Study on the regulatory effect of herbal cakepartitioned moxibustion on colonic CD206, AMPK and TSC2 in rats with Crohn disease
Dong Xiao-qing (董小慶), Li Xiao-ying (李曉瑩), Wang Xue-jun (王雪君), Guo Xiao-cong (郭瀟聰), Long Jun-yi(龍俊燚), Lu Yun-qiong (盧云瓊), Liu Li (劉力), Caoyao Jia-ni (曹姚佳妮), Zhang Dan (張丹), Lu Yuan (陸嫄),Wu Huan-gan (吳煥淦),, Xie Chen (謝晨), Ma Xiao-peng (馬曉芃),
1 Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China
2 Xi’an Traditional Chinese Medicine Hospital of Encephalopathy, Shaanxi Province, Xi’an 710032, China
3 Shanghai Research Institute of Acupuncture and Meridian, Shanghai 200030, China
Abstract
Keywords: Moxibustion Therapy; Indirect Moxibustion; Medicinal Cake-partitioned Moxibustion; Point, Tianshu (ST 25); Point,Qihai (CV 6); Crohn Disease; AMP-activated Protein Kinases; Tuberous Sclerosis Complex 2 Protein
Crohn disease (CD) is a chronic inflammatory disease of the gastrointestinal tract with protracted, recurrent,and gradually progressive symptoms[1]. The lesion involves entire digestive tract, mainly the ileum and the end of colon. The typical pathological features are segmental, asymmetry, and transmural infiltrating inflammation[2]. The clinical manifestations are right lower abdomen pain, chronic diarrhea and weight loss,as well as disease-associated symptoms such as fatigue and anorexia. Patients with colon involvement often have rectal bleeding or bloody diarrhea, and half of the patients have complications such as strictures, fistulas or abscesses[3]. Epidemiological investigations have shown that CD usually occurs in people over 20 years old, and the peak happens in 50-60 years old[4]. The incidence rate is relatively high in developed countries, e.g. it is 322 per 100 000 people in European countries. In recent years, the incidence rate of CD in Asian countries has also increased, reaching 0.54 per 100 000 people[5]. The onset of CD is related to many factors, currently believed to be mainly caused by innate and adaptive immune response disorders due to genetic susceptibility and environmental factors[1]. As a key cellular component of innate immunity[6], macrophages play important roles in the pathogenesis of CD.
Macrophage polarization refers to the phenotype and functional differentiation of mature macrophages induced by various factors[7]. According to different stimuli, surface molecular markers, and secreted cytokines and chemokines, macrophages can be divided into two polarized phenotypes: pro-inflammatory M1 type, the classically activated macrophages; antiinflammatory M2 type, the alternately activated macrophages[8]. It has confirmed that dextran sulfate sodium (DSS) induces increased M1 macrophages and decreased M2 macrophages in colitis mice[9-10].Promoting expression of colonic M2 macrophages in T cell transfer mouse model reduces intestinal inflammation[11]. AMP-activated protein kinase (AMPK)is closely related to macrophage activation. Activated AMPK directly phosphorylates tuberous sclerosis complex (TSC) 2, and competitively inhibits mammalian target of rapamycin complexes 1 (mTORC1) by promoting the formation of TSC1/2, thereby inhibiting nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome activation, promoting the polarization of macrophages to M2 phenotype to reduce inflammation[12-13]. In this study, a CD rat model was established by 2,4,6-trinitrobenzene sulfonic acid(TNBS) to observe the effect of herbal cake-partitioned moxibustion on the surface marker CD206 of colonic M2 macrophages and the upstream regulatory proteins of AMPK and TSC2; to explore the anti-inflammatory mechanism of herbal cake-partitioned moxibustion in CD treatment from the perspective of regulating colonic M2 macrophage expression.
1 Material and Methods
1.1 Experimental animal
Twenty-six specific pathogen free healthy male Sprague-Dawley rats, weighing (150±20) g, were provided by Shanghai Slack Laboratory Animal Co., Ltd.[SCXK (Hu) 2013-0016]. Before experiment, the rats were adaptively reared at room temperature (20±2) ℃ and humidity of 50%-70% for 7 d. All animal experiments followed the Guiding Opinions on the Treatment of Experimental Animals[14], which was issued by the Ministry of Science and Technology, and were approved by the Experimental Animal Ethics Committee of Shanghai University of Traditional Chinese Medicine.
