State of arts on the bio-synthesis of noble metal nanoparticles and their biological application
Kok Bing Tan,Daohua Sun,Jiale Huang,Tareque Odoom-Wubah,Qingbiao Li,3,*
1Department of Chemical and Biochemical Engineering,College of Chemistry and Chemical Engineering,Xiamen University,Xiamen 361005,China
2Department of Biomedical Engineering,College of Engineering and Computer Science,Wentworth Institute of Technology,550Huntington Avenue,Boston,MA 02115,United States
3College of Foodand Biology Engineering,Jimei University,Xiamen361021,China
ABSTRACT Nanomaterials are materials in which at least one of the dimensions of the particles is 100 nm and below.There are many types of nanomaterials,but noble metal nanoparticles are of interest due to their uniquely large surface-to-volume ratio,high surface area,optical and electronic properties,high stability,easy synthesis,and tunable surface functionalization.More importantly,noble metal nanoparticles are known to have excellent compatibility with bio-materials,which is why they are widely used in biological applications.The synthesis method of noble metal nanoparticles conventionally involves the reduction of the noble metal salt precursor by toxic reaction agents such as NaBH4,hydrazine,and formaldehyde.This is a major drawback for researchers involved in biological application researches.Hence,the bio-synthesis of noble metal nanoparticles (NPs) by bio-materials via bio-reduction provides an alternative method to synthesize noble metal nanoparticles which are potentially non-toxic and safer for biological application.In this review,the bio-synthesis of noble metal nanoparticle including gold nanoparticle (AuNPs),silver nanoparticle (AgNPs),platinum nanoparticle (PtNPs),and palladium nanoparticle (PdNPs) are first discussed.This is followed by a discussion of these biosynthesized noble metal in biological applications including antimicrobial,wound healing,anticancer drug,and bioimaging.Based on these,it can be concluded that the study on bio-synthesized noble metal nanoparticles will expand further involving bio-reduction by unexplored bio-materials.However,many questions remain on the feasibility of bio-synthesized noble metal nanoparticles to replace existing methods on various biological applications.Nevertheless,the current development of the biological application by bio-synthesized noble metal NPs is still intensively ongoing,and will eventually reach the goal of full commercialization.
Keywords:Noble metal nanoparticles Bio-synthesis Bio-templating Bio-material Bio-logical application Nanomaterials
1.Introduction
Nanomaterials are materials in which at least one dimension of the particle is 100 nm and below [1].They can be synthesized by atom assembly or bulk materials breaking to achieve nanometer dimension using various methods such as hydrothermal [2],impregnation [3],chemical vapor deposition [4],templating [5],sol-gel[6],and co-precipitation method[7].Depending on the synthesis methods and reaction conditions,nanomaterials can come in many different sizes and shapes,namely nanospheres [8],nanowires [9],nanorods [10],nanotubes [11],nanosheets [12],nanoflowers [13],and so on.In general,nanomaterials consist of inorganic nanomaterials such as metal nanomaterials [14],carbonaceous nanomaterials [15],and silica nanomaterials [16],as well as organic nanomaterials such as cellulosic [17]and chitosan nanomaterials [18].
Meanwhile,metal nanomaterials can be further categorized into noble metal NPs [19],non-noble metal NPs [20–22],metal oxide NPs [23–25],and metal sulfide NPs [26,27].Noble metal nanoparticles are metal nanomaterials that in general consist of Au,Ag,Pt,Ru,Rh,Os,Ir and Pd NPs [19].They are well known for their uniquely large surface area-to-volume ratio [28],optical[29],electronic properties [30],high stability [31],easy synthesis and tunable surface functionalization [32].Hence,they are widely used in various applications such as catalytic system [33–35],fuel cell [36–39],water and pollution control [40],and sensor [41,42].More importantly,noble metal NPs are known to have excellent compatibility with bio-materials [43].Therefore,noble metal NPs are also widely used in biological applications such as antimicrobial agents,wound healing,anticancer drugs,and bio-imaging.Conventionally,noble metals NPs are synthesized from their respective noble metal salt precursors,whereby the precursors are mixed with a chemical reducing agent such as NaBH4[44],hydrazine [45]and formaldehyde [46].These reducing agents will reduce the noble metal salt precursors into noble metal NPs.However,as these reagents are highly toxic [46–48],many researchers argue that noble metal nanoparticles synthesized via chemical method would add additional toxicity as a side-effect when used for biological applications [49].
To solve this problem,many researchers began to study the possibility of using non-toxic renewable bio-materials as an alternative bio-reducing agent for the bio-synthesis of noble metal NPs.Bio-synthesis of noble metal NPs is initiated first by mixing noble metal salt precursors with bio-materials such as plant,fruit extract,enzymes,and microorganisms [50].Bio-molecules such as alkaloids,flavonoids,proteins,reducing sugars,polyphenols contained in the bio-materials play the role of reducing and capping agents to bio-synthesize noble metal NPs from noble metal salt precursors[51].Throughout the years,many bio-materials have been reported to successfully bio-synthesize not only noble metal NPs but other metal NPs such as metal oxide NPs,metal sulfide NPs,and even bimetallic or alloy NPs [52].Our research group has widespread research experience in bio-synthesizing these metal nanoparticles with different types of bio-template on various applications such as catalytic [33–35,39,53,54],fuel cell [36],sensors [55–57],and antimicrobial [58].
However,knowing well the importance of bio-synthesized noble metal NPs on biological applications,we have henceforth decided to publish a review paper focusing on this area.Besides,we will also provide and discuss updated studies regarding this area both from our research group and other works.This review paper will first discuss the development of various bio-materials on the bio-synthesis of noble metal nanoparticles by various works.The biological applications which include antimicrobial,wound healing,anticancer drugs,and bio-imaging by biosynthesized noble metal NPs would be discussed.It is hoped that this review paper will provide insights and inspiration for existing and future researchers on various biological applications to bring the development of bio-synthesized noble metal nanoparticles into a more advanced level,but also for aspiring researchers who want to focus on various biological applications.
2.Bio-Synthesis of Noble Metal Nanoparticles
Generally,the process to produce bio-synthesized NPs begins by mixing noble metal salt precursors with bio-materials at a predetermined temperature and time.As it is mixed,the various biomolecules contained in the bio-materials will play roles such as reducing,stabilizing,and capping agents,to initiate the reduction of the noble metal salt precursors into noble metal NPs [59].An indication of the reduction process can be observed by the change in colloidal color,which is different for each type of noble metal precursor,as displayed in Table 1.It should be noted that until now,only bio-synthesized Au,Ag,Pt,Pd,Ru and Rh NPs are documented.Therefore,only these noble metal NPs are listed in Table 1.The state of arts and development for different types of biomaterials utilized to bio-synthesize noble metal NPs will be discussed below separately in different sub-sections.

Table 1 Changes on colloidal color for different noble metal salt precursor bio-reduction into Noble Metal Nanoparticles
2.1.Gold nanoparticles (AuNPs)
In our research group,Huanget al.[66]investigated the effect ofCinnamomum camphoraplant extract concentration on the biosynthesis of AuNPs.At 0.002 g·cm-3ofCinnamomum camphora,the number of bio-molecules from the plant extract,which were supposed to act as protecting/capping agents to prevent the growth and aggregation of the AuNPs,was insufficient.This led to the formation of nanotriangle and nanosphere AuNPs with an average size of 80 nm.By increasing the amount ofCinnamomum camphorato 0.01 g·cm-3,sufficient protecting agents were available,which led to the formation of nanospheres with much smaller sizes of 23.5 nm.The size of the AuNPs could be reduced further to averagely 21.4 nm with 0.02 g·cm-3Cinnamomum camphora,as a larger amount of the bio-material will prevent the growth of AuNPs.Similar trends were also been observed by Maddinediet al.[67].Meanwhile,Fayazet al.[68]also investigated the effect of pH on the bio-synthesis of AuNPsvia Maduca Longifolia.It was found that at pH 2,irregular nanoplates with particle sizes of 3000 nm was formed.However,as the pH was increased to 10,the particle sizes decreased significantly to 7 nm,forming Au nanospheres.This indicated the role of the hydroxyl group at high pH,as well as tyrosine,which was responsible for the reduction and stabilization of AuNPs.Similarly,Bogireddyet al.[69]investigated the effect of pH on the bio-reduction of AuNPsvia Coffea Arabicacoffee extract.The hydroxyl group from the phenolic functional group from the coffee extract was found to be responsible for the reduction and stabilization of AuNPs.As the pH was increased,the size of the particles was also found to decrease.This indicated that the hydroxyl group was also responsible to control the growth of the AuNPs,as displayed in Fig.1.However,such a trend was not observed from the work of Chenet al.[70].Lower pH was found to produce smaller AuNPs size by usingChenopodium formosanumshell extract,which is high in protein as a reducing agent.At higher pH,Chenet al.[70]postulated that such reducing agents will be degraded,which affected the formation of the AuNPs.
