Synthesis and characterization of caprolactone based polyurethane with degradable and antifouling performance
Abid Ali,Lina Song,Jiankun Hu,Jingxian Jiang,Qingqing Rao,Muhammad Shoaib,Shah Fahad,Yongjie Cai,Xiaoli Zhan,,*,Fengqiu Chen,Qinghua Zhang,,*
1 College of Chemical and Biological Engineering,Zhejiang Provincial Key Laboratory of Advanced Chemical Engineering,Manufacture Technology,Zhejiang University,Hangzhou 310027,China
2 Ningbo Research Institute,Zhejiang University,Ningbo 315100,China
Keywords: Antifoulant 4,5-dicholoro-2-octyl-isothiazolone Degradable polyurethane L-Lactide Caprolactone
ABSTRACT In this work,a degradable polyurethane composed of caprolactone (CL) and L-Lactide (LLA) as soft segments,and 4,4′-methylenebis(cyclohexyl isocyanate) (H12MDI) and polytetramethylene ether glycol(PTMEG) as hard segments,was prepared.Hydrolytic degradation experiment revealed that the degradable polyurethane (PU) could be degraded in artificial seawater.It also showed that caprolactone-copolyurethane (CL-PU) copolymer with higher crystallinity degraded much slower in artificial seawater.However,the introduction of LLA resulted in an increase in the hydrophilicity and reduction in the crystallinity of degradable PU,as demonstrated by the contact angle analysis.The result of the scanning electron microscope showed that the surface of degradable PU renewed under static condition.Moreover,degradable PU was able to be used as a carrier,and it controlled the release rate of 4,5-dichloro-2-octyl-isothiazolone (DCOIT).The anti-diatom (Navicula incerta) test demonstrated that the(caprolactone-co-L-lactide)-co-polyurethane 4 (CL/LAx-PU4) with DCOIT contents prevented the adhesion of diatom Navicula incerta(88.37%reduction)due to their self-polishing and the release of antifoulants.Therefore,the degradable PU consisted of CL,LLA,and DCOIT could be a durable resin with good antifouling activity for the application in the marine anti-biofouling field.
1.Introduction
Marine biofouling is one of the most urgent problems in maritime industries because the adhesion of marine organisms on the ship hull can damage its surface,which has very harmful effects on the environment and loads a heavy burden on the economy [1].Using antifouling coatings is a very effective strategy for the inhibition of marine biofouling.For example,they can inhibit the growth of adhered organisms by releasing toxic components[2].Tributyltin (TBT) based coatings are the most effective against biofouling,but they have an adverse effect on the marine environment;therefore,they were banned in 2008[3,4].The biocide based on non-metal or metal antifouling agents have been used as an alternative to TBT based antifouling in the paint.Though such biocides are very useful for marine biofouling,they are potentially toxic to non-target species in seawater because of their higher release rate [5,6].
In the current scenario,researchers have introduced ecofriendly antifouling system such as tin-free acrylate-based selfpolishing coatings [7,8],fouling release coatings [9–11],slippery liquid-infused surfaces[12],bioinspired surfaces[13],natural antifoulants [14],amphiphilic polymers [15–17],and zwitterionic polymers [18]into the marine antifouling field.Biodegradable polymers are recently recognized as antifouling materials due to their sustainable release under the attack of seawater or by the enzymatic effect.Therefore,they can reduce the biofouling colony by constantly renewing surfaces [19,20],and control the release rate of incorporated antifoulant,showing excellent antifouling performance [21].It is well understood that the antifouling performance of self-polishing acrylate copolymer in the side chain with the hydrolyzable ester unit depends mostly on seawater flow and a sailing speed of a ship.In the case of sluggish ships or those in harbor,these polymers generally show weak antifouling effect for a long time [22].
What’s more,polycaprolactone (PCL) biodegradable polymer is commonly used for the supply of antifouling materials in the biomedical field [23].PCL degrades very slowly due to its high crystallinity.As a result,the biodegradation level of the polyesters is significantly influenced by their tendency to crystallize.PCL has poor anti-adhesion performance due to its higher crystalline structure.Therefore,they cannot be used directly in the marine antifouling coatings.Meanwhile,it has been developed by physical or chemical modification or blending with L-lactide or deltavalerolactone[24–27]units and other polymers[28].This strategy is used to reduce crystallinity and improve the biodegradability of the PCL homopolymer as a carrier of eco-friendly antifoulant and the long-term capability of anti-biofouling in the marine environment [29].
In this study,a biodegradable polyurethane was fabricated by the modification of Poly (caprolactone-co-L-Lactide) P (CL/LAx)with 4,4′-methylenebis(cyclohexyl isocyanate)(H12MDI)and polytetramethylene ether glycol (PTMEG).The introduction of LLA reduced the spherulite size and increased the hydrolytic degradation performance of the degradable polyurethane.The properties of polyurethane were characterized by Fourier transform infrared spectroscopy (FTIR),adhesion test,gel permeation chromatography (GPC),contact angle,and thermogravimetric analysis (TGA).Furthermore,the release rate of DCOIT,hydrolytic degradation,and antifouling performance of degradable polyurethane has also been investigated.The as-prepared degradable PU is equipped with durability,nontoxicity,and has excellent potential to be used as a superior marine antifouling coating.