1.2 Main instruments and reagents
Tissue dehydrator, paraffin embedding machine, slicer,spreader and slide warmer (Leica, Germany); optical microscope and analysis software (Olympus, Japan);tissue homogenizer (Fluko, Germany); multifunctional microplate reader (Bio Tek, USA); electrophoresis instrument and gel imaging system (Bio-Rad Laboratories, USA); Light Cycler 480 real-time fluorescence quantitative polymerase chain reaction (RTqPCR) instrument (Roche Diagnostic, USA).
Moxa (Nanyang Hanyi Moxa Co., Ltd., China); Guifu No.1 prescription (Huaji Pharmaceutical Co., Ltd., China; 5%(W/V) TNBS (Sigma-Aldrich, USA); DAB chromogenic reagent, rabbit immunoglobulin G (IgG)-immunohistochemistry kit (Boster Biological Technology Co., Ltd.,China); BCA protein concentration determination kit, Cy3 labeled goat anti-rabbit IgG (H+L), SDS gel kit, HRP labeled goat anti-rabbit secondary antibody, ECL luminescent solution, Tris and TEMED (Beyotime Biotechnology Co., Ltd., China); GAPDH, AMPKα (5831),TSC2 (4308s) (Cell Signaling Technology, USA); tissue RNA extraction kit (RN001-plus, EZ Bioscience, USA); cDNA reverse transcription kit Prime Script? RT Master Mix,PCR amplification kit TB Green? Premix Ex Taq? Ⅱ(Takara Biomedical Technology, Japan); hematoxylineosin (HE) staining solution (Nanjing Jiancheng Technology Co., Ltd., China); sodium pentobarbital,absolute ethanol, neutral gum, methanol and xylene(Sinopharm Group Chemical Reagent Co., Ltd., China);trizol reagent (Thermo Fisher, USA).
1.3 Model preparation and identification
Twenty-six rats were randomly divided into a normal group (n=9) and a modelling group (n=17) according to the completely random segment grouping method[15].CD rat model preparation method: 5% (W/V) TNBS[100 mg/(kg·bw)] and 50% ethanol was mixed at 2:1(volume ratio) to prepare an enema solution, and enema was carried out at 3 mL/(kg·bw). Rats were fasted for 24 h to prepare intestine before enema. The enema needle was slowly inserted into rat anus by 6-8 cm in a head-down-tail-up position, the enema fluid was injected, and then the rats were kept upside down for 1 min to prevent the enema fluid from overflowing.Enema was performed once a week for 4 consecutive weeks[16]. One rat in the modelling group and one rat in the normal group were randomly selected for colonic histopathological observation to verify whether the CD model was confirmed successfully prepared. After the model was successfully prepared, group intervention started.
1.4 Grouping and intervention
The 16 CD rats were randomly divided into a model group and a herbal cake-partitioned moxibustion group,with 8 rats in each group. Qihai (CV 6) and bilateral Tianshu (ST 25) were selected, and the main ingredient of medicinal cake wasFu Zi(Radix Aconiti Lateralis Praeparata) powder in the herbal cake-partitioned moxibustion group. The mixture ofFu Zi(Radix Aconiti Lateralis Praeparata) powder and yellow wine was pressed into herbal cakes using a self-made special mold(0.6 cm in diameter, 0.3 cm in thickness). Moxa was pressed into cone-shaped moxa cones (0.4 cm in diameter, 0.4 cm in height, about 90 mg in weight) using a special mold. The herbal cake was first placed on the acupoints, the moxa cone was placed on the herbal cake,and then the moxa cone was lighted for moxibustion.Moxibustion was performed with 2 cones per acupoint,once a day, for 7 consecutive days. Rats in the normal group and the model group only received the same grasping and fixation as in the herbal cake-partitioned moxibustion group.
