How cancer stem cells cause disease recurrence in recovered patients
Zahra Farhoud Khomeirani and Sepehr Bozorgchenani
Zahra Farhoud Khomeirani is with Department of Veterinary Medicine Rasht Branch,Islamic Azad university,Rasht,Iran.E-mail:z.farhoudkhomeirani@gmail.com (Corresponding author).
Sepehr Bozorgchenani is with Department of Bioinformatics,Tehran branch,Science and Culture University,Tehran,Iran.
Abstract—Many tumors contain phenotypic and functionally diverse populations of cancer cells that differ in terms of metastatic and angiogenic potential,as well as medication resistance.Tumor recurrence and metastasis are two of the most difficult aspects of cancer treatment and even with conventional therapies (such as chemotherapy and radiotherapy),satisfactory results have not been obtained.The main source of cancer stem cell tumorigenesis is CSCs,which,by dividing cells,produce the same daughter cells and differentiate into different types and can create tumor tissue from a single cell.They are responsible for the development of cancer,progression,metastasis,recurrence,and treatment resistance,and are recognized to have a role in the failure of conventional therapy and tumor recurrence.To resolve this quandary,careful identification of CSC molecular targets and comprehensive disclosure of the underlying processes of CSC immune system properties are required.Traditional cancer therapies are insufficient to kill all intracellular cells,particularly those with great resistance to therapy,such as CSC.Targeting CSC metabolism has been proposed as a novel treatment strategy for preventing the development of different malignancies.In this study,the identification of cancer stem cell populations,the role of CSCs in drug resistance,metastasis,hypoxia,and the role of Nrf2 in CSC tumorigenesis has been reviewed.
Key words—Cancer stem cells,Nrf2,Metastasis,Hypoxia,Drug resistance,Cancer
INTRODUCTION
Despite recent scientific advances toward developing high-precision treatment schemes for tumor targeting,questions regarding how to break down tumor refractoriness to targeted therapy,which is known to be a primary cause of high death rates among patients with advanced malignancies persist [1].Surgery is requested as a treatment option for certain cancers.However,in the case of metastatic cancers,this technique creates a hypoxic microenvironment,which promotes aggressive tumor recurrence [2].Many cancers have phenotypically and functionally varied cancer cell populations that differ in terms of metastatic and angiogenic potential,as well as drug resistance [3-6].Patients undergoing radiotherapy and chemotherapy frequently have side effects from these treatments,which merely provide palliation.Although chemotherapy causes tumor reduction or even transitory elimination [7],patients eventually develop tumor resistance and relapse,the two primary causes of treatment failure [8].As a result,traditional treatments are not only unsuccessful in targeting advanced cancers,but they may also encourage tumor recurrence,necessitating an urgent expansion of current understanding regarding tumor behavior in response to therapy.Otto Warburg,a Nobel Laureate German researcher,was the first to discover that tumor cells employ a different metabolic route than healthy cells in the 1930s.Since then,the subject of cancer metabolism has grown in popularity,particularly in the recent decade.We now have a better understanding of the processes and function of metabolic reprogramming in cancer for tumor growth,metastasis,and treatment resistance thanks to the advancement of novel biochemical tools like metabolomics [9].Tumor relapse and metastasis are two of the most difficult areas of cancer therapy.As a result,even with standard therapies (such as chemotherapy and radiotherapy),sufficient outcomes were not obtained.Various efforts have been made to overcome these obstacles.One of these initiatives resulted in the discovery of the “first human CSCs in AML by Bonnet and Dick in 1997” [10,11].The discovery of the cause of cancer has sparked a lot of attention since it might lead to more comprehensive cancer therapy.For the past 20 years,several studies have shown that just a tiny subpopulation of cancer cells with tumor-initiating potential is the root cause of carcinogenesis,and this fraction of cancer cells has been dubbed cancer stem cells (CSCs).CSCs,as the name suggests,share some characteristics with regular stem cells.They may self-renew to create identical daughter cells by cell division and differentiate into distinct types of progenies [12].“Following chemotherapy for any tumor type,there are residual bodies enriched in cancer stem cells(CSCs)” [13,14].CSCs are slow-cycling,undifferentiated cells that can create cancerous tissues from a single cell.According to the “CSC model”,most malignancies have a complex and hierarchical cellular architecture,with a subpopulation of undifferentiated cells at the top.CSCs often reside as a minority subpopulation inside the tumor mass and are responsible for the formation of highly proliferative cancer cells that make up the majority of the tumor even when cancer recurs after therapy [15-17].CSCs have four properties with other stem cells in normal tissues:self-renewal,differentiation,tumorgenicity,and unique surface markers [18,19]and play a significant role in cancer start,development,metastasis,recurrence,and treatment resistance,as well as in conventional therapeutic failure and tumor relapse [20,21].In this study,the identification of cancer stem cell populations,the role of CSCs in drug resistance,metastasis,hypoxia,and the role of Nrf2 in CSCs tumorigenesis has been reviewed.