2.Experimental
2.1.Materials
Caprolactone (CL),L-lactide (LLA),poly (tetra-methylene ether glycol) (PTMEG) (M.W.850),4,4′-methylenebis(cyclohexyl isocyanate) (H12MDI),tetrahydrofuran (THF),dichloromethane(CH2Cl2),absolute methanol,dibutyltin dilaurate (DBTDL),4,5-dic hloro-2-octyl-isothiazolone(DCOIT) were purchased from Aladdin‘‘Chemical Reagent Co.,Ltd (China)”.The monomers were used without further purification.Titanium butoxide Ti(OBu)4was purchased from Sinopharm Chemical Reagent Co.,Ltd (China).The artificial seawater (ASW) (pH 8.2) was prepared by NaCl,MgCl2·6H2O,KBr,Na2SO4,NaHCO3,CaCl2,KCl,H3BO3,NaF,and SrCl2·6H2O,while pH adjusted by HCl and 1 M NaOH.
2.2.Synthesis of poly (caprolactone-co-L-Lactide) (P (CL/LAx))
P(CL/LAx) polymer was fabricated by the modified Fabienet al.[30]method.The P(CL/LAx) polymer was typically synthesized by the ring open polymerization of caprolactone (5.25 g,4.6 mmol)and LLA (0.288 g,0.2 mmol),while Ti(OBu)4(0.03 g,0.7% (mass)based on monomer)and CH2Cl2were used as the catalyst and solvent,respectively.They were first mixed under magnetic stirring,and deoxygenated by nitrogen gas for 15 min at ambient (25 °C)temperature in a 250 ml three-necked flask.The reaction of CL and LLA was conducted at 240°C for 40 min.The obtained product was cooled at room temperature(25°C and precipitated with cold absolute methanol,filtered,and finally kept into the vacuum oven at 40 °C for 12 hours (h) for drying.
2.3.Synthesis of degradable polyurethane
The degradable polyurethane was fabricated from P(CL/LAx)in two steps,as depicted in Fig.1.In the 1st step,the reaction of H2MDI with P(CL/LAx)was conducted at 80°C for 1 h in THF under nitrogen purging.In the 2nd step,PTMEG,and DBTDL (1% with respect to reactants) as a catalyst and extender,were introduced into the flask.The reaction was conducted at 85°C for 3 h to yield the prepolymer.The final product was dried for 24 h in the vacuum oven at 40 °C for curing the product.Five (caprolactone-co-L-lac tide)–co-polyurethane (CL/LAx-PU) were synthesized with different molar ratios,which showed in Table 1.

Table 1Molar ratio and characterization data of CL/LAx-PU
3.Characterization
3.1.Fourier transform infrared spectroscopy (FTIR)
FTIR test was performed by a Nicolet 5700 (FTIR) (Thermo Fisher,USA)instrument.The spectra were obtained by the KBr disk approach in 32 scans with a spectral resolution of 4 cm-1.
3.2.Adhesion test
The adhesion strength of CL/LAx-PU coatings to epoxy resin substrate was determined by adhesion tester (PosiTest AT-A automatic,USA) in compliance with ASTM Standard D 4541.The composition of the film was built by using the solution casting method at 60°C.The data for the adhesion test were collected by removing the aluminum dolly (20 mm in diameter) at a speed of 0.2 MPa·s-1.The average five different points were tested on the surface of each sample.
3.3.Gel permeation chromatography (GPC)

Fig.1.Schematic illustration for the synthesis of biodegradable CL/LAx-PU.
The number of average molecular weight and molecular weight dispersity were determined by the Water GPC system.The sample was dissolved into the THF solvent as eluent with a flow rate of 1 ml·min-1at 35 °C.The instrument was calibrated by the polystyrene standard.The GPC result was described in Table 1.
3.4.Contact angle (CA)
The contact angle measurements were carried out on a contact angle system(SINDIN Co.Ltd.,China)at room temperature(25°C).The degradable polyurethane CL/LAx–PU was prepared by the solution casting method,and 3 μl deionized water was dropped onto the polymer film surface.The average five different points were tested on the surface of each sample.
3.5.Thermogravimetric analysis (TGA)
TGA measurement was conducted on a TAQ500 (New Castle,USA) system in the atmosphere of nitrogen at a heating rate of 10 °C ·min-1between 50 and 500 °C.
3.6.Hydrolytic degradation test
The hydrolytic degradation test was performed in ASW at room temperature (25 °C).The composition of the film chip(15 mm×15 mm) was constructed by using the solution casting process.The 30% sample inn-butanol was dropped on the panel and was kept in the vacuum oven for 6 h at 60 °C.The chip was weighed (w1) and dipped into an artificial seawater container.We replaced the old water with fresh water every two weeks.The polymer chips were taken out from the container after a period of time,rinsed three times with deionized water,and dried in the vacuum at 60 °C till the mass remained constant.The chip was weighed (w2) after drying,and the following equation estimated the mass loss:

3.7.Release rate of DCOIT
UV-spectrophotometer (U-2900 UV/Vis spectrophotometer 200 V,Hitachi,Japan)measured the release rate of 10%DCOIT concentration under a static condition.The composition film chip(15 mm×15 mm)was prepared by using the solution casting process.The chips were dipped into artificial seawater in an individual container.After a day of immersion,5 ml synthetic seawater was collected from the single container and squeezed into the separating funnel and collected with 10 ml of xylene.Furthermore,The DCOIT intensity was determined at 291 nm by the UV–vis spectrophotometer.The release rate of DCOIT from the film was estimated from the DCOIT concentration using the following formula:

whereRis the release rate of DCOIT (μg·cm-2·d-1),Cis the concentration of DCOIT in xylene,Dis days of individual immersion container in ASW,andAis the area of the surface of the chip.
3.8.Scanning electron microscopy (SEM)
The surface morphologies of CL-PU and CL/LAx-PU film before immersion and after immersion in the ASW after 40 days were demonstrated by SEM (Phenom Prox-SED,USA) instrument.