1.5 Sample collection
After intervention, rats were fasted for 24 h and euthanized by intraperitoneal injection of sodium pentobarbital. Rats were fixed in a supine position, the abdominal wall was cut to collect all colons from 2 cm above the anus to the lower end of the cecum, and then cut longitudinally; after rinsed with 4 ℃ pre-cooled normal saline, about 1 cm severely damaged colon determined by gross observation was selected and fixed in 4% paraformaldehyde solution. The remaining colon was placed in a cryotube and stored in -80 ℃refrigerator after quickly frozen in liquid nitrogen.
1.6 Indicator detection
1.6.1 Morphological observation of colon tissues
After HE staining of colon tissues, the morphological changes were observed under an optical microscope.
The main operation steps included: tissue dehydration, paraffin embedding, sectioning (4 μm),baking slices at 60 ℃ for 1 h, dewaxing and hydrating in xylene and gradient alcohol, staining with hematoxylin for 10 min, washing with tap water for 10 min,differentiating with 1% hydrochloric acid and alcohol for 3 s, rinsing with tap water for 5 min, staining with 0.5%eosin for 2.5 min, and then dehydrating with 70%, 80%,90% and 100% gradient alcohol in sequence. After being transparentized with xylene, the slides were mounted with neutral gum.
1.6.2 Detection of surface marker CD206 of colonic M2 macrophages
The CD206 expression was detected by immunohistochemistry (IHC). Paraffin sections of colon tissues were pretreated with baking slices, xylene, and gradient alcohol, then immersed in citric acid buffer and heated in microwave oven for hot antigen retrieval.Endogenous peroxidase was blocked with 3% H2O2. The sections were incubated overnight at 4 ℃ in a humidified box after blocked with BSA and dropwise added with rabbit anti-CD206 antibody (1:1 000);reheated at 37 ℃ for 45 min, washed 3 times with PBS for 3 min/time, added with secondary antibody dropwise, and incubated at 37 ℃ for 30 min; added SABC dropwise and incubated at 37 ℃ for 20 min,washed 3 times with PBS for 3 min/time; added with DAB chromogenic solution dropwise and immersed in water to stop the color development after 3 min; stained the nucleus with hematoxylin for 2 min, rinsed with tap water for 10 min, differentiated with 1% hydrochloric acid alcohol for 3 s, rinsed with tap water for 5 min to reverse blue, dehydrated with 70%, 80%, 90% and 100%gradient alcohol; mounted the slides after transparentized with xylene; observed and photographed under a light microscope. Five fields of view were randomly selected from each slice to photograph, followed by analysis with Image-Plus Pro 6.0 software. The integrated optical density (IOD) of the positive target in each image was calculated, and the average value was used as IOD value for the slice.
1.6.3 Detection of AMPK and TSC2 protein expressions in colon
Western blot technology was used to detect the expression of colonic AMPK protein. One milliliter of RIPA lysate, 10 μL of PMSF protease inhibitor, and 20 μL of phosphatase inhibitor were added into every 0.1 g of colon tissue. After homogenization, the supernatant was collected, and the protein concentration was determined by BCA method to prepare the loading protein with 40 μg sample. Ten percent gel was prepared for electrophoresis, blocked with 5% BSA for 1 h after transfer, incubated with AMPK primary antibody (1:1 000)overnight at 4 ℃, added with horseradish peroxidase(HRP) labeled with secondary antibody (1:1 000),incubated at room temperature for 1 h, and then analyzed with Image J software after development.
Immunofluorescence technique was used to detect colonic TSC2 protein expression. Paraffin sections of colon tissues were baked at 60 ℃ for 1 h, dehydrated by xylene and gradient alcohol, boiled in citric acid buffer for 8 s, incubated with 3% H2O2for 25 min in dark,blocked with 5% BSA at room temperature for 25 min,and incubated with primary antibody at 4 ℃ overnight;added with fluorescent secondary antibody dropwise;mounted with DAPI-containing mounting fluid and exposed to different wavelengths. Semi-quantitative analysis was performed with Image J software to detect the average fluorescence intensity of positive expression(arbitrary units, AU).
