LnCu3(OH)6Cl3(Ln=Gd,Tb,Dy): Heavy lanthanides on spin-1/2 kagome magnets?
Ying Fu(付盈) Lianglong Huang(黃良龍) Xuefeng Zhou(周雪峰) Jian Chen(陳見(jiàn)) Xinyuan Zhang(張馨元)Pengyun Chen(陳鵬允) Shanmin Wang(王善民) Cai Liu(劉才) Dapeng Yu(俞大鵬)Hai-Feng Li(李海峰) Le Wang(王樂(lè)) and Jia-Wei Mei(梅佳偉)
1Joint Key Laboratory of the Ministry of Education,Institute of Applied Physics and Materials Engineering,University of Macau,Avenida da Universidade,Taipa,Macao SAR 999078,China
2Shenzhen Institute for Quantum Science and Engineering,and Department of Physics,Southern University of Science and Technology,Shenzhen 518055,China
3Department of Physics,Southern University of Science and Technology,Shenzhen 518055,China
4Institute of Functional Crystals,Tianjin University of Technology,Tianjin 300384,China
5Institute of Resources Utilization and Rare-earth Development,Guangdong Academy of Sciences,Guangzhou 51065,China
6Shenzhen Key Laboratory of Advanced Quantum Functional Materials and Devices,Southern University of Science and Technology,Shenzhen 518055,China
Keywords: kagome lattice,hydrothermal method,frustrated magnetism,spin-1/2
1. Introduction
The kagome antiferromagnet (KAFM) has been intensively investigated both theoretically and experimentally as a long-standing platform to search for quantum spin liquid (QSL),[1–5]which is highly entangled quantum matter and features fractional excitations and no symmetrybreaking down to absolute zero temperature. Two famous KAFMs,herbertsmithite(Cu3Zn(OH)6Cl2)[6,7]and Znbarlowite (Cu3Zn(OH)6FBr),[8,9]have been regarded as the prototype for QSL. Both of them show no phase transition down to low temperatures and exhibit fractional spinon excitations revealed by inelastic neutron scattering (INS) and nuclear magnetic resonance(NMR).Beyond QSL,additional interactions like Dzyaloshinskii–Moriya (DM) interactions and single-ion anisotropies may lead KAFM into other exotic ground states. For example, V3+(S= 1) ions in NaV6O11[10,11]build a kagome lattice and form spin-singlets.KFe3(OH)6(SO4)2(S=5/2)[12]presents a long-range order with positive chirality, while CdCu3(OH)6(NO3)2(S=1/2)forms 120°spin structure with negative chirality.[13,14]Theoretically, a suitable combination of geometric frustration,ferromagnetism, and spin–orbit interactions in kagome magnets would realize high-temperature fractional quantum hall states and superconducting state.[15–18]Experimentally, the Kondo physics scenario of non-magnetic impurities screened by spinons in QSL has been proposed according to the muon spin relaxation (μSR) study on ZnCu3(OH)6SO4,[19]analogous to the Kondo effect usually observed in 3d–4f heavy fermion metals, where local spins are screened by itinerant electrons.
Recently,YCu3(OH)6Cl3with perfect Cu-kagome layers and free of the Y–Cu anti-site disorder has been proposed as an ideal quantum KAFM,[20]which has a“q=0”type(i.e.,the magnetic unit cell is identical to the structural unit cell with uniform chirality) antiferromagnetic (AFM) order with negative chirality due to a large DM interaction.[21,22]Replacing yttrium with light lanthanides,RCu3(OH)6Cl3(R=Nd,Sm,Eu)compounds still show strongly frustrated behaviors in despite of forming the canted AFM order with Neel temperatures(TN)ranging from 15 K to 20 K.[23,24]With expecting that the heavy rare earths may further affect the magnetic frustration,we synthesized the polycrystalline samples of LnCu3(OH)6Cl3(Ln= Gd, Tb, Dy) by a universal way. The magnetic susceptibilities and heat capacity were measured. We discussed the magnetic contributions of Cu-kagome lattice and heavy lanthanides. With these results, we conclude that the heavy lanthanides ions in LnCu3(OH)6Cl3have little impact on the intrinsic magnetism of kagome-Cu2+.