3.9.Anti-diatom assay
Navicula incerta diatom settlement assays were conducted on the films (20 mm×20 mm) with epoxy resin panels.First,each sample was immersed in the tank filled with 10 ml of ASW containing diatoms.The cell density of the culture suspension of diatoms were approximately 5 μg·ml-1,and each treatment was repeated in triplicate.Then,the diatoms were left to settle for 60 hours at room temperature statically.After 60 h,the substrates were gently washed in filtered ASW to remove cells that had attached to the surface.Afterward,chlorophylls were extracted from the Navicula incerta diatom with 90% acetone,and the chlorophyll contents were determined by a UV–vis spectrophotometer at 664 nm.
4.Results and Discussion
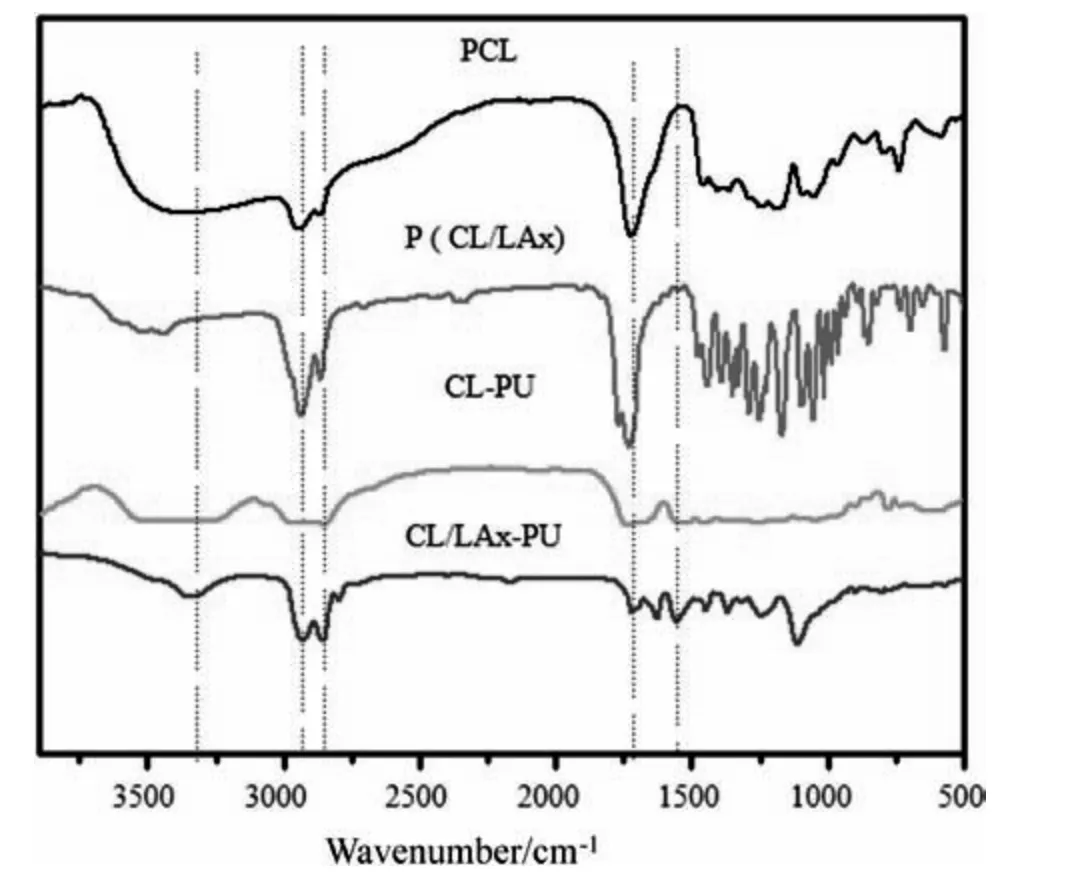
Fig.2.FTIR spectra of the PCL,P (CL-LAx),CL-PU,and CL/LAx-PU.
The FTIR spectra of CL/LAx-PU is shown in Fig.2.The characteristic band obtained at 1719 cm-1was attributed to the carbonyl in the 1st amide band,and 3332 cm-1could be assigned to hydrogen bond N-H,respectively.Whereas,the band at 1556 cm-1corresponded to the combination bands of N-H and C-O (2nd amide band),and the band at 2800–3000 cm-1was attributed to symmetric and asymmetric stretching of -CH3.The disappearance of the -NCO band at 2275 cm-1confirmed the PU was all cured[31,32].These results indicated that CL/LAx-PU was successfully prepared.
The related adhesion strength detail is depicted in Table 1 and Fig.3.The adhesion strength for CL-PU was around 1.83 MPa.The strength of the adhesion varied when LLA content was added.The adhesion strength amount for CL/LAx-PU1,CL/LAx-PU2,CL/LAx-PU3,and CL/LAx-PU4 were 1.63,1.52,1.48,and 1.19 MPa,respectively.We realized that CL-PU had the most considerable strength to adhesion.Therefore,CL-PU had a more versatile chain than other polymers,which comprised much polar interaction between epoxy and substrate.When the LLA content increased in the polymer,the adhesion strength became much weaker than CL-PU.