1.6.4 Detection of AMPK and TSC2 mRNA expressions in colon
RT-qPCR was used to detect the mRNA expressions of AMPK and TSC2 in colon tissues. Trizol was used to extract total RNA from colon tissues, and Prime Script?RT Master Mix kit was used to generate the first cDNA strand of mRNA. The 10 μL reverse transcription reaction system was as follows: 5× Prime Script RT Master Mix 2 μL, total RNA 500 ng, and RNase free dH2O to make up to 10 μL. Reverse transcription conditions were: 37 ℃for 15 min, 85 ℃ for 5 s. RT-qPCR amplification was performed using SYBR Green dye method. The 20 μL reaction system was as follows: 10 μL of TB Green Premix Ex Taq Ⅱ, 0.8 μL of PCR forward primer (10 μmol/L),0.8 μL of PCR reverse primer (10 μmol/L), 2 μL of cDNA template, 6.4 μL of dH2O. The cyclic reaction program was: 95 ℃, 30 s, 1 cycle; 95 ℃, 5 s, 60 ℃, 30 s,40 cycles; 95 ℃, 5 s, 60 ℃, 1 min, 1 cycle; 50 ℃, 30 s,1 cycle. The 2-△△Ctmethod was used to calculate the relative expression level of mRNA[17], △Ct for each sample = Ct value of target gene - Ct value of internal reference; 2-△△Ctfor each sample = 2-△Ctfor each sample ÷ Mean value of 2-△Ctfor all samples in the normal group. The primers used in this experiment are shown in Table 1.
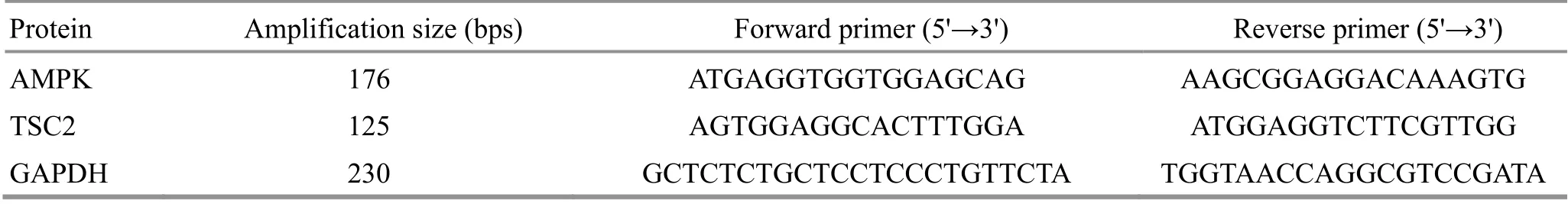
Table 1. RT-qPCR primer design
1.7 Statistical methods
2 Results
2.1 Morphological changes of rat colon in each group
Observed under light microscope, the rat colonic epithelium in the normal group was intact, with uniform distribution of monolayer columnar epithelial cells,continuous intestinal mucosa, neatly arranged glands,without congestion, edema or ulcers. In the model group,the colonic mucosa was damaged, the epithelial layer was partially missing, and the glands were hyperplastic and irregularly arranged; the lamina propria and submucosa had obvious hyperemia and edema; the submucosal muscle layer was thickened; large number of monocytes and lymphocytes were infiltrated with fissure-like ulcers reaching the muscle layer. Rat mucosal epithelium structure in the herbal cake-partitioned moxibustion group was still intact, but some glands were swollen and deformed, and new mucosal epithelium appeared (as shown by the arrow in Figure 1), suggesting the formation of healed ulcers, significantly reduced submucosal hyperemia and edema, and a small amount of inflammatory cell infiltration (Figure 1).
2.2 Expression of CD206, a surface marker of colonic M2 macrophages
It can be seen from Figure 2 that colonic CD206 is mainly expressed in lamina propria. Compared with the normal group, the colonic CD206 expression in the model group was significantly reduced (P<0.01);compared with the model group, the CD206 expression in rat colon tissues in the herbal cake-partitioned moxibustion group was significantly increased (P<0.01),suggesting that herbal cake-partitioned moxibustion upregulated the expression of colonic CD206, promoted colonic macrophage polarization to type M2.