Microorganisms can also be used for the bio-synthesis of AuNPs.Like plant extract,the microorganism concentration is also responsible for the growth control of the AuNPs.Wadhwaniet al.[71],reported that a larger amount ofActinobacterled to the formation of uniform spherical AuNPs with smaller sizes.Uniform AuNPs were also observed in the work of Bakiret al.[72]and Tripathiet al.[73],whereby AuNPs were bio-synthesized withLyngbya majusculaandTrichoderma harzianum,respectively.The ability of microorganisms to synthesize AuNPs was due to roles played by the intercellular,and subsequently,extracellular of the microorganisms[74].In the intercellular part,reducing sugar,fatty acids,and reducing enzymes all play roles in reducing AuNPs.Meanwhile,the exocellular of the microorganism leads to the formation of exopolysaccharide that adsorbed Au3+and reduced it[74].Additionally,Yanget al.[75]managed to synthesized Au in the form of nanowires instead of nanospheresvia E.coliassisted by hexadecyltrimethylammonium bromide (CTAB) and Ascorbic Acid (AA),as displayed in Fig.2 (a).It was postulated that Au3+was first bounded onE.coli,while the other Au3+reacted with CTAB to form a ligand-substituted anion.The bounded Au3+onE.coliwas then reduced by AA,which initiated the preferential nucleation sites onE.coli.Finally,CTAB directed the linear fusion of AuNPs,forming a long chain of Au nanowires.The mechanism is summarized below as Fig.2 (b).

Fig.1.Effect of pH on the size and shape of AuNPs bio-synthesized by Coffea Arabica coffee extract (This figure is reprinted with permission from Bogireddy et al. [69].Copyright (2016),Royal Society Chemistry).
Unlike microorganisms and shells,plant extract tends to produce AuNPs that are polydisperse and irregular nanospheres.To investigate this problem,Jianget al.[76]from our group utilizedArtocarpus heterophyllus Lamplant extract as a sample biomaterial to bio-synthesize AuNPs.It was found that the AuNPs consisted of polydisperse AuNPs with various shapes that included nanotriangles and nanospheres,as displayed in Fig.3 (a).This was due to different bio-reducing agents that existed in theArtocarpus heterophyllus Lam.To prove this,Jianget al.[76]tested the bio-material sample before and after the reducing reaction.They found that reducing sugar,polyphenols,and flavones were collectively responsible for the reduction of AuNPs.Therefore,Jianget al.[76]attempted to separate these reducing agents by first adsorbing the plant extract with AB-resin,and then it was washed with de-ionized water,which desorbed reducing sugar.Further rising with ethanol would desorb the flavones,and finally,polyphenol was desorbed.These parts of the separated extracts were then used as bio-reducing agents on AuNPs.As a result,relatively monodisperse AuNPs were formed from these individual reducing agents.Jianget al.[76]observed that spherical AuNPs were formed by reducing sugar,which plays a role in preventing the NPs from aggregation.This led the AuNPs to self-assemble into a spherical shape [77],as displayed in Fig.3 (b).Meanwhile,flavones enabled fast nucleation and growth of Au monomers,while reinforcement from ketones steric hindrance enabled the formation of Au nanoflowers [76].On the other hand,polyphenols molecule first chelates with Au3+ions on phenolic hydroxyl,which were inductively oxidized to carbonyls and ketones.These functional groups were selectively bound to Au (111) plane and initiated isotropic growth on this plane,and finally formed nanoplates [76].Hence,Jianget al.[76]proved that polydisperse nanoparticles formed from the same plant extract were due to various reducing agents contained in the plant extract.Our group also introduced another method to produce monodisperse uniform AuNPs by using a simple method of density gradient.Viatheoretical and experimental work,Zhenget al.[78],were able to optimize the centrifugation force and time for the separation of AuNPs of various size reduced byCacumen Platycladileaves.As a result,3 fractions of(18.0±0.9),(25.2 ± 1.6),and (37.5 ± 2.3) nm AuNPs were formed.Table 2 tabulated the list of bio-materials utilized for the synthesis of AuNPs.
2.2.Silver nanoparticle (AgNPs)
Throughout the years,our research group has also investigated the potential of several bio-material on the bio-reduction of AgNPs.For example,Fuet al.[79]investigated the potential ofAeromonassp.SH10microorganism for the bio-reduction of AgNPs.As it took months for the reduction to complete,our group decided to add NaOH,as the OH-tends to promote the reduction of Ag+[80].As a result,within hours,relatively uniform spherical AgNPs were formed with an average size of 6.4 nm,as calculated by the Scherrer equation.Furthermore,uniformly distributed AgNPs were also observed under SEM,suggesting that the AgNPs were not only uniformly bounded onto the surface of the cells during the reduction reaction but also uniformly deposited onto the cell surface during the drying process before SEM observation.In our other work,Huanget al.[66]managed to develop AgNPs bio-synthesized withCinnamomum camphoraplant extract,in addition to AuNPs in the same work.It was soon discovered that the shape of AgNPs and AuNPs were different,especially when 0.02 g·cm-3ofCinnamomum camphoraplant extract was used.While spherical AuNPs were formed at this plant extract concentration,polydisperse AgNPs with a mixture of nanospheres and nanotriangles were formed instead.This was because,in addition to the rapid reduction,the bio-molecules protective agent from the plant extract was insufficient to resist the growth of anisotropic AgNPs,leading to Oswald ripening.Meanwhile,the bio-molecules protective agent was found to be sufficient and could firmly prevent the Oswald ripening of AuNPs at the same plant extract concentration.This resulted in the formation of nano-spheres.Meanwhile,mild reduction condition at 0.01 g·cm-3ofCinnamomum camphoraplant extract enabled the formation of quasi-spherical AgNPs,with an average size of 64.8 nm.Such a trend of results was also observed in our other works,where a high concentration ofCacumen Platycladiplant extract caused Oswald ripening,leading to the formation of polydisperse AgNPs [58].
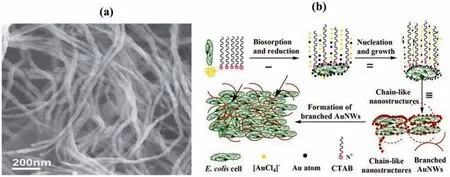
Fig.2.(a) SEM image of Au Nanowire (AuNw) bio-synthesized by E.coli assisted by hexadecyltrimethylammonium bromide (CTAB) and Ascorbic Acid (AA);(b) AuNw formation mechanism (This figure is reprinted with permission from Yang et al. [75].Copyright (2014),Wiley).
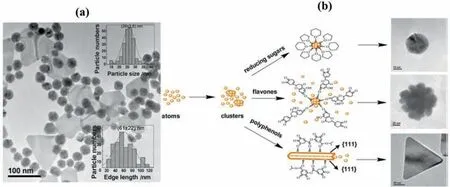
Fig.3.(a) Various shapes of AuNPs bio-synthesized with Artocarpus heterophyllus Lam;(b) Individual shapes of AuNPs bio-synthesized by reducing sugar,flavones,and polyphenols isolated from Artocarpus heterophyllus (This figure is reprinted with permission from Jiang et al. [76].Copyright (2013),Springer).