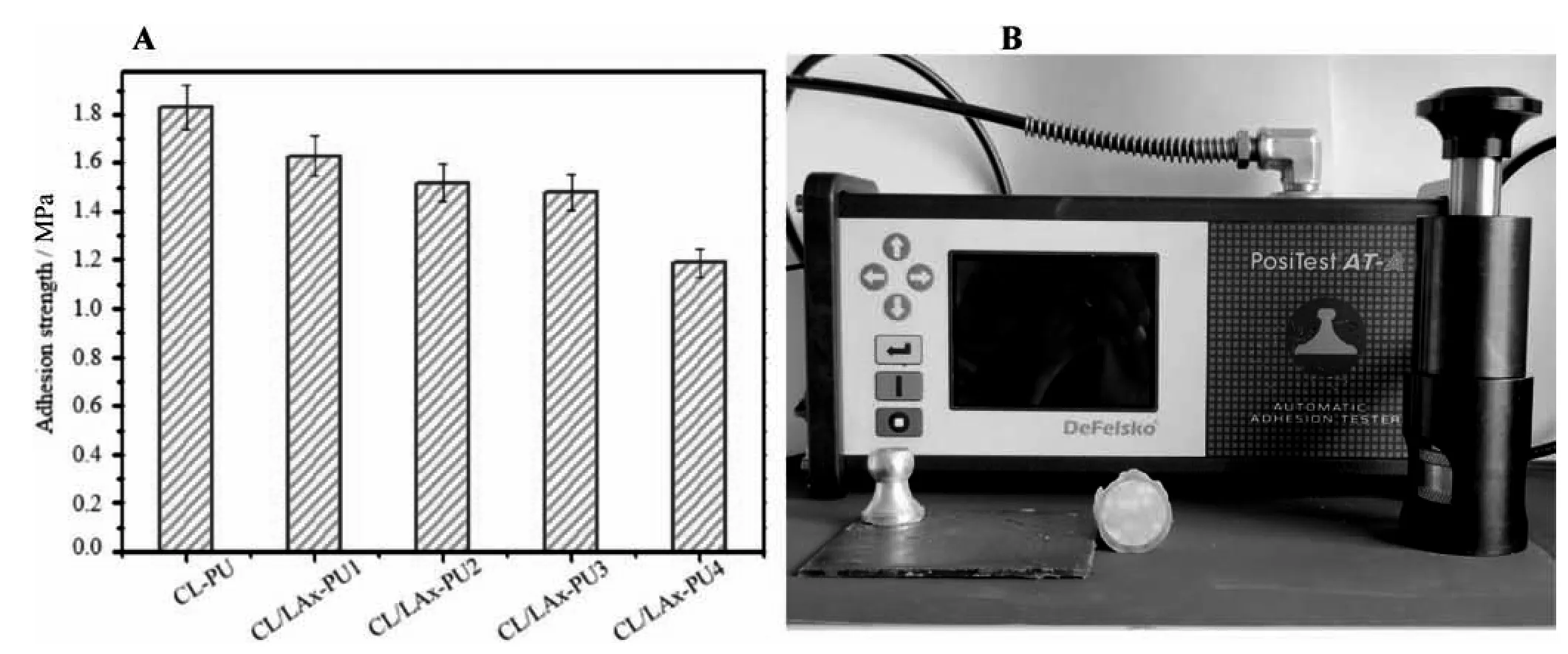
Fig.3.The adhesion strength of degradable PU with different LLA contents(A),and the adhesion test was performed by adhesion tester(PosiTest AT-A automatic,USA)(B).
The contact angle analysis is an effective method for the identification of hydrophilicity and hydrophobicity [32].In Fig.4,the contact angles of all CL/LAx-PU surfaces were lower than the CLPU surface because PCL as the soft segment was dispersed better in the entire hard segment; therefore,the obtained CL-PU film is almost hydrophobic[33].The contact angles of CL/LAx-PU samples were gradually decreased from 81°to 55°with the increase in LLA content,and CL/LAx-PU4 was the most hydrophilic.The increase in LLA content has decreased the spherulite size and crystallinity of PCL and increased the amorphous region in the polymer [34].
The thermogravimetric analysis provides information about the thermal stability of the CL/LAx-PU.The decomposition temperature(T50%)of CL/LAx-PU decreased with the LLA content segments in the polymer(Table 1 and Fig.5).The decomposition of TGA thermograms at low temperatures correlated to the decomposition of CCL/LAx-PU moieties,which was decreased by the addition of LLA blocks by 4–76 °C.While the decomposition temperature of CL/LAx-PU4 was lower than other polymers that were indicated that the introduction of LLA would make the CL/LAx-PU copolymers thermally weaker than the CL-PU.
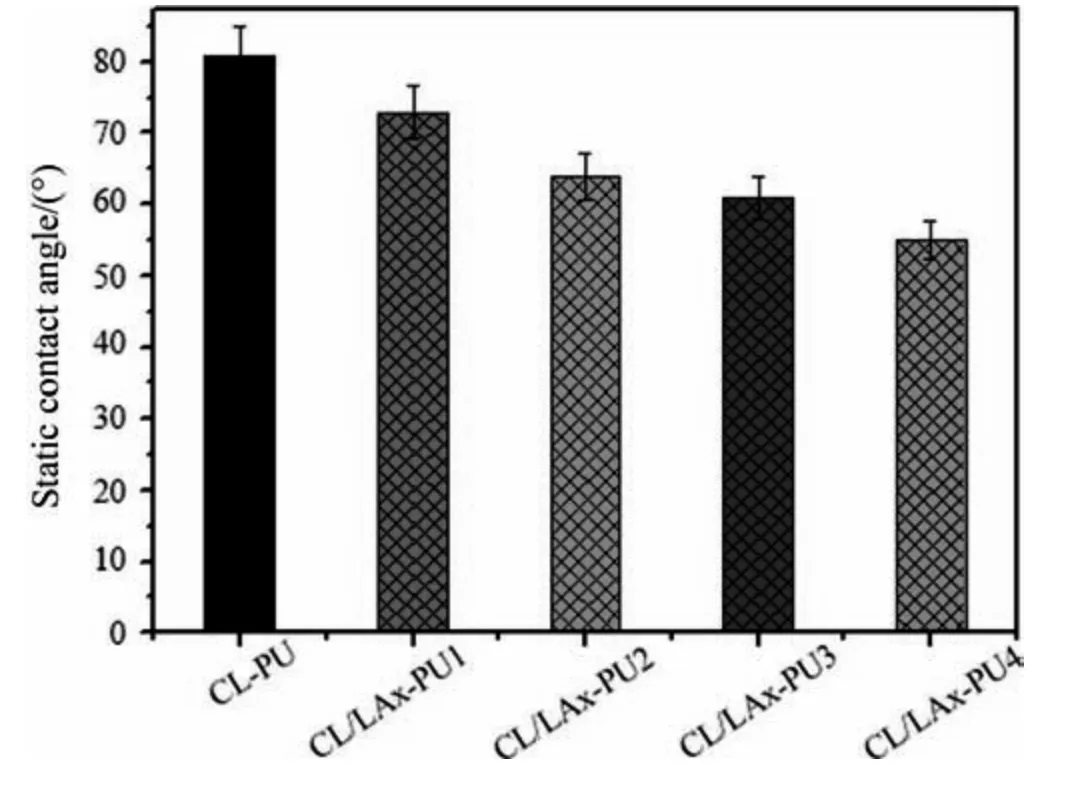
Fig.4.Water contact angle of degradable polyurethane with different content of LLA.