2. Experimental details
Although the structure of GdCu3(OH)6Cl3was reported by Sunet al.[23]with tiny crystals, the high-purity sample was not obtained for further investigation of magnetic properties. In this work,we efficiently synthesized LnCu3(OH)6Cl3(Ln = Gd, Tb, Dy) samples with high purity by a hydrothermal method. The starting reagents were GdCl3·6H2O(99.9%, Alfa Aesar), TbCl3·6H2O (99.99%, Energy Chemical), DyCl3·6H2O (99.99%, Energy Chemical), and CuO(99.9%, Alfa Aesar). LnCl3·6H2O was ground thoroughly with CuO in a ratio of 1:3, and the mixture was transferred into an autoclave and heated at 200°C for about 10 h. Finally, the blue polycrystallined powder of LnCu3(OH)6Cl3was obtained after washed repeatedly by alcohol.This method avoids impurities and is also suitable for the preparation of YCu3(OH)6Cl3,SmCu3(OH)6Cl3,and EuCu3(OH)6Cl3.
The temperature-dependent powder x-ray diffraction(PXRD) was performed fromT=300 K to 4 K on Rigaku Smartlab-9 kW diffractometer with CuKαradiation (λKα1=1.54056 °A,λKα2=1.54439 °A,and intensity ratioIKα1:IKα2=2 : 1). The scanning step width of 0.01°was applied to record the patterns in a 2θrange of 10°–100°. The structures of LnCu3(OH)6Cl3were refined by Rietveld profile methods using the FULLPROF suite of programs.[25]The magnetic and specific heat measurements of LnCu3(OH)6Cl3were performed with Quantum Design (QD) magnetic property measurement system(MPMS)SQUID magnetometer and physical property measurement system(PPMS),respectively.
3. Results and discussion
3.1. Crystal structure