IDENTIFICATION OF CANCER STEM CELLS
The creation of CSCs is based on two ideas:1) Gene changes in normal stem and progenitor cells resulting from genetic and epigenetic instability,changing them into CSCs.2) Tumor cells change into stem cells as a result of oncogene-induced plasticity [15].“The population of CSCs varies depending on the type of cancer and the biomarker used for their identification.In breast cancer,cell population with high CD44 and low CD24 expression was designated as CSCs”.CD44 expression has been associated with a poor prognosis.In addition,the expression of these markers differs amongst intrinsic subtypes [22].Due to difficulty in isolating pure populations,a scarcity of CSC cell lines,and trustworthy characterization methodologies,dissecting the full profile of cancer stem cells has been a major issue.CSCs may now be studied using a variety of approaches such as tumorsphere assays,colony formation assays,and flow cytometry studies.The most dependable method for isolating and enriching CSCs has relied on“surface markers and aldehyde dehydrogenase (ALDH)expression” [23,24].Common CSC markers include“CD133,CD44,CD24,ALDH1,oct4,Nanog,and sox2”.CD44 and CD133 are two of the most often utilized for isolation and characterization.CD44 is important in CSCs for communicating with the microenvironment,as well as for “maintaining stemness” [25].The stem/progenitor cell marker CD133 is present in adult organs such as the kidney,brain,prostate,and liver.It is a useful marker for separating CSCs from non-CSCs inside malignancies [15,26].
The role of CSCs in drug resistance
Chemoresistance and recurrence after chemotherapy are two important challenges in cancer treatment [27,28].Most of the time,this resistance is linked to the existence of aggressive cancer cell populations inside tumors that have acquired chemoresistance mechanisms,resulting in failed treatments and worse survival rates for cancer patients [29,30].These aggressive cancer populations have been identified as cancer stem cells (CSCs) in several investigations [31-36].Chemoresistance mediated by CSCs is based on numerous biological processes,including reduced growth rates and the capacity to inactivate chemotherapeutic drugs intracellularly and enzymatically[29].
Cancer cells have chemicals on their surface that the immune system can recognize.These molecules are referred to as tumor antigens.Immunotherapy uses tumor antigens to change the balance between cancer cell death and proliferation to achieve full tumor cell eradication.Despite recent advancements in immunotherapy,a substantial minority of patients remain unresponsive or develop resistance to these treatments [37-39].In most solid tumors,CSC is thought to be the origin of tumor growth and the driver of treatment resistance [40].Chemotherapy works by causing DNA damage and inhibiting mitotic division in highly proliferative cells;however,it has little effect on slow and non-dividing cancer cells such CSCs [41-44].Indeed,numerous studies have demonstrated that CSCs derived from various tissues may withstand the effects of a variety of medicines,including doxorubicin,temozolomide,cisplatin,paclitaxel,etoposide,and methotrexate.As a result,after chemotherapy,which kills the majority of the tumor,CSCs remain on the site and can restart tumorigenic and metastatic processes.To avoid recurring malignancies,finding treatments that target latent or slow dividing cells is critical [45].Tumors that return after an initial response to treatment frequently act more aggressively and are more resistant to chemoradiotherapy.According to the classic paradigm of treatment resistance,one or more cells in the tumor population develop genetic alterations that give drug or radioresistance.These cells have a selective growth advantage under treatment settings,allowing them to overrun the tumor cell population following therapy [46,47].
Van Rhenen and colleagues established the significance of CSC in AML tumor recurrence and therapeutic resistance.In 92 individuals,they looked at the relationship between the existence of CSC and clinical outcome.A significant percentage of CSC at baseline was associated with a high prevalence of posttreatment minimum residual illness and a poor outcome [48].Shlush and colleagues found that the biological genesis of recurrence in certain AML patients is a rare population of cells with a CSC phenotype that is already present at diagnosis before medication commencement.CSC may also go dormant and hence avoid antineoplastic treatments.Dormancy may potentially play an important role in tumor progression and the creation of clinically undetected metastatic foci [49].Tumor mass dormancy is defined as the length of time in which tumor cell populations remain undetected until the reappearance of a clinically apparent illness [50].