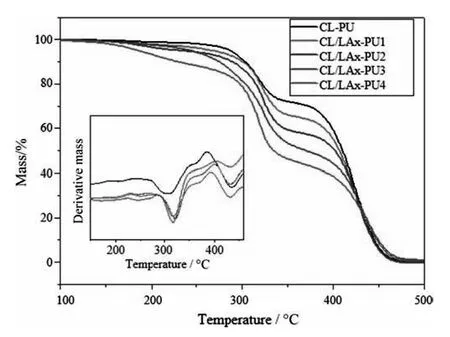
Fig.5.TGA curves of degradable PU with different content of LLA.
The hydrolytic degradation of the CL/LAx-PU chip was influenced by its molecular structure,ester group density,amorphous size,spherulite size,and crystallinity [33].The experiment of hydrolytic degradation was performed in ASW with the same salt concentration and pH as seawater,and the effect of microorganism adsorption was neglected.The hydrolytic degradation of the polymer occurs through surface erosion,and it also depends on the balance between the hydrolysis rate of the attached group and the diffusion of water kinetics inside the polymer matrix.If water diffusion is higher than the hydrolysis of the attached group,the matrix degrades through thin surface erosion.On the contrary,if the chain cleavage is faster in comparison with the diffusion of water,the material triggers surface degradation [33].The films were prepared by the solution casting method from polymer CLPU,CL/LAx-PU1,CL/LAx-PU2,CL/LAx-PU3,and CL/LAx-PU4.The mass loss of films was monitored for 35 days.The hydrolytic degradation performance of CL/LAx-PU chips in the ASW at 25 °C is shown in Fig.6A.The mass loss in all CL/LAx-PU chips started after 3 days.The CL-PU chip gave a 5.8%mass loss because it had a lower rate of degradation owing to its significantly higher crystallinity and less hydrophilicity.The crystallinity of the polymer strongly hampered the water uptake because crystallinity was dominant in almost the entire region of the hard segment.Therefore,the water molecules hardly penetrated the polymer matrix,and it only degraded the surface film [35].The percentage of mass loss of CL/LAx-PU1,CL/LAx-PU2,CL/LAx-PU3,and CL/LAx-PU4 were reached at 7.9%,8.4%,9%,and 10.96% after 35 days,respectively.
CL/LAx-PU1,CL/LAx-PU2,CL/LAx-PU3,and CL/LAx-PU4 showed a higher degradation owing to the coordination between their solubility and the surface erosion.We can monitor the erosion by changing the polymer composition of LLA groups to ester units in the main chain.The CL/LAx-PU4 showed the fastest degradation rate because LLA reduced the spherulite size and crystallinity of CL and also increased the amorphous region in the hard segment.The hydrolytic degradation of the CL/LAx-PU chip was increased in ASW with the increase in LLA content because LLA influenced the irregularity of the spherulite size of the CL,and it also caused higher hydrophilicity,which was confirmed by CA measurement(Fig.4).As shown in Fig.6B,the apparent thickness,and level of degradation rate were measured unless the thickness indicated a significant decrease.The level of erosion of CL/LAx-PU4 rose enormously during the initial stage(~12 days)owing to higher LLA content,as depicted in Fig.6A.Generally,the decrease of the thickness can indicate that the polymer surface is eroded; in addition,the thickness is significantly influenced by the degree of erosion and fouling.Therefore,the thickness is a vital factor in measuring the self-polishing polymer coating system.The mechanism for the hydrolytic degradation process of polymer is shown in Fig.7,which shows that the polymer can degrade into small fragments in seawater.
If erosion is sufficiently high,then the biodegradable resin itself would be highly antifouling,though a high degradation speed indeed leads to limited service life [33].Thus,improving the antifouling property of biodegradable polymer by incorporating environmentally friendly antifoulants would be a good strategy because successful biofouling security on ship hulls can be accomplished by antifouling coatings,which is present at the coatingwater interface,and can stop fouling species from settling [36].The constant concentration of DCOIT is desired for the successful prevention of fouling to the surface coating.The release process of antifoulant from antifouling coatings have to be continued for a prolonged period.So it is essential to regulate the release rate of antifoulant from the antifouling coating in seawater,which is controlled by coating resins [37].In principle,the CL/LAx-PU coating surfaces would be degraded and decomposed underneath seawater to evaluate the eco-friendly release of antifoulants.