The site disorder remains mired in controversy in this series of compounds. For YCu3(OH)6Cl3,single-crystal diffraction revealed a splitting disorder of Y3+and no anti-site disorder between Cu2+and Y3+.[20]However, the neutron scattering results[26]supported no splitting disorder for Y3+. The no site-splitting for the rare earth ions was also proposed for LnCu3(OH)6Cl3(Ln=Nd, Sm, Gd, Eu).[23,24]In this experiment,we found no obvious improvement for the refinements after taking into account the splitting disorder for the rare earth ions. Therefore,we performed Rietveld profile refinement on LnCu3(OH)6Cl3(Ln = Gd, Tb and Dy) without considering the site-splitting of Ln3+.
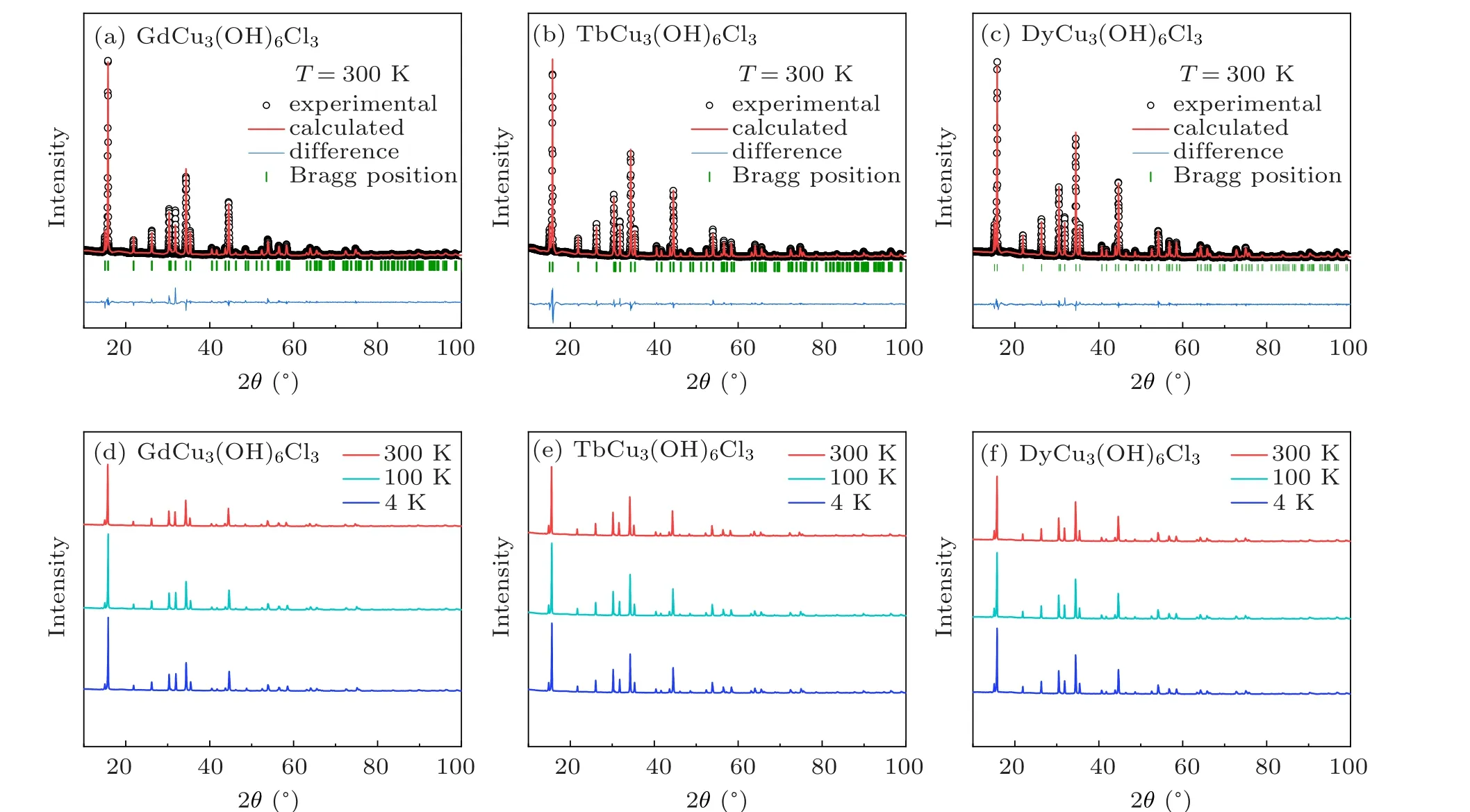
Fig. 1. Powder XRD patterns and refinements for LnCu3(OH)6Cl3. (a)–(c) Refinements for PXRD at 300 K. (d)–(f) XRD patterns of LnCu3(OH)6Cl3 at T =300 K,100 K,4 K.
As shown in Figs. 1(a)–1(c), the good refniements for LnCu3(OH)6Cl3in space groupP3m1(No.164)suggest that our powder sample is of high quality. The detailed lattice parameters are listed in Table 1.As excepted at 300 K,the lattice parameters decrease from Gd3+to Dy3+in coincidence with the decreasing of ion radius(r=1.053 °A,1.04 °A,1.027 °A for Gd3+, Tb3+, Dy3+, respectively). However, at 4 K,aandbshow a contrast behavior withc,which is associated with the anisotropic thermal expansion. The temperature-dependent XRD patterns, as shown in Figs. 1(d)–1(f), have no peaksplitting or new peaks appearing as the temperature decreases down to 4 K,suggesting that no structure transition happens to LnCu3(OH)6Cl3.
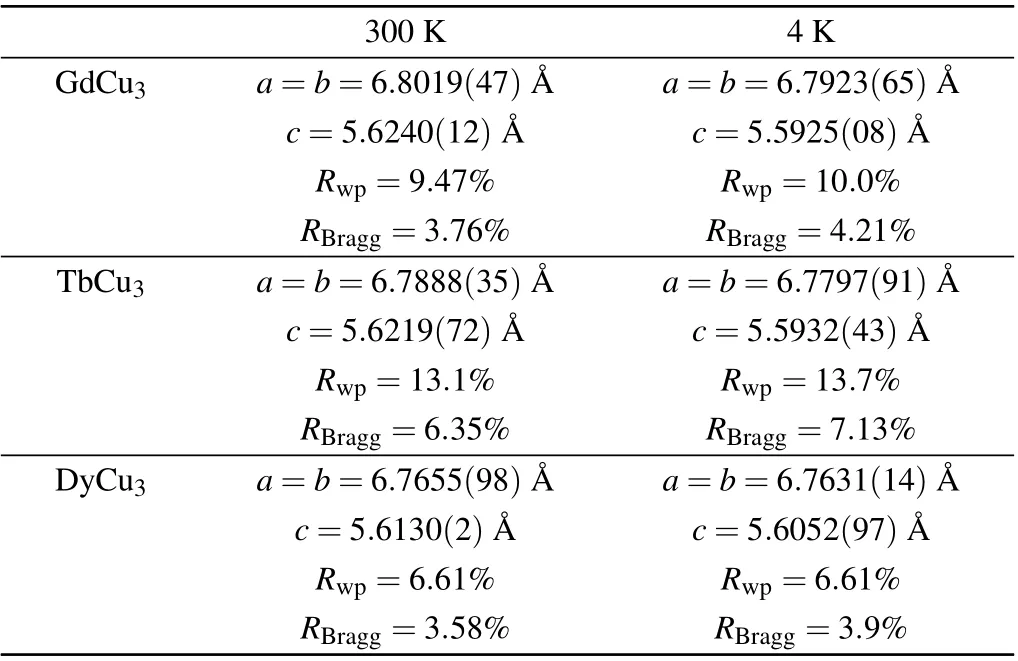
Table 1. Comparison of lattice parameters at 300 K and 4 K forLnCu3(OH)6Cl3. LnCu3(OH)6Cl3 is abbreviated to LnCu3.
