that promotes survival and spreading of cancer cells[33,34].
Stanilovaet al[14]demonstrated for the first time the role of TGF-β in CRC depending on the gender of the patients.Their findings emphasized the significance of TGF-β and its functional polymorphism in CRC development in both male and female patients.
We did not find significant differences in the present study when we compared TGF-β1 serum levels among early and advanced CRC cases and healthy controls.However,when put together all data on TGF-β1 serum levels in regards to early and advanced CRC and healthy people above 50 years,we found significant differences between higher levels of TGF-β1 serum levels in healthy controls above 50 years and CRC patients at the early and advanced CRC,significant differences were calculated only for the GG genotype.
Additionally,TGF-β1 serum levels were also increased in healthy persons above 50 years with homozygous TGF-ΒR2G[-875]G genotype compared to GA + AA genotype.
Our study enlarges the data with a new entry regarding the effect of the TGF-ΒR2 gene -875G/A promoter polymorphism on serum acid-activated latent TGF-β1 quantities in serum samples of healthy persons and CRC patients.This study first examined serum TGF-β1 levels associated with the TGF-ΒR2 -875G/A polymorphism in a large group of Bulgarian healthy control subjects.We generally observed significant differences in serum TGF-β1 quantities depending on age and gender combined with genotype in the healthy control group,where the highest levels of the cytokine occurred in healthy males above 50 years.
One can suggest that decreased TGF-β combined with TGF-ΒR2 -875GG genotype might be connected with uncontrolled chronic inflammation,including in the gastrointestinal tract.The hypothesis for altered levels of cancer-associated cytokines,i.e.,decreased TGF-β1 and IL-10 in peripheral blood,together with cancer-associated reprogramming of gene expression in blood cells,was supported by many investigators,including us[35,36].Thus,we believe that normal concentrations of circulating TGF-β1 may suppress tumorigenesis by controlling the systemic and local gut inflammation.Furthermore,TGF-β may suppress tumor growth by inhibiting IL-6 trans signalling in CRC[9,36].
Moreover,a CRC cancer-specific overall survival has been shown to correlate with high TGF-β and low TGF-ΒR1 and TGF-ΒR2[37].
No differences in TGF-β1 serum levels in female CRC patients and healthy persons were found in our study,but the trend was similar;however,without reaching significance.Additional studies should further explain the observed discrepancies.
CONCLUSION
In conclusion,our results demonstrated that TGF-ΒR2 -875AG and AA genotypes were associated with a reduced risk of CRC,as well as circulating levels of TGF-β could prevent CRC development in a gender-specific manner.Notably,male carriers of TGFΒR2 -875A allele genotypes had a lower risk of CRC development and progression.Suggesting that TGF-ΒR2 -875A/G polymorphisms significantly affect protective biological factors,influencing the risk of colon and rectal carcinogenesis.
Although there is growing evidence that TGF-β signaling alterations(both mutations and polymorphisms of TGF-β receptors,etc.)contribute to CRC development and progression,there are still many unknown mechanisms.However,our data could help determine the greater risk for CRC development,especially in those patients that possess at least one C allele in their genotype.Ongoing advances in understanding TGF-β’s role in the pathogenesis and development of CRC may enable new approaches to CRC prevention and treatment.
ARTICLE HIGHLIGHTS
Research background
The role of transforming growth factor beta(TGF-β)signaling,which includes both the cytokine and its receptors,in the etiology of colorectal cancer(CRC)has been investigated recently.TGF-β-associated cancer pathways must be disrupted in the early stages of tumor growth,while TGF-β activation can promote cancer invasion and metastasis.
Research motivation
Given the importance of the TGF-β1 signaling pathway in CRC production and the fact that TGF-β1 exerts its effects through these receptors,we could hypothesize that genetic polymorphisms in the TGF-β1 gene and genes for TGF-β receptors may also play a role in CRC susceptibility.Previously,we recorded that circulating TGF-β1 and the -509C/T functional promoter polymorphism(rs1800469)within the TGF-β1 gene(TGF-Β1)plays a gender-dependent role in the resistance,development,and prognosis of CRC in Bulgarian patients.Therefore,we were interested in gender-associated differences in the frequency of TGF-ΒR2G[-875]A promoter polymorphism and CRC risk.
Research objectives
We performed a case-control gene association research approach to examine the association between TGF-β receptor 2 TGF-ΒR2G[-875]A promoter polymorphism and CRC risk in a cohort of Bulgarian patients,as well as TGF-β1 protein levels in the peripheral blood.We also estimated the role of this polymorphism at different stages of the disease,defined as early and advanced in men and women.
Research methods
One hundred eighty-four CRC patients and 307 sex and age-matched stable participants were recruited in the study.Primer-introduced restriction analysispolymerase chain reaction methods were used for genotyping the TGF-ΒR2G[-875]A(rs3087465)polymorphism.
Research results
The GG genotype was shown to have the greatest chance of developing colorectal neoplasia in this case-control study.Male CRC patients who were homozygous for the GG genotype had an elevated risk of developing CRC.Male carriers of TGF-ΒR2 -875A allele genotypes,on the other hand,had a lower chance of CRC growth and progression.TGF-β1 serum levels were higher in the GG genotype in people over 50 years old than in CRC patients.
Research conclusions
TGF-ΒR2 AG and AA genotypes were associated with a lower risk of CRC in our study.Besides,circulating TGF-β levels could inhibit CRC production in a genderspecific manner.
Research perspectives
Since we documented that male carriers of TGF-ΒR2 -875A allele genotypes had a lower risk of CRC formation and progression,we can imply that the TGF-ΒR2 -875A/G polymorphism has a direct effect on the protective biological factors that influence the risk of colon and rectal carcinogenesis.
 World Journal of Gastrointestinal Oncology2021年11期
World Journal of Gastrointestinal Oncology2021年11期
- World Journal of Gastrointestinal Oncology的其它文章
- Hepatocellular carcinoma biomarkers,an imminent need
- Anatomical vs nonanatomical liver resection for solitary hepatocellular carcinoma:A systematic review and meta-analysis
- Atezolizumab plus bevacizumab versus sorafenib or atezolizumab alone for unresectable hepatocellular carcinoma:A systematic review
- Cell-free DNA liquid biopsy for early detection of gastrointestinal cancers:A systematic review
- Colorectal cancer in Arab world:A systematic review
- Induction chemotherapy with albumin-bound paclitaxel plus lobaplatin followed by concurrent radiochemotherapy for locally advanced esophageal cancer