Hypoxia and CSCs
Cancer,perivascular,endothelial,and other cells make up the tumor microenvironment,which sends out immunosuppressive signals that inhibit the function of tumor-infiltrating leukocytes in a variety of ways [51].To avoid antitumor immune responses,cancer cells employ a variety of strategies.The absence of effective oxygenation inside the tumor core has been demonstrated to cause hypoxia and hypoxia-related variables,which are linked to tumor development [52].In tumor tissue,hypoxia is a prevalent occurrence [53].Hypoxia regulates the transcription of hundreds of genes that control carcinogenesis,angiogenesis,invasion,metastasis,treatment resistance,and immune suppression,and therefore has a significant impact on the tumor microenvironment.Hypoxia-inducible factor-1 (HIF-1) is a key transcription factor that is activated in tumor cells in response to oxygen deprivation and regulates cancer cell survival and development.Hypoxia influences not only CSC production but also immune system regulation.Hypoxia increases the ability of lymphoid-primed multipotent progenitors to differentiate into B cells via HIF-1,resulting in the generation of B cells [54].HIF-1 controls innate immune responses,generates regulatory T cells (T-regs),and facilitates immunological escape from cytotoxic T lymphocytes and other complicated immune responses under hypoxic circumstances [55-57].HIF-1-mediated tumor immunity has been recommended as a way to overcome difficulties connected with tumor therapy in the past several years [58-60],thus the immune response of CSCs mediated by HIF-1 is also worth examining while researching anti-cancer medicines.Hypoxia is a common occurrence in tumor tissue [53].In CSCs,hypoxia increases drug resistance [61]and decreases tumor response to radiation [53]and chemotherapy [62].Hypoxia improves EMT-associated CSC characteristics such as selfrenewal [61],and CSC enrichment occurs in vivo when cells are exposed to HIF-1; this is for improving chemotherapy resistance (Figure 1) [63].Hypoxia promotes early EMT by promoting snail via Notch activation and reducing E-cadherin (an epithelial marker)via twist activation [64].Hypoxia-inducible factors (HIFs),especially HIF-1 and HIF-2,are essential mediators of the cellular response to hypoxia,controlling many cellular functions such as survival,proliferation,metabolism,EMT,angiogenesis,and metastasis throughout cancer formation.In several tumor forms,high HIF expression has been related to a poor prognosis [65-68].Hypoxia activates the unfolded protein response (UPR) signaling (indirectly through endoplasmic reticulum [ER]stress) [69],which keeps CSC reactive oxygen species (ROS) at low levels[70].Hypoxia increases the production of immunosuppressive factors,inhibits monocyte phagocytosis,inhibits T cell proliferation,and activates and inducts T-regs in glioblastoma multiforme-related CSCs,according to studies [71-73].This is accomplished through the phosphorylated STAT3/HIF-1/vascular endothelial growth factor (VEGF) pathway.Hypoxic conditions have been linked to influencing CSC “biology,including the maintenance of self-renewal/stemness,EMT,quiescence,and drug resistance traits” [74].Several in vitro studies have shown that non-CSCs can acquire stem-like characteristics by expressing genes such as OCT4,SOX2,and NANOG,which are required for the maintenance of self-renewal in stem cells,or by activating the Notch signaling pathway,which regulates cell self-renewal and differentiation [75-77].Interestingly,hypoxia-induced CSC enrichment is mostly reliant on the HIF-2 pathway [78].
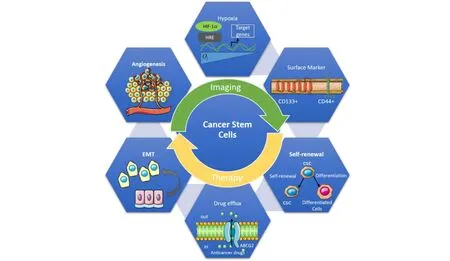
Fig.1 CSC characteristics in their regenerative capacity and differentiation into heterogeneous subpopulations have significantly contributed to tumor growth and metastasis.
The role of CSCs in metastasis
During their multistage growth,cancer cells can acquire a variety of biological skills.Hanahan and Weinberg identified 10 cancer hallmarks that change cell physiology to promote malignant growth:1) Sustained proliferation,2)Evasion of growth suppression,3) Cell death resistance,4)Replicative immortality,5) Evasion of immune destruction,6) Tumor-promoting inflammation,7) Activation of invasion and metastasis,8) Induction of angiogenesis,9)Genome instability,and 10) Alteration of metabolism” [79,80].It has recently been proposed that cancer is characterized by a breakdown of multicellular cooperation caused by instances of cellular "cheating" that disturb all of the following:suppression of cell proliferation,cell death control,division of labor,resource transfer,and extracellular environment maintenance Furthermore,dysregulation of differentiation has been indicated as another key component of tumorigenesis [81].The formation of secondary tumor growth in a distant organ or tissue from the main tumor location is known as metastasis,and it is responsible for more than 90% of cancer-related fatalities.CSCs are intrinsically capable of metastasizing because of their resistant character; this population is termed as metastatic CSC [82-84].CSCs have been shown to have a role in orchestrating the metastatic cascade in the tumor microenvironment,“via interactions with the cellular components of the tumor microenvironment to establish the new metastatic sites,termed the pre-metastatic niche for their arrival through distinct cellular and molecular mechanisms” [85,86].