As depicted in Fig. 2, each Cu2+is surrounded by four equivalent O2?and two Cl?, forming a distorted [CuO4Cl2]octahedron with the Cu–Cl bond (~2.82 °A) significantly longer than the Cu–O bond (~1.97 °A). The [CuO4Cl2] octahedrons connect to each other by sharing the O–Cl edges to build the Cu-kagome plane. Ln3+is 8-coordinated by six O2?and two Cl?to form[LnO6Cl2]dodecahedron and locates at the center of the Cu-hexagon to constitute a Ln-triangular lattice(Fig.2(c)).
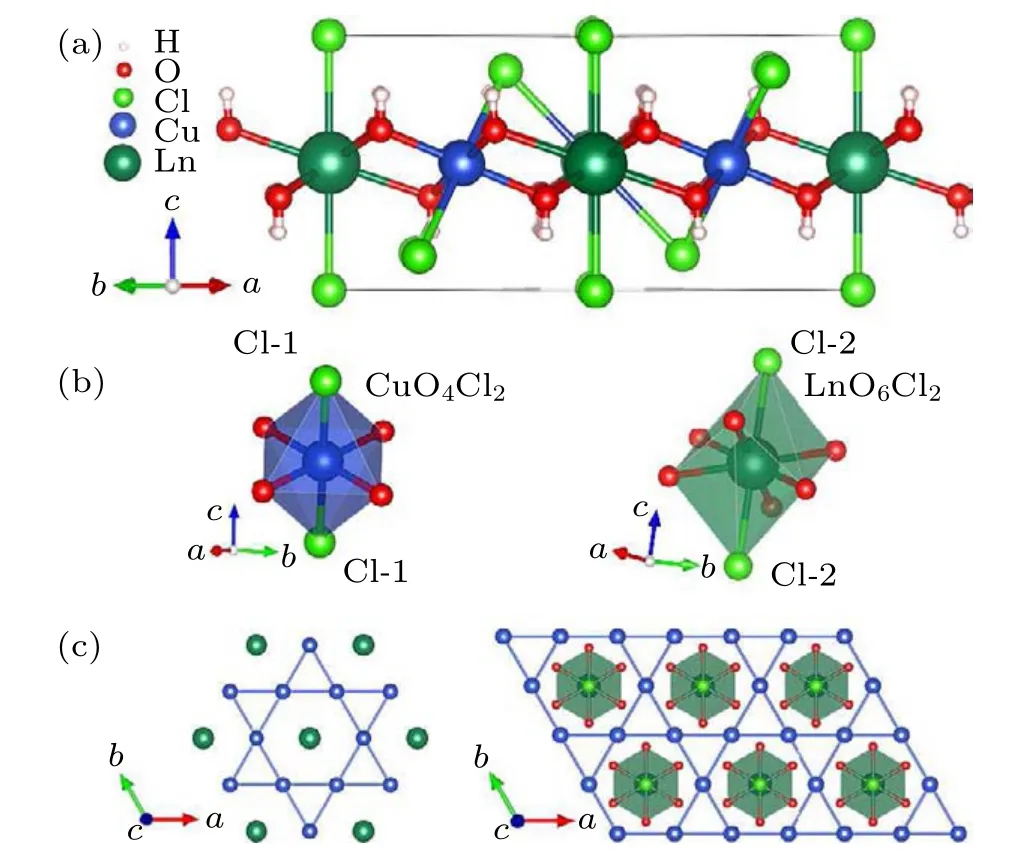
Fig. 2. Crystal structure of LnCu3(OH)6Cl3 (Ln = Gd, Tb, Dy) without considering the site-splitting of Ln3+ ions. (a)Unit cell structure. (b)Coordinations of Cu and Ln atoms. (c)The illustration of Cu-kagome lattice and Ln-triangular lattice.
It is worth noting that analogous distorted octahedra[CuO4Cl2] in herbertsmithite and Y3Cu9(OH)19Cl8increase the splitting in the ligand-field of d orbitals and lower the energy level of dz2with a large d–d gap around 1–2 eV,unveiling the insulating nature of a charge transfer insulator,[27]which would be adapted to YCu3(OH)6Cl3and LnCu3(OH)6Cl3.