2.3 Expressions of AMPK protein and mRNA in colon
It can be seen from Figure 3 that compared with rats in the normal group, the expressions of colonic AMPK protein and mRNA in the model group were statistically significantly decreased (bothP<0.01); compared with the model group, the AMPK protein and mRNA expressions in rat colon tissues in the herbal cakepartitioned moxibustion group were statistically significantly increased (bothP<0.05).
2.4 Expressions of TSC2 protein and mRNA in colon
It can be seen from Figure 4 that compared with rats in the normal group, the expressions of colonic TSC2 protein and mRNA in the model group were statistically significantly decreased (bothP<0.01); compared with the model group, the TSC2 protein and mRNA expressions in rat colon tissues in the herbal cakepartitioned moxibustion group were statistically significantly increased (P<0.05,P<0.01).
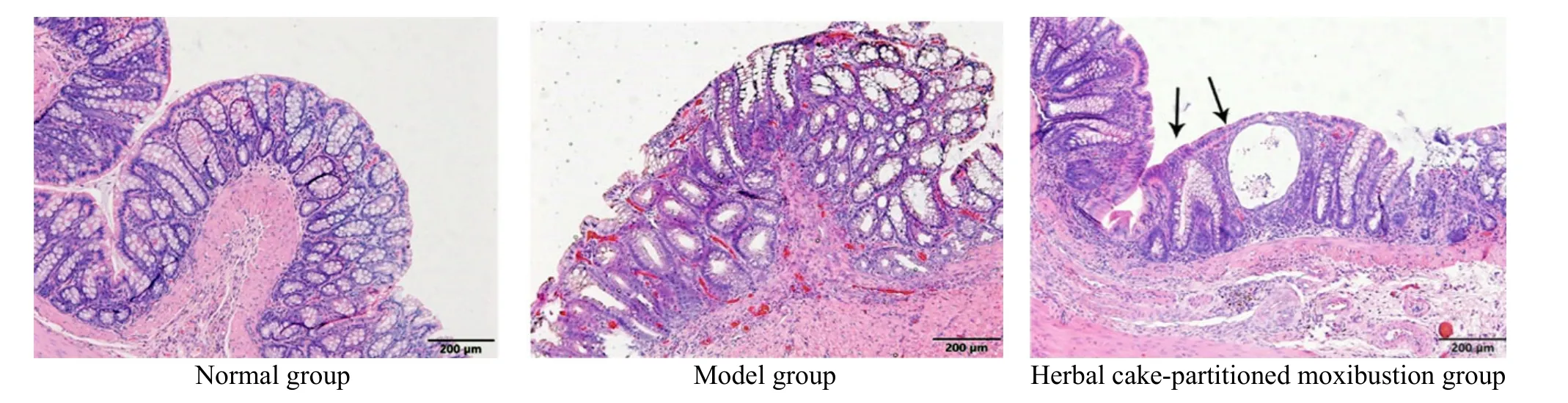
Figure 1. HE staining of rat colon tissues in each group (HE, ×100)
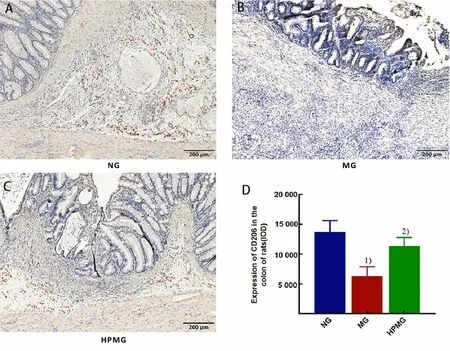
Figure 2. CD206 expression in rat colon tissues in each group (immunohistochemical staining, ×100)
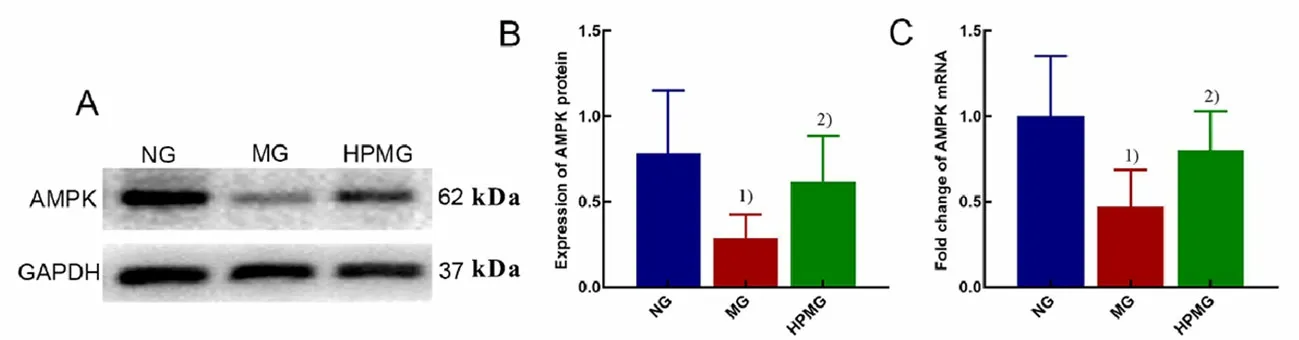
Figure 3. AMPK protein and mRNA expressions in rat colon tissues in each group
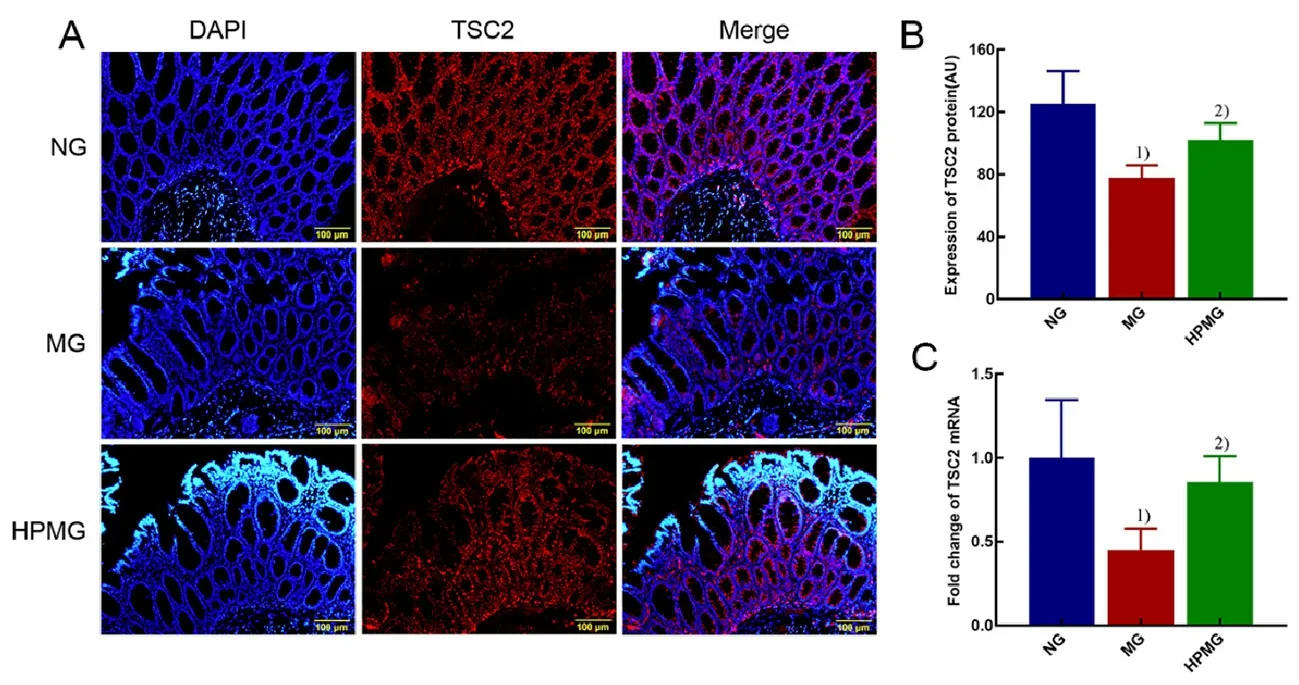
Figure 4. TSC2 protein and mRNA expressions in rat colon tissues in each group
3 Discussion
The pathogenesis of CD is complicated, but currently believed to be mainly caused by genetic susceptibility,environmental factors and interaction of intestinal flora,resulting in abnormal mucosal immune response and impaired epithelial barrier function[18-19]. CD is characterized by alternately clinical remission and recurrence, lingering and