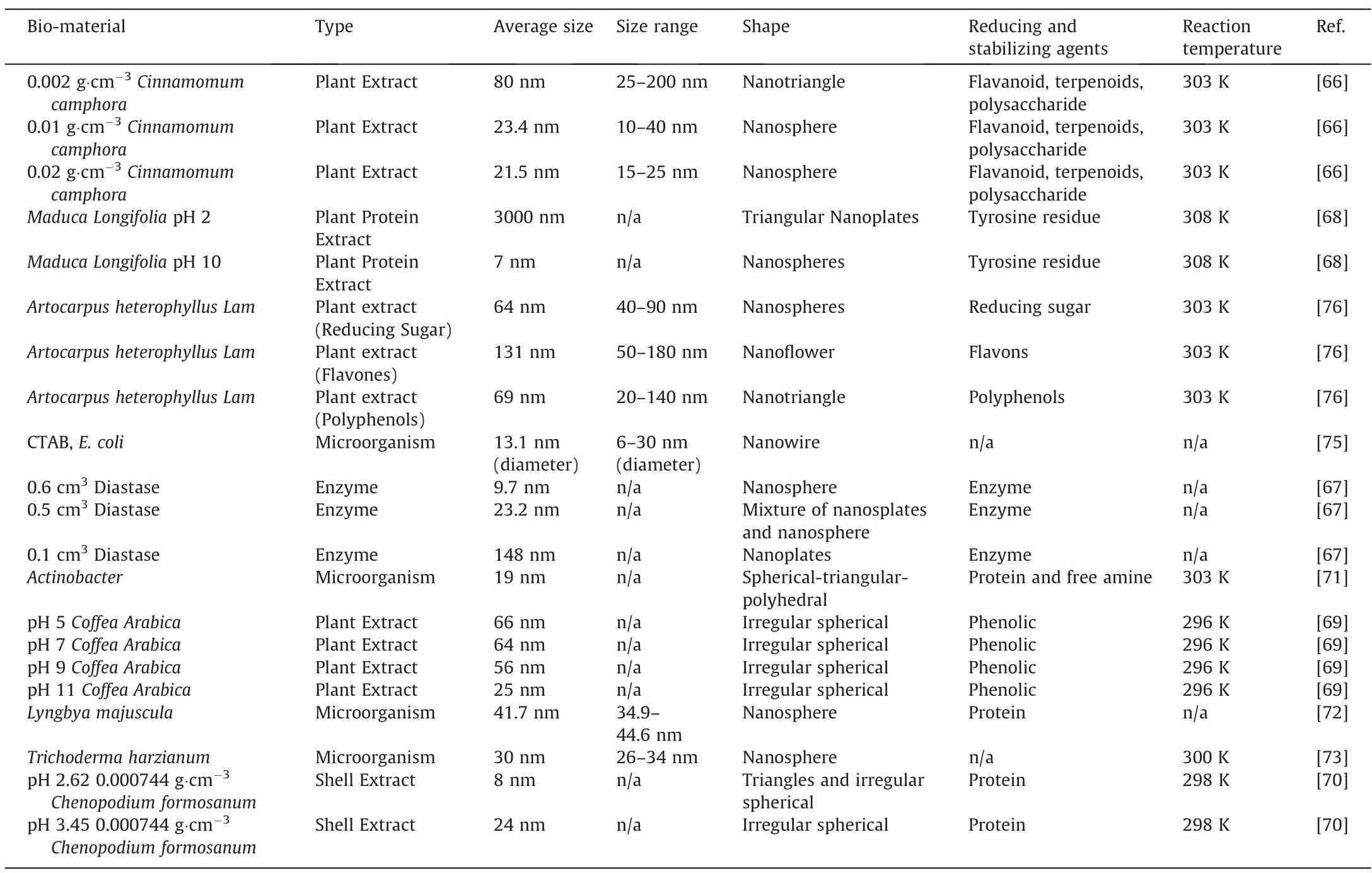
Table 2 Lists of Bio-template utilized for the synthesized of AuNPs
To improve the uniformity and reduce the size of the AgNPs,our group extended the research from a batch reactor [66]into a continuous tubular reactor.Huanget al.[81]investigated the effect of AgNO3precursor solution flow rate and tube inner diameter on the uniformity and the size of the AgNPs using the sameCinnamomum camphoraplant extract as the bio-reducing agent.At 0.0083 cm3·s-1flowrate,0.5 mm of tube inner diameter,quasi-spherical AgNPs of average 10.5 nm within the range of 5–18 nm was formed.This effectively decreased the size and increased the uniformity,as compared to that of batch reaction.However,when the flowrate was increased to 0.017 cm3·s-1,polydisperse and larger AgNPs were formed,regardless of the tube inner diameter.This was because lower flowrate led to longer resident time and thus higher fluid temperature.This resulted in more rapid nucleation while the growth remained slow,leading to the formation of smaller and quasi-spherical AgNPs.Such a trend of results were also observed by Huanget al.[58],where higher reaction temperature on the reduction of Ag byCacumen Platycladiplant extract lead to the formation of spherical AgNPs.On the other hand,it was found that lower temperature generally leads to the formation of polydisperse AgNPs.Linet al.[82]of our research group also developed Ag nanowires withCassia fistulaleaf plant extract as a reducing agent at a low reaction temperature of 303 K and a reaction time of 48 h.The Ag nanowires formed were measured,and it was found that the diameter was averagely 40 nm,with 1 μm in length.In order to study the growth mechanism of the nanowires,Linet al.[82]studied the change of AgNPs shape and size with time.From here,our group concluded that by 0.5 h of reaction,a mixture of spherical AgNPs with 12 nm average diameters and larger sizes of 50 nm were formed.As the surface energy of the larger particles is lower than smaller ones,AgNPs will be dissolved in solution and grow into larger particles.Thus by 8 h,the size of the AgNPs was mostly larger at 60 nm.This larger AgNPs eventually adhered to each other with time.As a result,newly formed Ag atoms were deposited onto the concave regions of the connected NPs via capillary phenomenon,which led to the formation of nanorods.Meanwhile,the ends of these nanorods were bounded by (111) facets with weak interaction with bio-molecule,leading it to be reactive to the newly formed Ag atoms [82].The reaction enabled the diffusion of Ag atoms onto the ends of the nanorods,resulting in the formation of AgNPs by 24 h.The formation mechanism is summarized and displayed below as Fig.4.
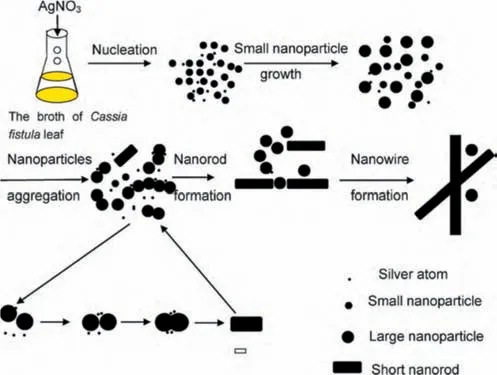
Fig.4.Schematic diagram of the formation mechanism of Ag nanowire (AgNW)biosynthesized with Cassia fistula leaf(This figure is reprinted with permission from Lin et al. [82].Copyright (2010),Elsevier).