The Cu–O–Cu super-exchange bond angles are 118.78(12)°for GdCu3(OH)6Cl3, 118.19(10)°for TbCu3(OH)6Cl3, and 117.7(4)°for DyCu3(OH)6Cl3, respectively.The values are comparable to the antiferromagnets like barlowite (117.4°)[28]and herbertsmithite (119°).[29]The Cu–Cu distances are 3.40330(11) °A for GdCu3(OH)6Cl3,3.39115(16) °A for TbCu3(OH)6Cl3, and 3.3830(3) °A for DyCu3(OH)6Cl3,equal to the corresponding Ln–Cu distance.
3.2. Magnetic properties
Figure 3 shows the temperature-dependent magnetizaion for LnCu3(OH)6Cl3(Ln=Gd,Tb,Dy),with YCu3(OH)6Cl3served as a reference.For YCu3(OH)6Cl3(Fig.3(a)),the magnetic susceptibilities increase suddenly at 15 K,which is associated with the negative-vector-chirality 120°magnetic structure, confirmed by the neutron scattering study.[22]Continuously lowering the temperature,the drops at 3–4 K correspond to possible spin-glass state.[21]Compared to the small magnetic moment of Cu in YCu3(OH)6Cl3, the magnetic susceptibilities of LnCu3(OH)6Cl3are much larger, suggesting that the dominant contribution to the magnetization arises from lanthanides, especially at low temperatures. As shown in Figs.3(b)–3(d),under low fields,LnCu3(OH)6Cl3compounds present similar temperature-dependent magnetization curves as YCu3(OH)6Cl3,with a rapid increase at about 16 K or 17 K and followed by a drop at lower temperatures. The zero fieldcooling(ZFC)and field-cooling(FC)data at 0.005 T begin to split after the rapid increase, which could be ascribed to the possible in-plane canted ferromagnetic component, as previously pronounced inRCu3(OH)6Cl3(R=Nd,Sm,Eu).[23,24]It indicates that the Cu-kagome lattice of LnCu3(OH)6Cl3may have the same physics as YCu3(OH)6Cl3, and form a magnetic structure atTN~16 K, which necessitates a neutron scattering study. In contrast to the robust magnetic order at 15 K in YCu3(OH)6Cl3, the magnetic phase transition of LnCu3(OH)6Cl3can not be identified easily with increasing field. WhetherTNwas suppressed by fields or the magnetic responses of Ln3+ions masked the magnetic order of Cu2+needs a further measurement of the specific heat.
As shown in Fig.4,the high temperature behavior of the inverse magnetic susceptibility above 150 K was fit to the Curie–Weiss lawχ=C/(T ?θ) (whereCis the Curie constant, andθis the Weiss temperature) in red lines withC=9.52 K·emu·mol?1,13.76 K·emu·mol?1,16.05 K·emu·mol?1andθ=?13.98 K,?16.37 K,?13.72 K for Gd-, Tb-, and Dy-compounds, respectively. Considering the crystal-field splitting for Ln3+ions, we also applied the Curie–Weiss fitting between 20 K to 50 K, shown in black lines. The deducedθare?1.71 K,?4.24 K,?1.40 K for GdCu3(OH)6Cl3,TbCu3(OH)6Cl3, and DyCu3(OH)6Cl3, respectively. The absolute values ofθare smaller than those at high temperatures. The small Weiss temperatures are significant different from Nd-,Sm-,and Eu-analogues,[23,24]whoseθranges from?100 K to?300 K with a distinct spin frustration compared toTN~15 K.Two main reasons for the reduction ofθare proposed: One is that the Cu–Cu AFM-interaction is weakened by Ln3+; the other is that Ln3+ions form a FM-interaction that competes with the Cu–Cu AFM-interaction.