hard-to-heal, which brings great challenges to clinical treatment and seriously affects the quality of life in patients. At present, the basic treatment with Western medicine primarily includes induction and maintenance therapy, such as immunosuppressive agents of glucocorticoids,aminosalicylic acid, and thiopurine; biological agents such as adalimumab. Although these therapies can delay disease progression, the serious side effects such as diabetes, osteoporosis, lymphoma, and infection cannot be avoided[20-22]. Thus it is important to actively explore treatment strategies without side effects that can treat the disease, reduce complications, and control disease progression[23]. Preliminary studies of our group have confirmed that herbal cake-partitioned moxibustion can effectively alleviate CD intestinal inflammation and improve mucosal damage[24]. This study further found that herbal cake-partitioned moxibustion promoted the expression of colonic M2 macrophage marker CD206,up-regulated the expressions of colonic AMPK and TSC2 protein and mRNA in CD rats, and reduced intestinal inflammation. As innate immune cells, macrophages respond quickly to environmental signals through many receptors, maintaining a specific and optimized activation status, and are considered to be the main effector cells in many chronic inflammatory diseases[25].Macrophages are the most common mononuclear phagocytes in the lamina propria of the intestinal mucosa, which play an important role in maintaining homeostasis of intestinal immune system. Chronic intestinal inflammation can greatly change the number and sub-population diversity of macrophages[26]. Tissue microenvironment changes can induce abnormal differentiation of macrophages, resulting