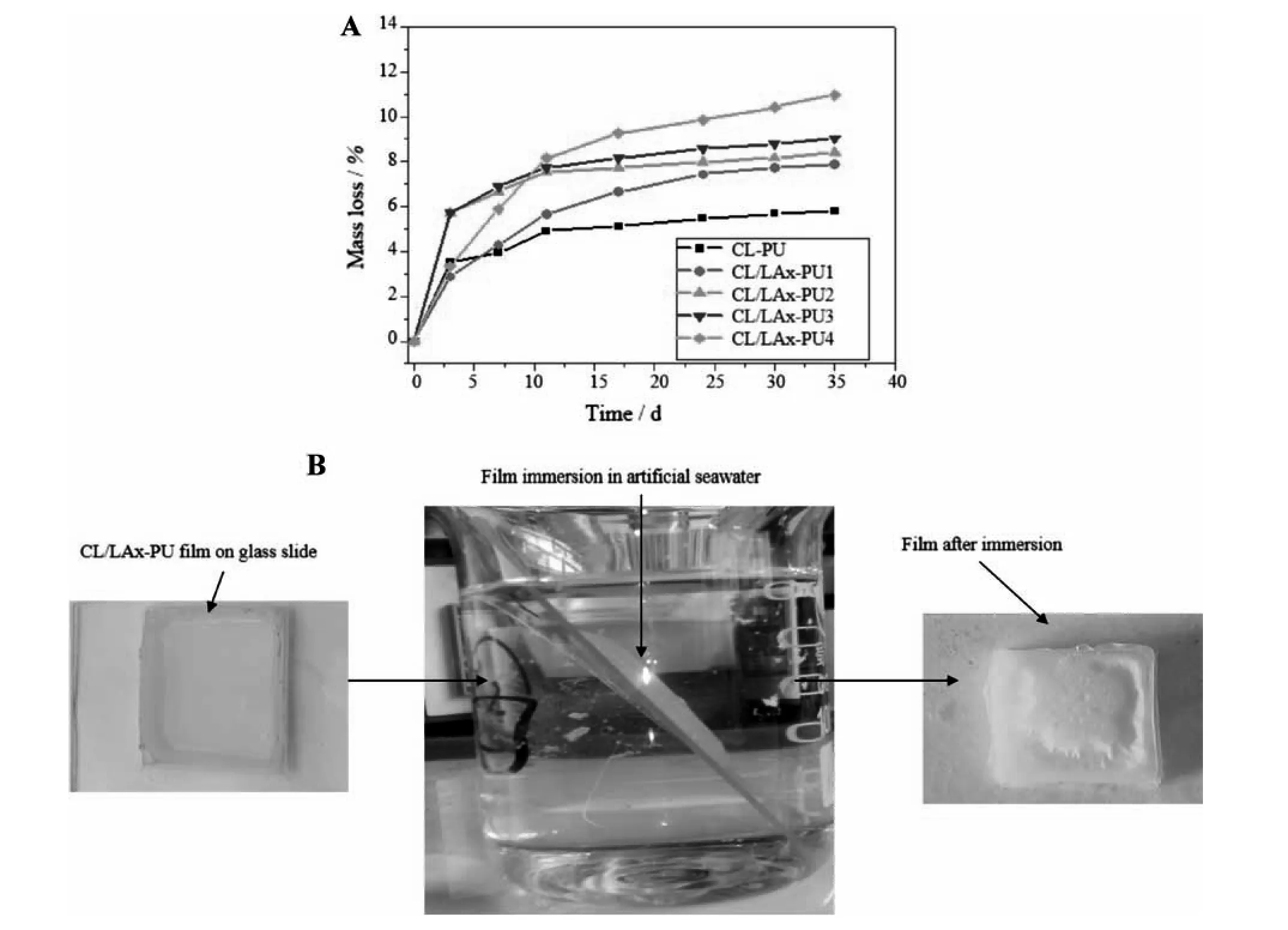
Fig.6.(A)Time dependence of mass loss of degradable PU with different LLA contents in artificial seawater at 25 °C,and (B)Left side of the image illustrate the CL/LAx-PU casting solution film prepared onto the glass slide immersed in artificial seawater (mid).The right side indicates the effect of immersion on the film (after 35 days).
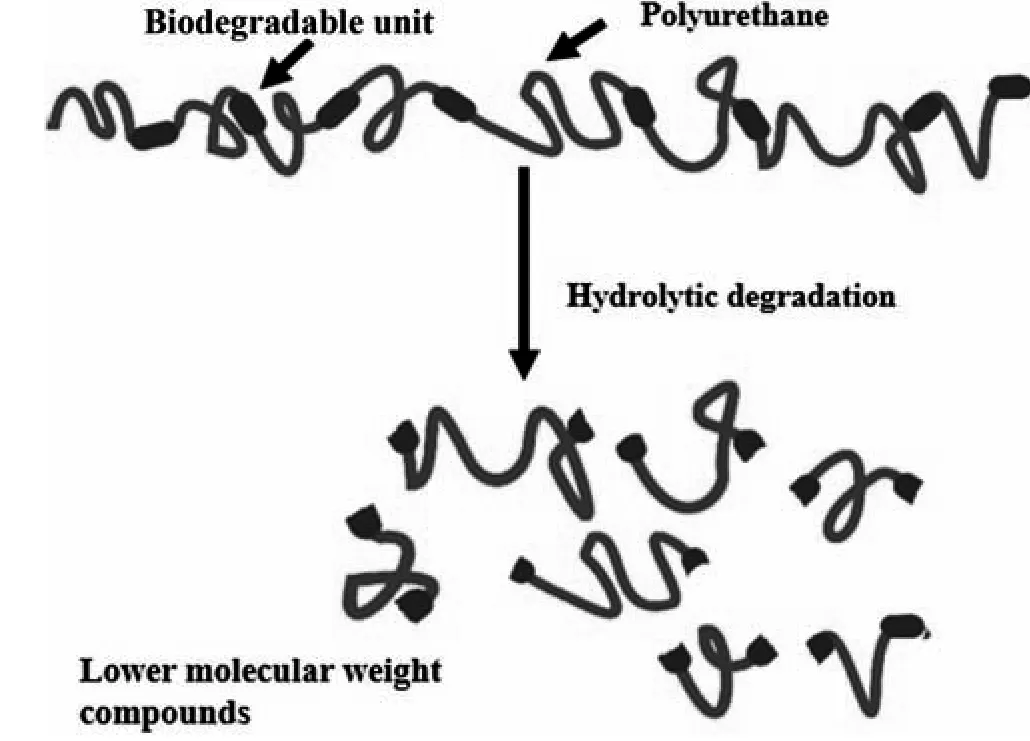
Fig.7.Possible hydrolysis reaction mechanism of biodegradable PU in seawater.