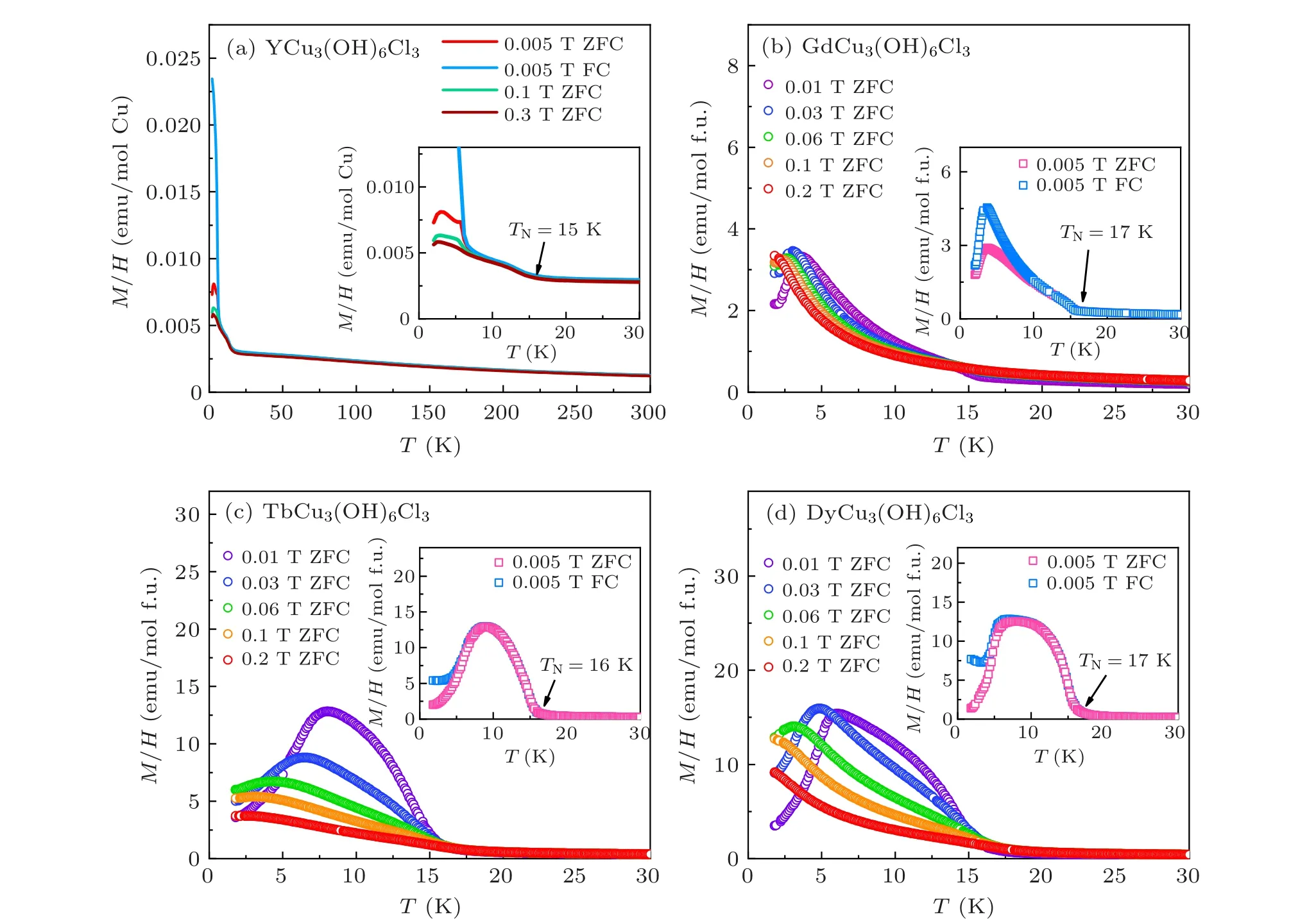
Fig. 3. Temperature-dependent magnetization of YCu3(OH)6Cl3 and LnCu3(OH)6Cl3 (Ln = Gd, Tb, Dy) at selected fields. Inset in (a) is the zoom-in data,and insets in(b)–(d)are the ZFC and FC curves collected at 0.005 T.
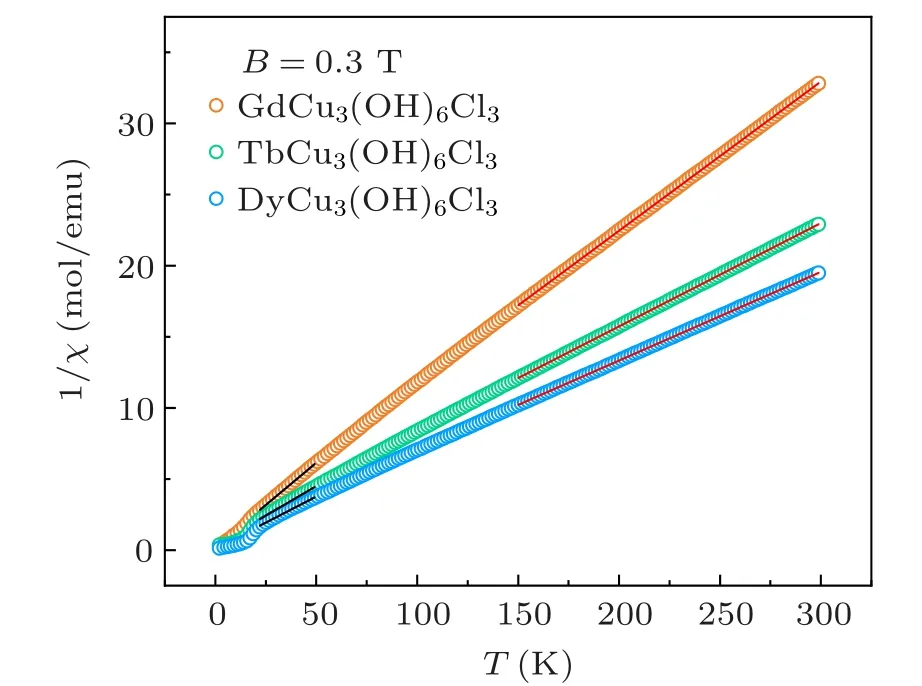
Fig. 4. Temperature dependence of inverse magnetic susceptibilities 1/χ under a field of 0.3 T.The red and black lines are the fitting-plots of Curie–Weiss law at high temperatures (150–300 K) and low temperatures (20–50 K),respectively.