In other research groups,Narayananet al.[83]investigated the bio-synthesis of AgNPs with hytopathogenXanthomonas oryzae pv.OryzaeStrain BXO8 bacteria as bio-reducing agent.It was found that although Ag is toxic to these bacteria,it has a special detoxification system that expresses small periplasmic silver-binding proteins.It binds the Ag at the cell surface,and by efflux pumped propulsions of metal from the cytoplasm[84].It is this protein that acted as nucleation centres for the formation of AgNPs,with size ranging within 3.06–74.16 nm.Furthermore,it was also determined that protein was also responsible for the stabilization of AgNPs.This was also verified by Syedet al.[85],where it was determined that the protein inHumicola sp.was responsible for the extracellular synthesis of high dispersity and stable spherical AgNPs with sizes in the range of 5–25 nm.However,protein from microorganisms is not necessary the only compound responsible for the formation of AgNPs.Wanget al.[86]demonstrated that instead of protein,nitrate reductase secreted by theB.methylotrophicus DC3bacteria was responsible for the Ag bio-reduction.This resulted in the formation of spherical AgNPs with size in the range of 10–25 nm.Fruits extracts were also reported to be utilized as a bio-reduction template for the formation of AgNPs.Donget al.[87]were able to synthesize AgNPs bio-reduced by tannias,flavonoids,ascorbic acid,and alkaloids containedLycium barbarumfruit extract.These compounds were adsorbed on the surface of AgNPs,forming spherical AgNPs with size in the range of 5–40 nm.Meanwhile,it was determined by Renet al.[88]that polysaccharides,amino acids,polyphenols,and flavonoids in apple extracts all played roles as reducing and capping agents for the formation of spherical AgNPs.Table 3 tabulated the list of bio-materials utilized for the synthesis of AgNPs.
2.3.Platinum nanoparticle (PtNPs)
Songet al.[90]investigated the effect ofDiopyros kakiplant extract concentration on the size of bio-synthesized PtNPs,and the average size of the PtNPs wasfoundto decreasewithincreasing plant extract concentration.Similartrends were also observed in ourwork,where Zhenget al.[91]investigated the effect ofCacumen Platycladion the bio-synthesis of PtNPs.As the plant extract concentration was increased,stronger interaction occurred between the PtNPs and the protective bio-molecules,which inhibited the growth of the PtNPs.Additionally,both Songet al.[90]and Zhenget al.[91]of our group also investigated the effect of H2PtCl6precursor at a fixed concentration ofDiopyros kakiandCacumen Platycladi,respectively for the biosynthesis of PtNPs.It was found that as the amount of precursor increased at a fixed plant extract concentration,there was lesser number of the protective bio-molecules available.Hence,it was sufficient to inhibit the growth of PtNPs,leading to an increase in particle size.Such a trend was also observed by Linet al.[92],who used wood nanomaterial extract as a reducing agent for the synthesis of PtNPs.As the Pt precursor was increased from 0.4 mmol·dm-3to 4 mmol·dm-3,the average particle size was found to increase from 11 nm to 101.3 nm.Also,Linet al.[92]investigated the effect of wood nanomaterial extract’spH.It was found that as the pH was changed from3.1 to 4.1,the shape of the particle was observed to change from cubic to aggregated cluster.Meanwhile,the size was found to decrease from averagely 11 to 4.5 nm.However,as thepHwas increased further to 11.3,the shape became a spherical nanocluster,with an average size of 21 nm.Linet al.postulated that this effect was mainly contributed by the hydrolysis of hemicellulose at differentpH,which affects its performance as stabilizing agents,and thus,the shape and size of the PtNPs.Similarly,cluster PtNPs were also produced upon reduction with lignin at relatively highpHof 7[93].
However,it should be noted that unlike Au and AgNPs,which can be easily reduced at room temperature,PtNPs requires significantly high reaction temperature,often close to 373 K,as tabulated in Table 4.As explained by Thirumuruganet al.,this could be because more energy is required for the initial formation of Pt nuclei,as compared to that of Au and Ag [95].Hence,in order to have sufficient energy for the formation of PtNPs,high reaction temperature is essential.This was proven by both Songet al.[90]and Zhenget al.[91]from our group,who investigated the effect of reaction temperature for bio-reduction byDiopyros kakiandCacumen Platycladi,respectively for the production of PtNPs.It was determined that the reduction rate of Pt2+into Pt on both studies increased significantly close to 100% as the reaction temperature was closer to 373 K,resulting in higher PtNPs formation rateand yield.Furthermore,the reduction rate at higher reaction temperature occurs so rapidly that secondary reduction processes on the surface of preformed nuclei can occur [96].This led to an increase in particle sizes at higher reaction temperature in both studies.Despite the increase in particle size,complete reduction at higher temperatures facilitated homogenous nuclei growth of PtNPs [97],which explains the synthesis of the narrower size distribution of PtNPs with higher reaction temperature in both studies.
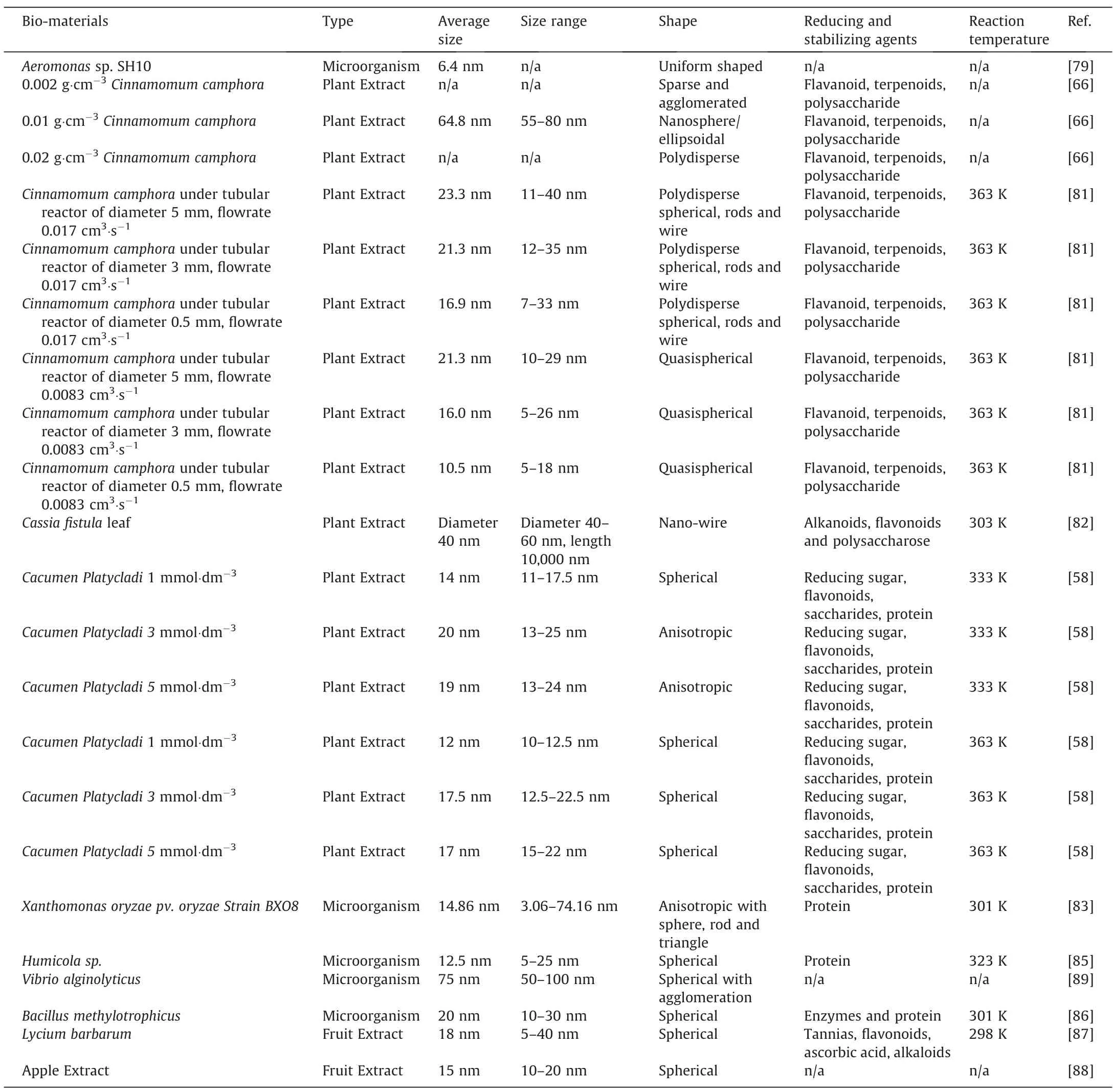
Table 3 Lists of Bio-template utilized for the synthesized of AgNPs
2.4.Palladium nanoparticle (PdNPs)
Similar to Au,Ag,and Pt,many factors affect the size and shape of PdNPs.Sathishkumaret al.[98]utilizedCurcuma longa tuberplant extract for the bio-synthesis of PdNPs.It was found that plant extract concentration had not much of an effect on the size and shape of PdNPs.As a result,spherical particles of 15–20 nm were synthesized regardless of plant extract concentration.However,the amount of PdNPs synthesized increased with increasing plant extract concentration,as there were more reducing agents avail-able for the formation of PdNPs.On the other hand,pH has a mild effect on the size of the PdNPs.It was reported that PdNPs with 15–20 nm could be formed under pH 5,while the size was found to increase to 20–25 nm as the pH was increased beyond 5.Similar trends of results were also reported in another work of Sathishkumaret al.[99],but withCinnamom zeylanicumbark as the bioreducing agent.Meanwhile,our research group investigated the effect of Pd precursor concentration on the formation of PdNPs by usingCinnamomum camphoraplant extract,and it was reported that the PdNPs size decreased with increasing Pd precursor concentration [100].However,this was in contradiction with PdNPs reduced via synthetic reducing agent[101].This was because while higher concentration beyond 0.003 mmol·dm-3·cm-3of plant extract would lead to a greater rate of Pd atom generation [101],the growth rate remained slow due to the sufficient protective effects by the bio-molecules inCinnamomum camphoraplant extract.Hence,Oswald ripening was inhibited [101],leading to the decrease in PdNPs size from 4 to 3 nm,as the plant extract concentration was increased from 0.003 mmol·dm-3·cm-3to 0.005 mmol·dm-3·cm-3.Such protective bio-molecules,however,did not exist in synthetic reducing agents reported by Hoet al.[101].On the other hand,as the Pd atom formation rate was low at 0.001 mmol·dm-3·cm-3of plant extract,freshly produced Pd atoms were inclined to attach to a more stable surface of larger PdNPs.Therefore,Oswald ripening was promoted [102],leading to the formation of larger,irregular spherical PdNPs of particle size 5 nm,with a large size distribution of 3.5–10 nm.