The release rate of CL/LAx-PU films depended on their contact time with the artificial seawater at 25 °C,as shown in Fig.8.All the CL/LAx-PU films started the release of DCOIT once they were immersed in the artificial seawater.The release rate of CL/LAx-PU was faster due to the presence of the LLA component.While the release rate of CL-PU was very slow because CL-PU was less hydrophilic and had higher crystallinity; therefore,the penetration of water molecules with polymer became difficult,as aforementioned.The release rate of the CL-PU became steady after 27 days.The release rate of CL/LAx-PU4 was the fastest due to the highest LLA content,which resulted in the decreased spherulite region and led to an increase in the amorphous region.Because higher LLA content led to a higher degradation,it could also improve the antifouling activity and resulted in a short life period of antifouling material.Meanwhile,the hydrolyzable units controlled the release rate of antifoulant,and the controlled release rate of DCOIT could show good antifouling activity.The release rate of antifoulant was faster at the initial stage,and then it became stable over time.The ester group also had an effect on the degradation process because the LLA had higher ester group density than PCL;therefore,higher ester group density resulted in a higher rate of degradation.The range of the average release rate was between 0.78 and 1.71 μg·cm-2·d-1until 30 days,but the release rate decreased between 30 and 45 days was 0.27 and 1.39 μg·cm-2·d-1.The polyurethane with polyester as soft segments would be degraded because of the hydrolysis of ester link in the marine environment,but its degradation and release rate could be independent on the shipping speed and enzymatic condition in natural seawater.Consequently,the system would exhibit strong antifouling activity in both dynamic and static marine environments.
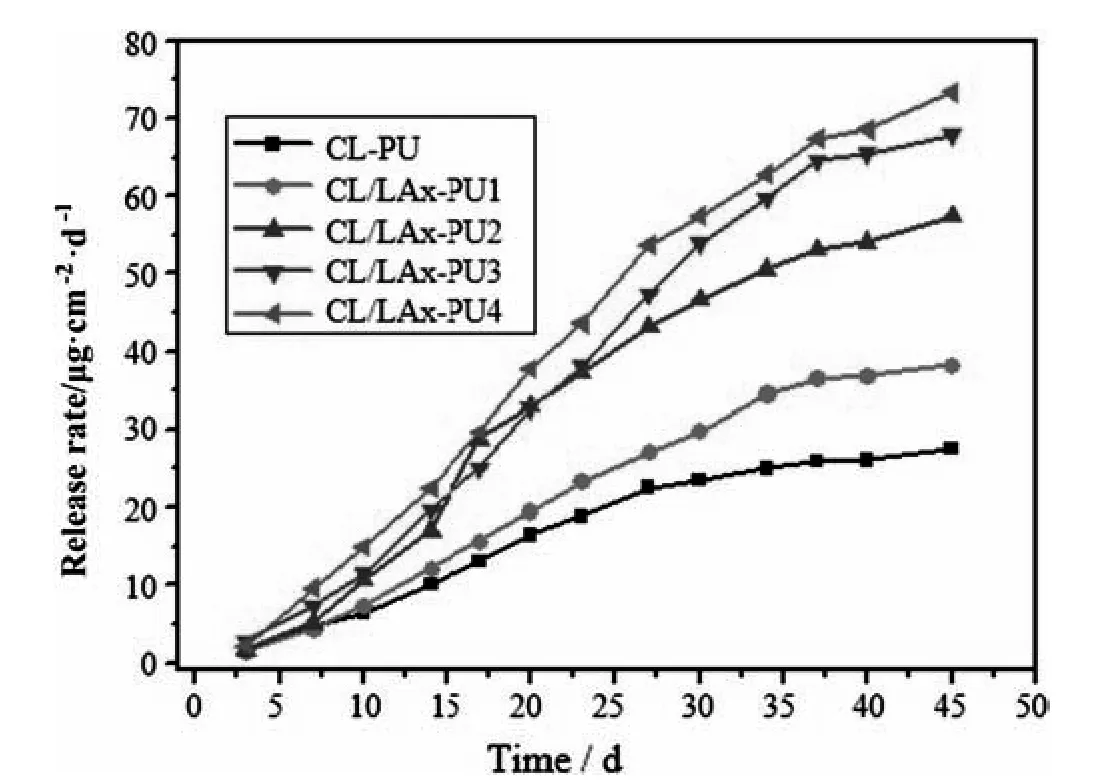
Fig.8.Time dependence of release rate of degradable PU with different LLA contents in artificial seawater at 25 °C.