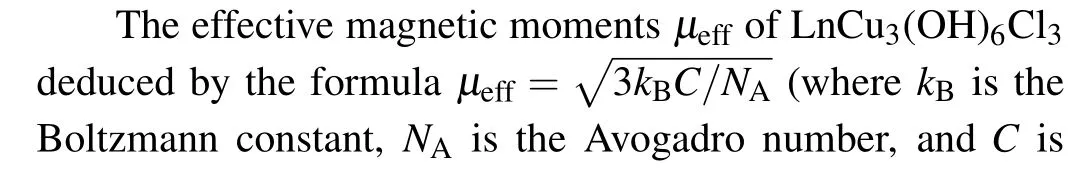
The field-dependent magnetization curves (M–H) of LnCu3(OH)6Cl3(Ln = Gd, Tb, Dy) are shown in Fig. 5,referred to YCu3(OH)6Cl3collected at 2 K. With decreasing temperature, the magnetization of LnCu3(OH)6Cl3increases and grows rapidly below a field of about 2 T. At 2 K, GdCu3(OH)6Cl3saturates to a large value of 7.69μBat 7 T, suggesting that spins of Gd3+ions are polarized.TbCu3(OH)6Cl3and DyCu3(OH)6Cl3seem to be saturated with a linear increase under higher fields, especially for DyCu3(OH)6Cl3,which could be ascribed to the temperatureindependent Van Vleck paramagnetism. It is noted that the magnetic moment of each Cu2+at 2 K is only 0.065μBin the strong frustrated material, YCu3(OH)6Cl3(μ0H= 7 T)(Fig.5(a)),which is two orders of magnitude smaller than that of LnCu3(OH)6Cl3. Thus, we deduce that lanthanide has a dominated magnetic contribution in LnCu3(OH)6Cl3at low temperatures and can easily dominate the magnetic response of the Cu-kagome lattice.

Fig.5. Field-dependent magnetization of(a)YCu3(OH)6Cl3 at 2 K and(b)–(d)LnCu3(OH)6Cl3 (Ln=Gd,Tb,Dy)at selected temperatures.
3.3. Specific heat
Figure 6 shows the specific heat results of LnCu3(OH)6Cl3(Ln = Gd, Tb, Dy) and YCu3(OH)6Cl3.As shown in Fig. 6(a), under zero field, a shoulder anomaly is observed at around 15–17 K for each compound, consistent with the rapid increase of magnetic susceptibilities atTN,representing a formation of magnetic order for kagome-Cu2+.Moreover, the low temperature (below 10 K) specific heat of LnCu3(OH)6Cl3shows more features,in contrast to decaying to zero for YCu3(OH)6Cl3, relating to the low-temperature magnetic correlation of Ln3+ions.
For further understanding the origin of the magnetic phase transition, we measured the specific heat with applied magnetic fields. Since the intrinsic nearest neighboring interaction in YCu3(OH)6Cl3is around 80 K,[30]the applied magnetic field (5 T) has little impact on the specific heat and entropy (see Figs. 6(b) and 6(c)), in line with previous report on YCu3(OH)6Cl3[21]and EuCu3(OH)6Cl3.[24]However, as shown in Figs.6(d)–6(f),Cp/Tof LnCu3(OH)6Cl3responses notably to the external magnetic field. For GdCu3(OH)6Cl3,the upturn ofCp/Tis generally evolved into a broad peak and pushed to high temperatures by field withTNkeeping constant. For TbCu3(OH)6Cl3and DyCu3(OH)6Cl3,the lowtemperature broad peak ofCp/Tis efficiently pushed to aboveTNby a field of 7 T and merges with the high-temperature broad peak induced by the magnetic phase transition. This behavior of driving the specific heat peak position to high temperatures by applied magnetic fields may indicate a formation of short-range ferromagnetic order belowTN.