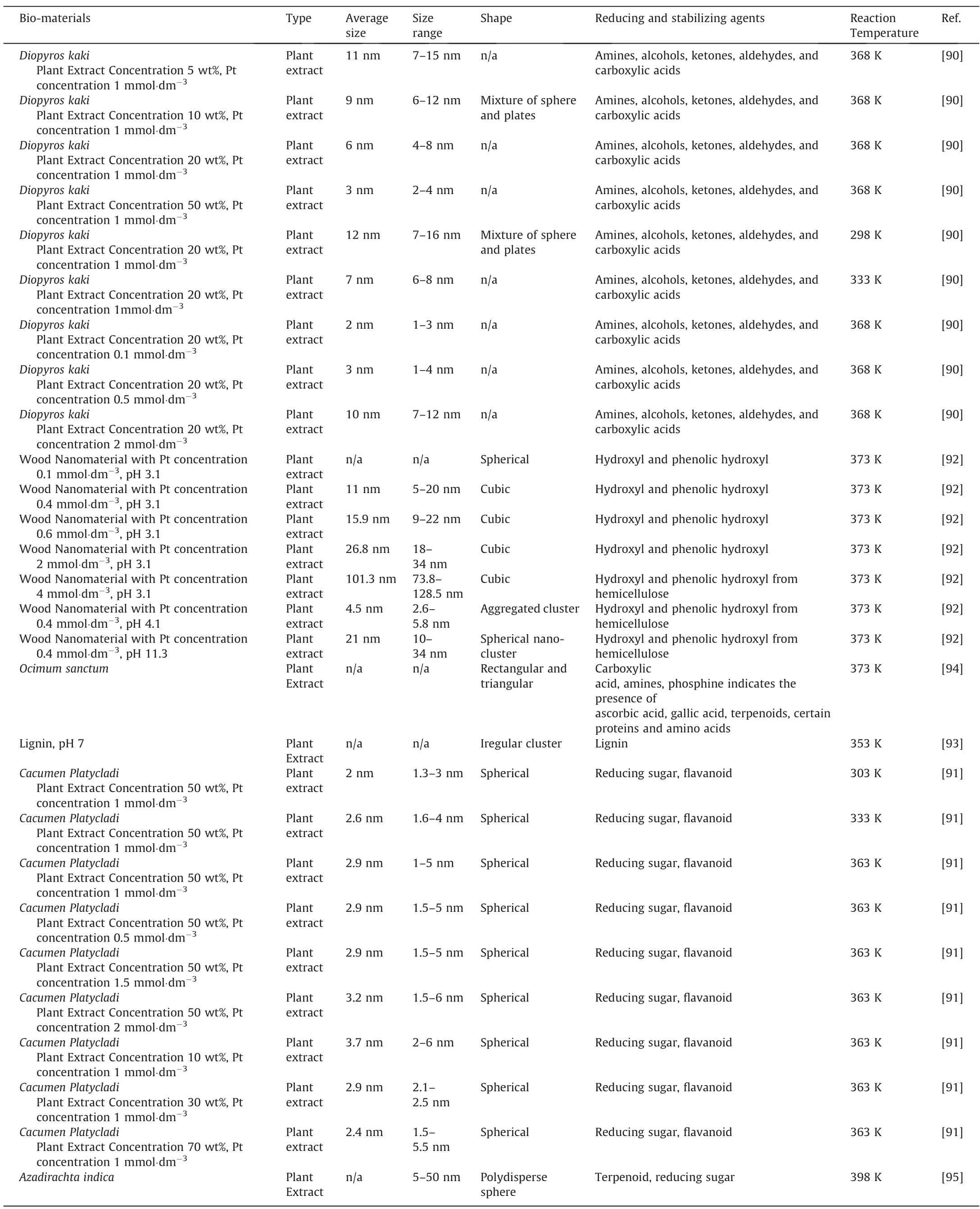
Table 4 Lists of Bio-materials utilized for the synthesized of PtNPs
In addition to pH,bio-reducing agent,and Pd precursor concentration,modification on bio-reducing agents would also affect the size and shape of the PdNPs.Cocciaet al.[93]modified lignin plant extract with different ligand consist of ammonium and calcium.As a result,the resulting spherical PdNPs sizes were determined to be averagely 16 and 20 nm,respectively [93].Meanwhile,Chenet al.[103]of our group utilizedPichia pastoriscell for the bio-synthesis of spherical PdNPs with an average size of 8.7 nm and size distribution of 2–17 nm.However,as the cell was treated with HCl,NaOH,and amino group methylation while maintaining the Pd precursor initial concentration,it was found that the PdNPs sizes decreased with a narrower size distribution,as listed in Table 5.This was because modification of these chemicals on the cell enhanced the adsorption of Pd2+ion onto the surface of the cell.Therefore,more nuclei were formed over the cell surface,while a lesser amount of unabsorbed Pd2+ions were available for the growth of the nuclei at the same concentration of Pd precursor.This led to narrower size distribution and smaller size.Meanwhile,our group had also investigated the effect of various halide ions treatment onCacumen platycladeplant extract [108].Among all the halide ions,only I-ion treatedCacumen platycladeplant extract was able to decrease the size of PdNPs to 1.62 nm as compared to that of untreatedCacumen platycladeplant extract (6.23 nm),as displayed in Fig.5.This was because I-ion enhance the reduction rate while being a strong reducing agent,could be easily oxidized into I2[108].As a steric stabilizer,starch can also be used to prevent the agglomeration of NPs [110].This was demonstrated by Hazarikaet al.[109],whereby the addition of 0.3% of starch intoG.pedunculata Roxbplant extract was sufficient to synthesize PdNPs with no agglomeration.