SEM was used to gain an understanding of the surface degradation of CL/LAx-PU polymer films.All thin film surfaces were not perfectly smooth or either displayed multiple clusters on the surface on 0 day.Notably,as the degree of hydrolysis approached an advanced stage (40 days),CL/LAx-PU film encountered changes to their surface morphology.Fig.9,reveals the surface morphologies of the polyurethane film during degradation for 40 days immersion in ASW was determined by SEM.After 40 days,the film CL-PU showed no significant change because CL-PU hydrolyzed very slowly due to hydrophobicity and higher crystallinity.CL/LAx-PU copolymer showed a more significant change because its surface became smooth owing to the hydrolysis of LLA ester groups,which indicated that hydrolysis was slightly affected on the surface morphology.The surface of CL/LAx-PU4 became the smoothest as compared to other polymers,but many small holes appeared on the surface film because of the higher hydrolytic degradation rate.In other words,the roughness of the CL/LAx-PU4 surface became gradually smooth.The degradation of PU could control this erosion during the lifetime of the coating due to the self-renewal surface.The film could detach the accumulated biofouling due to the degradation of the surface.On the other side,the degradable polymer could control the release rate of DCOIT in the seawater due to self-renew surface and exhibited excellent efficiency against biofouling.
In general,the biofouling of underwater species begins directly through the adhesion of protein(1 min)and then diatoms and bacteria (1–24 h) when the clean substrate is submerged in natural seawater.Herein,the adhesion of Navicula incerta diatom cells were examined on the surfaces of the CL/LAx-PU4.CL/LAx-PU4 were composed of 0%,2.5%,5%,10%,and 20% of DCOIT,respectively.Fig.10A and 10B demonstrated the number of Navicula incerta diatoms adhesion to CL/LAx-PU4 after 60 hours.Fig.10A revealed that the development of biofilm on CL/LAx-PU4 with an absorbance of 0.301,demonstrating that CL/LAx-PU4 without antifoulant was unable to tolerate the creation of biofilm.By contrast,slightly fewer biofilm produced at 2.5% containing DCOIT with absorption of 0.094,which showed that the antifoulant plays a very important function in the prevention of biofilm formation.In addition,CL/LAx-PU4 with 5%,10%,and 20% containing DCOIT showed good anti-diatom absorption capacities of 0.074,0.044,and 0.035,respectively.Fig.10B was also observed that the volume of diatoms adhered to CL/LAx-PU4 without antifoulant was greater than all other samples,which indicated that CL/LAx-PU4 without DCOIT was weak resistance to diatom adhesion.However,it noticed that the lower number of Navicula incerta diatoms(11.63%)were on the CL/LAx-PU4 with 20%containing DCOIT,indicating that the antifoulant DCOIT on the surface successfully prevented the diatoms.The number of diatom cells were approximately 31.2%,24.58%,and 14.61% attached on the surface of CL/LAx-PU4 with 2.5%,5%,and 10% containing DCOIT.The anti-diatom performance improved with the antifoant DCOIT content in the polymer.
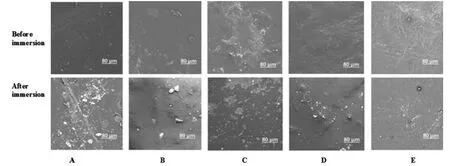
Fig.9.SEM images of polymer before and after immersion in ASW (A),CL-PU,(B) CL/LAx-PU1,(C) CL/LAx-PU2,(D) CL/LAx-PU3,and (E) CL/LAx-PU4.

Fig.10.(A) The absorbance of Chlorophyll-a concentration of diatom and (B) the abundance of diatoms on the CL/LAx-PU4 copolymer surfaces with different content of DCOIT.
5.Conclusions
We have prepared degradable polyurethane synthesized with caprolactone and L-Lactide by ring open polymerization and condensation reaction.Our studies show that the increase in LLA content with a constant CL amount reduce the crystallinity and improve the amorphous interfacial region in the polymer.The experiment of hydrolytic degradation shows that synthesized degradable PU degraded when immersed into artificial seawater.The result also indicates that higher LLA content in degradable PU accelerated its degradation rate in ASW.CL-PU film has been shown to degrade gradually in artificial seawater,having lower kinetics of degradation than that of CL/LAx-PU.The erosion performance of CL-PU film depicts that the hydrophobicity hindered bulk erosion; therefore,CL-PU film shows a slow surface erosion as observed by SEM.Furthermore,the SEM result also shows that the CL/LAx-PU4 was highly renewed and kept active surface during degradation.The release rate of DCOIT from CL-PU has become steady about 27 days period as compared to all other CL/LAx-PU.The CL/LAx-PU copolymer reveals promising degradation and release rate under hydrolytic condition.Moreover,the results of anti-diatom(Navicula incerta)indicated that the CL/LAx-PU4 polymer with 20% containing DCOIT had excellent antifouling ability than other polymers.These results indicate that the degradable CL/LAx-PU and DCOIT would have remarkable durability and excellent antifouling activity for marine biofouling.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21776249,21878267,and 21576236).
 Chinese Journal of Chemical Engineering2021年6期
Chinese Journal of Chemical Engineering2021年6期
- Chinese Journal of Chemical Engineering的其它文章
- Effects of coagulation-bath conditions on polyphenylsulfone ultrafiltration membranes
- Functional monodisperse microspheres fabricated by solvothermal precipitation co-polymerization
- Synthesized graphene oxide and fumed aerosil 380 dispersion stability and characterization with partially hydrolyzed polyacrylamide
- Removal of lead (Pb(II)) and zinc (Zn(II)) from aqueous solution using coal fly ash (CFA) as a dual-sites adsorbent
- Catalytic performance improvement of volatile organic compounds oxidation over MnOx and GdMnO3 composite oxides from spent lithium-ion batteries:Effect of acid treatment
- Application of fracturing technology to increase gas production in low-permeability hydrate reservoir:A numerical study