The function of Nrf2 in cancer development and carcinogenesis in CSCs
Nuclear factor erythroid 2-like 2 (NFE2L2),also known as NRF2,is a key transcription factor for the cytoprotective response to oxidative and electrophilic stress.Under the oxidative stress condition,NRF2 dissociates from its molecular inhibitor Kelch-like ECH-associating protein 1(KEAP1) and translocates into the nucleus.Then,NRF2 binds to the antioxidant response element (ARE) in the regulatory region of its target genes to induce their expression [87].“NRF2 target genes include NAD(P)H quinone oxidoreductase-1 (NQO-1),Aldo-Keto Reductase 1C1 (AKR1C1),GSH generating enzymes,and drug efflux transporters such as BCRP” [88].Additional noncanonical routes for NRF2 activation have been revealed:p62,which was discovered as an autophagy linker protein,stimulates NRF2 activation via competitive binding to the KEAP1 protein and autophagic degradation of KEAP1 [89,90].Although NRF2 has a wide range of positive effects in normal cells,its excessive activity has been linked to unfavorable tumor phenotypes.NRF2 expression is often elevated in a variety of tumor types,including lung,breast,colon,and ovarian cancer [91],and high NRF2 levels play a key role in tumor cell proliferation and chemoresistance by increasing ROS-inhibiting enzymes and drug efflux transporters [87].NRF2 signaling has been found to be involved with CSC-like characteristics in a variety of cancer cells in recent research.Glioma stem cells' ability to self-renew was reduced when NRF2 was knocked down[92].In spheroid cultivated breast and colon cancer cells,NRF2 signaling is activated,and elevated NRF2 activity in these CSC-enriched systems promoted spheroid development and chemotherapeutic resistance [93,94].In malignancies,the NRF2 gene (NFE2L2) is commonly changed (in a gain-of-function manner),resulting in excessive Nrf2 activation to protect cells from adverse oxidative stresses.These alterations have been associated with treatment resistance,which leads to poor clinical outcomes [95].Furthermore,some Keap1 mutations might cause cancer by suppressing Nrf2,reducing the cell's capacity to eliminate chemical carcinogenesis [96].The secretome of colon CSC-like cells revealed a high level of NRF2-induced antioxidant and detoxifying proteins,indicating that Nrf2 is important for CSC metabolism [97].The Nrf2 and Notch signaling pathways are commonly mutated in cancers.Mutant Nrf2 and Notch mutual interaction are thought to be a key carcinogenesis mechanism in lung tissue cells [98].CD44 is a CSC marker that has been associated with a high level of Nrf2 expression.In breast CSCs,CD44 regulates Nrf2 activation via p62 expression to establish stem cell properties [99,100].
CONCLUSION
Scientists are drawn to the challenge of using the host immune system to target CSC and treat cancer patients.To that purpose,careful identification of CSC molecular targets and full disclosure of the processes underlying CSC immunoevasive properties are required [40].Traditional cancer medications,which target the vast majority of cancer cells,are ineffective in killing all cancer cells in the tumor,particularly those that are resistant to therapy,such as CSCs.It's important to remember that CSCs are surrounded by a complex network of cells called the CSC niche,which secretes a range of substances that help CSCs survive while also allowing them to be flexible and resistant to drugs.Concentrating on the niche components
is a viable therapeutic method since the CSC niche is critical for CSC survival and medication resistance [101].Current studies reveal that metabolic “reprogramming in CSCs is essential for tumorigenesis,metastasis,treatment resistance,and tumor recurrence.CSC metabolic targeting has been postulated as a possible therapeutic strategy for avoiding the development of various cancers”,although a paucity of research on the mechanisms of metabolic plasticity in CSCs has been done [102].
ACKNOWLEDGMENT
The authors did not receive any funding for this study.
- Cancer Advances的其它文章
- Efficacy and prognosis of mastoscopic axillary lymph node dissection for breast cancer:a systematic evaluation and meta-analysis
- The preferences and values of patients treated with acupuncture for cancer pain:a qualitative systematic review
- How Can We Give Hope for Cancer Patients to Cure This Disease?
- Common mechanisms in chronic obstructive pulmonary disease and lung cancer