While most bio-synthesized PdNPs generally are spherical in shape [103,104,106,107],our group also investigated the possibility of synthesizing PdNPs with other shapes.For example,Odoom-Wubahet al.[105]produced nanoflower-shaped PdNPs by modifyingPichia pastoriscell with AA and Hexadecyltrimethylammonium chloride(CTAC).The formation mechanism began with the interaction ofPichia pastoriscell with Pd2+ions from the precursor,which led to the reduction of Pd+ions on the cell surface.AlthoughPichia pastoriscell can reduce Pd+ions further into PdNPs[103],the addition of AA plays a role in enhancing the reduction process,whilePichia pastoriscell function as both a reducing agent and preferred nucleation sites.Meanwhile,CTA+ion from the CTAC is adsorbed onto the crystal nucleus to coordinate the shape evolution of PdNPs.Eventually,nanoflower morphology begins to take shape as a result of the Ostwald ripening process,as displayed in Fig.6.
2.5.Other noble metal nanoparticles
While bio-synthesized Au,Ag,Pt and Pd NPs are well documented throughout these years,there are very limited studies or otherwise,none on the bio-synthesis of other noble metal nanoparticles.Nevertheless,back in 2017,Ismailet al.[64]successfully bio-synthesized RhNPs by usingAspalathus Linearisnatural extract as bio-reducing agent.Spherical non-agglomerated nanoparticles within the size range of 0.8–1.6 nm was synthesized.However,it is not revealed whether this bio-synthesized RhNPs are useful for biological application or otherwise.Meanwhile,Aliet al.[111]bio-synthesized RuNPs withDictyota dichotomaMarine algae as bio-reducing agent.As a result,RuNPs particle size of averagely 30 nm was formed.RuNPs with size range 25–90 nm can also be bio-synthesized viaGloria superbaleaf extract as bio-reducing agent [65].
3.Biological Application of Bio-Synthesized Noble Metal Nanoparticles
3.1.Antimicrobial
One of the major applications of noble metal nanoparticles is as antimicrobial agents.Different bio-synthesized nanoparticles from different bio-materials would demonstrate different antimicrobial activity on different types of microorganisms.In general,the antimicrobial activity is measured based on the diameter of the inhibition zone surrounding the nanoparticle in a cultured microorganism sample by using the disc diffusion method [73].The larger the inhibition zone,the stronger the antimicrobial activity.Sathiyarananet al.[112]utilizedBacillus Megaterium MSBN04for the bio-synthesis of spherical AuNPs of 5–20 nm.Its antimicrobial activity was tested onE.coli,Bacillus cereus MTCC 430,andKlebsiella Pneumoniae MTCC 432.The inhibition diameters were determined to be 14,12,and 17 mm,respectively as the nanoparticle concentration was 100 μg·cm-3.More importantly,the inhibition zones were found to be larger than that of chemicallysynthesized AuNPs via sodium citrate,which were 4,7 and 8 nm,respectively.It is not clearly explained how the bio-molecules from bio-synthesized AuNPs could contribute to the enhanced inhibition zone as compared to that of chemically-synthesized AuNPs,Nevertheless,the results clearly have shown that the synergistic effect between the bio-molecules from the bio-material and AuNPs contributed to the enhanced inhibition zone.Tripathiet al.[73]synthesized spherical AuNPs with sizes between 32–44 nm by usingTrichoderma harizianumextract at various AuNPs concentration on the antimicrobial activity onE.coli.It was found that larger AuNPs concentration led to a larger inhibition zone,and by 60 μg·cm-3of AuNPs,E.coliwas found to be completely inhibited.Similar trends of results were also observed by Chenet al.[70]on the antimicrobial activities ofE.coliandStaphylococcus aureusin AuNPs concentration range of 10–90 μg·cm-3.In this study,Chenet al.[70]utilizedChenopodium formosanumfor the bio-synthesis of AuNPs.However,instead of the inhibition zone diameter,Chenet al.[70]measured the antimicrobial activity by using the inhibition ratio percentage,where a larger inhibition ratio percentage indicates better antimicrobial activity.Chenet al.[70]also explained that the antimicrobial activity mechanism begins withthe accumulation of AuNPs on the bacterial cell wall,causing cell perforation.This enabled the AuNPs to be diffused into the nucleus,causing nucleus degrading,which hinders the DNA replication and thus cell lysis of the microorganism.Alternatively,AuNPs could also be accumulated within the bacterial membrane and cytoplasm,while directly inhibit bacterial growth by interaction with the protein synthesis machinery of the microorganism[113].Chenet al.[70]also found that overall,AuNPs have higher antimicrobial activity onE.colithanStaphylococcus aureusas the latter has a thicker layer of peptidoglycan.This caused difficulty in the diffusion of AuNPs intoStaphylococcus aureuscell wall [70].
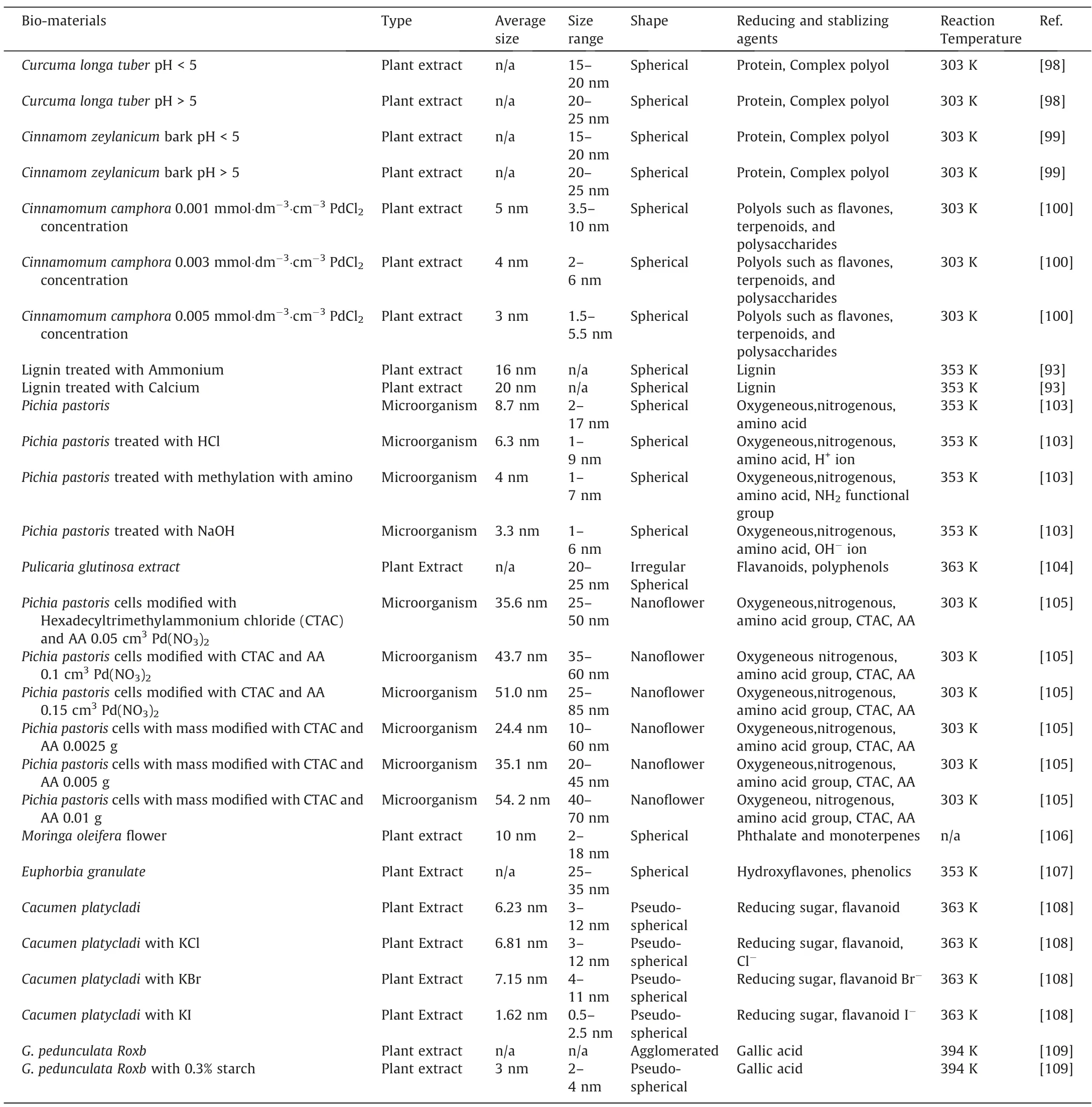
Table 5 Lists of Bio-materials utilized for the synthesized of PdNPs
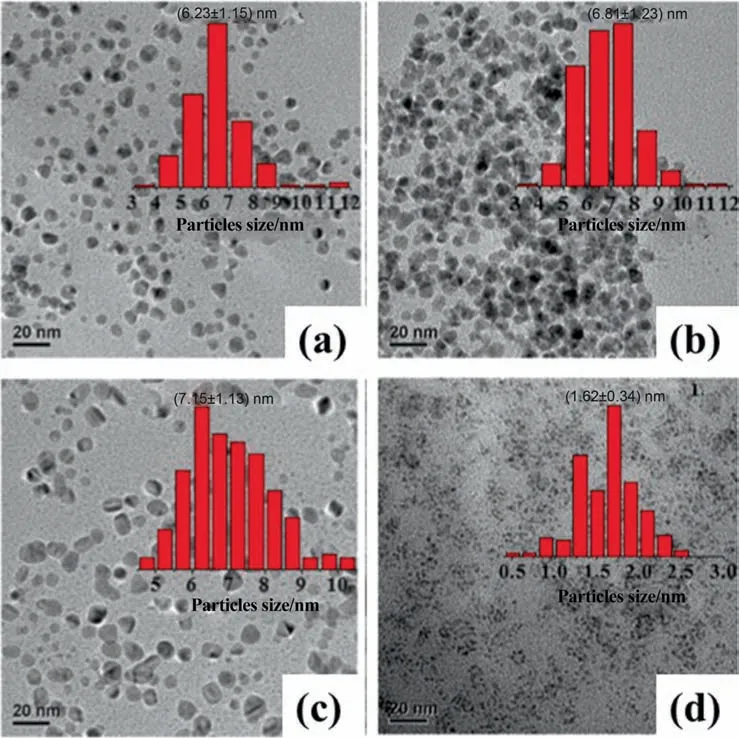