Considering that YCu3(OH)6Cl3forms a robustq= 0 type AFM order andRCu3(OH)6Cl3(R= Nd, Sm, Eu) enters a canted AFM phase belowTN,we speculated reasonably that LnCu3(OH)6Cl3(Ln = Gd, Tb, Dy) also has a canted AFM phase transition atTNwith a large ferromagnetic component. The ferromagnetic correlation is influenced obviously by fields and even screens the signal of AFM ordering in magnetic susceptibility, but the AFM phase indeed exists and is robust under large fields,like the case in YCu3(OH)6Cl3.
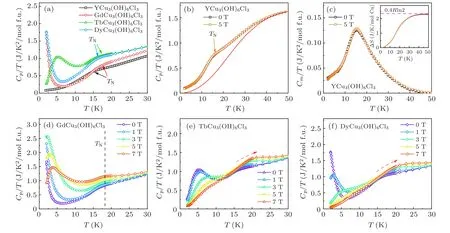
Fig. 6. Specific heat for YCu3(OH)6Cl3 and LnCu3(OH)6Cl3 (Ln = Gd, Tb, Dy). (a)Cp/T for YCu3(OH)6Cl3 and LnCu3(OH)6Cl3 under zero field.(b) The Cp/T of YCu3(OH)6Cl3. The red solid line is phonon-contribution fitting. (c) Magnetic specific heat Cm/T of YCu3(OH)6Cl3 after subtracting phonon-contribution. Inset is magnetic entropy per Cu2+. (d)–(f)Temperature-dependent specific heat under different magnetic fields for GdCu3(OH)6Cl3,TbCu3(OH)6Cl3,and DyCu3(OH)6Cl3,respectively.
3.4. Discussion and conclusion
Theq=0 type magnetic structure with negative-chirality in YCu3(OH)6Cl3is interesting, which was also reported in other kapellasite-type compounds like CdCu3(OH)6(NO3)2withTN=4 K[14]and CaCu3(OH)6Cl2withTN=7.2 K.[31,32]As demonstrated recently,with light lanthanides(Sm and Eu)replacing yttrium, SmCu3(OH)6Cl3and EuCu3(OH)6Cl3still feature canted antiferromagnetic ordering with strong spin frustration.[23,24]The light lanthanides with small magnetic moment may have limited influence on the magnetism of Cukagome lattice.
In our work,the magnetic and thermodynamic behaviors of LnCu3(OH)6Cl3(Ln = Gd, Tb, Dy) exhibit two significantly different characteristics: large magnetic moment compared with YCu3(OH)6Cl3and a ferromagnetic-like spin correlation belowTN. According to our experimental results,heavy lanthanides(Gd,Tb,Dy)probably modulate the DM interaction and induce a large ferromagnetic correlation, which can mask the intrinsic low-temperature magnetic properties of kagome-Cu2+, but can not prevent the AFM ordering of Cukagome as revealed in specific heat. The Curie–Weiss law no longer works for evaluating the intrinsic interactions. The spectroscopy technology, like electron spin resonance (ESR)orμSR,is hopeful to further detect the detailed magnetic interactions for LnCu3(OH)6Cl3(Ln = Nd, Sm, Eu, Gd, Tb,Dy).
In summary, we have successfully synthesized the polycrystalline samples of LnCu3(OH)6Cl3(Ln = Gd, Tb and Dy). The heavy lanthanides significantly change the magnetic and thermodynamic behaviors,which keep the intrinsic magnetism of Cu-kagome lattice. LnCu3(OH)6Cl3(Ln=Nd,Sm,Eu, Gd, Tb, Dy) compounds provide a good platform to further investigate systemically the effect of lanthanides on the frustrated magnetism of Cu-kagome lattice.
Acknowledgement
We thank Dr. L.Zhang,Dr. J.M.Sheng,and Prof. L.S.Wu for useful discussion.
- Chinese Physics B的其它文章
- Physical properties of relativistic electron beam during long-range propagation in space plasma environment?
- Heterogeneous traffic flow modeling with drivers’timid and aggressive characteristics?
- Optimized monogamy and polygamy inequalities for multipartite qubit entanglement?
- CO2 emission control in new CM car-following model with feedback control of the optimal estimation of velocity difference under V2X environment?
- Non-peripherally octaalkyl-substituted nickel phthalocyanines used as non-dopant hole transport materials in perovskite solar cells?
- Dual mechanisms of Bcl-2 regulation in IP3-receptor-mediated Ca2+release: A computational study?