in two phenotypes, namely classic activation (M1 type) and alternate activation (M2 type) macrophages[27]. Different macrophage differentiation types express different surface markers. The surface of M2 type macrophages highly expresses mannose receptors of CD206 and CD163, arginase 1, anti-inflammatory factors of interleukin (IL)-4 and IL-10. It plays an important role in tissue repair, angiogenesis and metabolism[28]. CD206 is a highly specific marker for M2 macrophages. Therefore,this study used CD206 expression to characterize the expression level of M2 type macrophages. M2 type macrophages have anti-inflammatory properties, and activated M2 type macrophages and their cytokines may be immunotherapeutic targets for anti-inflammatory effects. The study of Zhu W,et al[10]showed that promoting the polarization of M2 macrophages can induce the production of IL-10 and regulatory T cells,which can effectively reduce intestinal inflammation.Yang R,et al[29]treated mice with DSS-induced colitis by intraperitoneal injection of purified M2 macrophage exosomes, and found that M2 macrophage exosomes up-regulated the expression of Treg cells and IL-4, and reduced the production of pro-inflammatory cytokines of IL-1β, IL-6 and IL-17A, effectively alleviated colon inflammation in DSS mice. In this study,immunohistochemical staining of colon tissues showed that the CD206 expression in colonic lamina propria of CD rats was significantly reduced, and herbal cakepartitioned moxibustion treatment significantly increased the colonic CD206 expression, suggesting that promoting colonic M2 macrophage expression should be one of the effective mechanisms of herbal cakepartitioned moxibustion in alleviating intestinal inflammation.