Fig.5.TEM images of PdNPs bio-synthesized with Cacumen platyclade plant extract(a)Untreated;(b)Treated with Cl-;(c)Treated with Br;(d)Treated with I-(This figure is reprinted with permission from Xu et al. [108].Copyright (2017),American Chemical Society).

Fig.6.Schematic diagram of the formation of Pd Nanoflower bio-synthesized with Pichia pastoris cell modified by AA and CTAC(This figure is reprinted with permission from Odoom-Wubah et al. [105].Copyright (2015),Elsevier).
Our group also demonstrated that AgNPs could also be used as an antimicrobial agent.Huanget al.[58]developed spherical AgNPs of 18.4 nm size bio-reduced byCacumen Platycladi.Similar to that of Chenet al.[70],Huanget al.[58]also demonstrated that AgNPs had higher antimicrobial activity onE.colithanStaphylococcus aureus,regardless of AgNPs concentration in the range of 1.4–55 μg·cm-3.In fact,the antimicrobial activity of AgNPs was found to be much effective than several antibiotics.This was demonstrated by Wanget al.[86],where it was determined that all antibiotics such as vancomycin,penicillin G 10,oleandomycin,cycloheximide had no inhibition effect onCandidaalbicans,Salmonella enterica,E.coli,and Vibrio parahaemolyticusmicroorganism.However,only AgNPs with 3 μg·cm-3concentration had an inhibition effect on these microorganisms,clearly demonstrating AgNPs as an excellent alternative antibiotic.Furthermore,unlike chemically reduced AgNPs,especially using toxic NaBH4as a reducing agent,AgNPs synthesized from bio-reducing agents by Wanget al.[86]and Huanget al.[58]were free of toxic chemicals.Hence,this further reinforces the potential of biosynthesized AgNPs as excellent antibiotics that are safe for human consumption.However,not all bio-synthesized AgNPs can be an effective antimicrobial agent.For example,AgNPs synthesized fromProtem Seretumby Mohantaet al.[114]could not inhibit the growth ofStaphylococcus aureus(AgNPs in the range of 31.2 5–500 μg·cm-3).On the other hand,AgNPs synthesized from apple pomace was able to inhibit the growth ofStaphylococcus aureusby Renet al.[88]even at 10 μg·cm-3AgNPs concentration.This group explained that this was due to the shape and size of the AgNPs.Ideally,the synthesized AgNPs has to be as small as possible to ensure a larger specific surface area to enhance the interaction between bio-molecules.This includes phosphatide,amino groups in protein,carbonyl group,negative oxygen ions,thiol,and disulfide bond in DNA [88].Hence,Ag+can be released from the AgNPs to be adsorbed more efficiently on the microorganism cell surface and form free radicals to damage the cell structure [115].Besides,the shape of the particles should be spherical as it can easily pass through the cell membrane to kill the cell [116].This explained the poor results onStaphylococcus aureusinhibition mentioned above by Mohantaet al.[114]as compared to that of Renet al.[88].The AgNPs by Mohantaet al.[114]were found to be larger at 100 nm with an irregular shape,while the Renet al.[88]synthesized spherical AgNPs and much smaller at 10–20 nm in size.
Some researchers also attempted to use Pd and PtNPs as antimicrobial agents.Dineshet al.[117]synthesized spherical PdNPs with a diameter of 30 nm withPhyllanthus emblicaas a bio-reducing agent.This bio-synthesized PdNPs demonstrated maximum inhibition zone diameter forStaphylococcus aureus and P.aeruginosaat 9.6 and 7.6 mm,respectively,as the PdNPs concentration was fixed at 0.1 g·cm-3.Meanwhile,Manikandanet al.[118]were able to bio-synthesize PdNPs with an irregular shape between 50–150 nm.It demonstrated an inhibition zone of diameter 6 and 5 mm forBacillus subtilisandPseudomonas aeruginosa,respectively at 250 μg·cm-3PdNPs concentration.Hazarikaet al.[109]biosynthesized spherical PdNPs with 2–4 nm in size usingG.pedunculataRoxb as a bio-reducing agent,which demonstrated an inhibition zone with a diameter of 31.47 mm against MDRCronobactersakazakiAMD04 strain at PdNPs concentration of 12,800 μg·cm-3.In addition to synthesizing PdNPs,Aryaet al.[119]also synthesized PtNPs usingBotryococcus brauniias a bioreducing agent.The synthesized Pt and PdNPs were both spherical with 86.96 nm and 4.89 nm in size,respectively.In the ranges of nanoparticle concentration within 100–400 μg·cm-3,the inhibition zone diameter achievable againstPseudomonas aeruginosa,E.coli,Klebsiella pneumonia,andStaphylococcus aureuswere found to be between 7–16 mm.Overall,the antimicrobial activity for Pd and PtNPs is generally weaker as compared to that of Au and AgNPs.This was as evident by the range of nanoparticle concentration utilized by Au and AgNPs are generally lower (0.01–0.5 μg·cm-3[70,88]) than that of Pd and PtNPs (0.1–100 μg·cm-3[117,119])to achieve comparable inhibition zone diameter.Moreover,since Pd and PtNPs are more precious and expensive than that of Au and AgNPs [120],it is deduced that Au and AgNPs are more economical and feasible as antimicrobial agent than that of Pd and PtNPs.Table 6 displayed the bio-synthesized noble metal nanoparticles which were used as antimicrobial agent.