AMPK is an important regulator of intracellular energy metabolism and widely present in gastrointestinal tissues. Phosphorylation and activation of AMPK closes some anabolic pathways to reduce the consumption of adenosine triphosphate (ATP), and to activate the catabolic pathway that produces ATP, which plays an important regulatory role in the inflammatory response[30-31]. Studies believe that AMPK activation promotes the expression of caudal type homeobox 2(CDX-2) and improve the barrier function of intestinal epithelial cells[32]; experiments have confirmed that AMPK activation reduces the levels of intestinal proinflammatory cytokines of tumor necrosis factor (TNF)-α,IL-1β and IL-6, and significantly reduces colonic inflammation[33]. Regulatory effect of AMPK on inflammation may be related to macrophage polarization. Wang Y,et al[34]found that AMPK activation promotes M2 macrophage polarization to reduce neuroinflammation. Garami A,et al[35]found that knocking down AMPK expression with siRNA promoted the polarization of M1 type macrophages, enhanced the secretion of TNF-α and IL-6, and promoted inflammation.The role of AMPK in regulating macrophage polarization may be related to the mammalian target of rapamycin(mTOR)-related signaling pathways. Research by Qing L,et al[12]confirmed that AMPK/mTOR/NLRP3 inflammasome signal pathway was involved in regulating the polarization of M2 macrophages. AMPK inhibited mTORC1 activity by phosphorylating multiple regulatory factors in mTORC1 pathway. First, activated AMPK directly phosphorylated tuberous sclerostin TSC2 to promote TSC1/2 complex formation, which directly acted on GTPase Rheb and inhibited its activity[36], and Rheb was an upstream active molecule that directly interacted with mTORC1[37], so the activity of mTORC1 was inhibited. The mTOR was also the binding partner of NLRP3[38], which inhibited the activation of NLRP3 inflammasomes by binding to NLRP3. Therefore,activated AMPK inhibited the mTORC1 expression through phosphorylation of TSC2, thereby reducing NLRP3 inflammasome activation. Reduced activation of NLRP3 inflammasomes in macrophages inhibited the polarization of M1 macrophages and the production of IL-1β[39]. At this time, the phenotype of macrophages changes towards to M2 type, which is manifested as increased M2 type macrophages and reduction of inflammation. The results of this experiment also showed that the protein and mRNA expressions of AMPK and CD206 in rat colon tissues were reduced under CD inflammation, and their expression levels were significantly increased after herbal cake-partitioned moxibustion.
In summary, AMPK and TSC2 proteins are closely related to colonic M2 macrophages. Herbal cakepartitioned moxibustion increased the colonic CD206 expression in CD rats, up-regulated the expressions of AMPK and TSC2 proteins and mRNAs in colon of CD rats,and reduced intestinal inflammation, suggesting that herbal cake-partitioned moxibustion may increase the protein and mRNA expressions of colonic AMPK and TSC2, promote colonic macrophage polarization to type M2, and improve intestinal inflammation. However, this experiment has not been able to confirm the potential association between regulating AMPK and TSC2 with the polarization of macrophages by herbal cake-partitioned moxibustion. Therefore, further research is needed.
Conflict of Interest
Authors Ma Xiao-peng and Wu Huan-gan are members of the Editorial Board of Journal of Acupuncture and Tuina Science. The paper was handled by other editors and has undergone rigorous peer review process. Authors Ma Xiao-peng and Wu Huan-gan were not involved in the journal’s review or decisions related to this manuscript.
Acknowledgments
This work was supported by National Natural Science Foundation of China (國家自然科學(xué)基金項(xiàng)目, No.81674073, No. 81904303); Natural Science Foundation of Shanghai ( 上海市自然科學(xué)基金項(xiàng)目, No.20ZR1453000, No. 19ZR1451600).
Statement of Human and Animal Rights
The treatment of animals conformed to the ethical criteria in this experiment.
Received: 15 October 2020/Accepted: 14 April 2021
 Journal of Acupuncture and Tuina Science2021年5期
Journal of Acupuncture and Tuina Science2021年5期
- Journal of Acupuncture and Tuina Science的其它文章
- Acupuncture combined with medication for postherpetic neuralgia affecting the head and face:a randomized controlled trial
- Tendon-regulating and bone-setting manipulation plus endurance resistance exercises for female with chronic neck pain
- Efficacy and effect on related brain-gut peptides of acupoint sticking therapy for functional dyspepsia
- Effects of acupuncture plus language training on language function and cerebral blood flow in patients with motor aphasia after ischemic stroke
- Clinical observation on moxibustion therapy plus tuina in treating children with recurrent respiratory tract infections due to qi deficiency of spleen and lung
- Clinical efficacy observation of ‘Tong Du Yun Pi’manipulation for infantile diarrhea in autumn