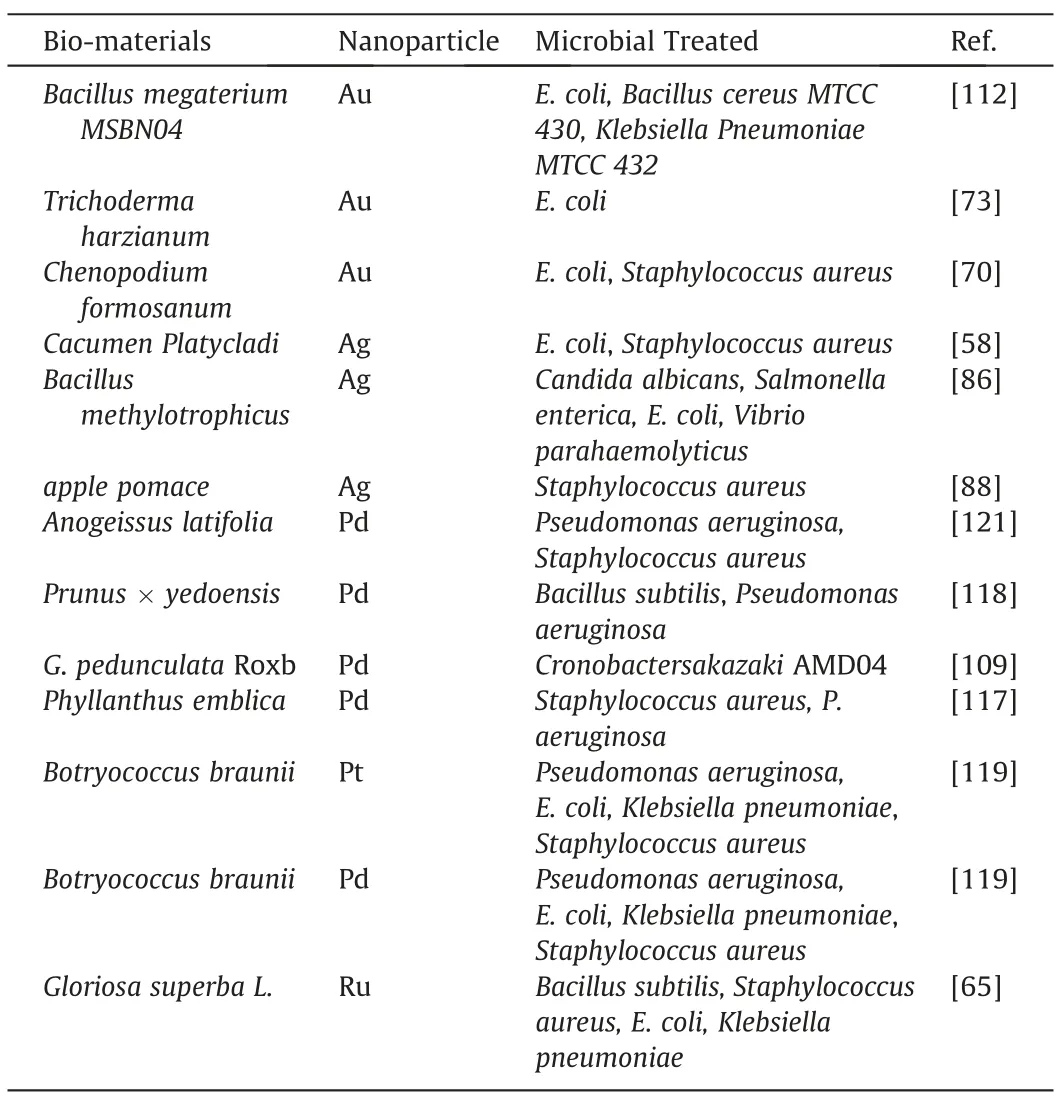
Table 6 Lists of Bio-synthesized Noble Metal Nanoparticle utilized as Antimicrobial Agent
3.2.Wound healing
Bio-synthesized noble metal nanoparticles can also be utilized for wound healing applications.In most cases,wound healing test are conducted on rats,whereby the wound closure percentage in a period of few days are evaluated [122].Naragintiet al.[122]utilizedColeus forskohliiroot extract for the bio-synthesis of AuNPs with various shapes and AgNPs with a spherical shape.The wound closure percentage was compared with Soframycin drug and ointment base.By day 14,the wound closure percentage by Soframycin drug and ointment base was determined to be 83% and 40%,respectively,which were lower than that of AuNPs (95%) and AgNPs(92%),as displayed in Fig.7.Meanwhile,Shamugasundaramet al.[123]synthesized AgNPs of 30.5 nm in size and AuNPs of 14.5 nm in size by usingStreptomyces sp.B5as the bio-reducing agent.The wound closure percentage of Ag and AuNPs on rats was compared with relatively more toxic Nitrofurazone ointment.The wound closure rate of Ag and AuNPs were found to be comparable to Nitrofurazone.By day 21,the wound closure percentage was determined to be close to 100%.Alternatively,wound healing performance of bio-synthesized noble metal nanoparticle can also be conducted on rabbit.For example,Muhammadet al.[124]synthesized Glucuronoxylan-mediated AgNPs in the form of wound healing dressing film.The AgNPs-loaded dressing was tested on the rabbit,and the performance was compared with commercial band-aid dressing.Both AgNPs-loaded dressing and commercial band-aid dressing were able to completely heal the wound on the rabbits by 15 days.
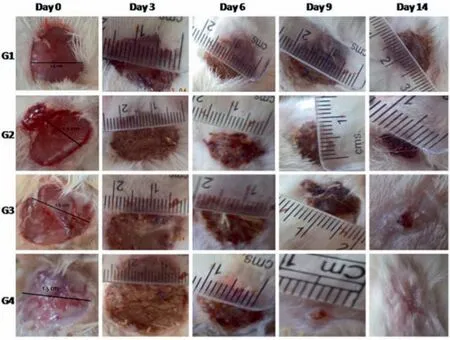
Fig.7.Comparisons of wound healing on rats by G1(Ointment base),G2(Soframycin drug),G3 (AuNPs) and G4 (AgNPs) (This figure is reprinted with permission from Naraginti et al. [122].Copyright (2016),Elservier).
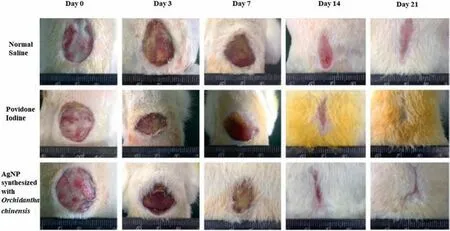
Fig.8.Comparisons of wound healing on rats by Normal saline,povidone-iodine,and AgNPs(This figure is reprinted with permission from Wen et al.[126].Copyright(2016),Dove Medical Press).
These papers have shown the potential of Au and AgNPs as alternative wound healing agents as compared to many conventional wound healing agents.Yet,the mechanism behind the wound healing effect by Au and AgNPs is not clearly explained.Despite that,it is known that for the wound to be healed,the wounded area must be free of bacteria and pathogen,as they will affect the healing process [125].Therefore,wound healing agents must have certain antibacterial/antimicrobial properties to inhibit the growth of microorganisms.As explained in Section 3.1,many studies have shown that Au and AgNPs have excellent antimicrobial properties,which explained consequently the reason why Au and AgNPs are also excellent wound healing agents.For example,spherical 40 nm AgNPs bio-synthesized viaArnebia nobilisby Garget al.[125]was able to inhibit the growth ofE.coliandStaphylococcus aureusand achieved 95%wound closure percentage in 21 days.Meanwhile,Wenet al.[126]bio-synthesized AgNPs withP.spinulosumOC-11.The synthesized spherical AgNPs of 25 nm inhibited the growth ofPseudomonas aeruginosa,E.coli,andStaphylococcus aureus.By day 21,it was found that 95%wound closure percentage was achieved by the synthesized AgNPs.This was higher than that of normal saline (75%) and povidone-iodine (85%).The comparisons of wound healing by AgNPs,normal saline,and povidoneiodine are displayed in Fig.8.It should be noted that to our knowledge,there was no publication related to bio-synthesized Pd and PtNPs as wound healing agents.As explained in Section 3.1,this could be due to both Pd and PtNPs are not as effective as antimicrobial agents,as compared to that of Ag and AuNPs.Table 7 displaysthe bio-synthesized noble metal nanoparticles which were used as a wound-healing agent.
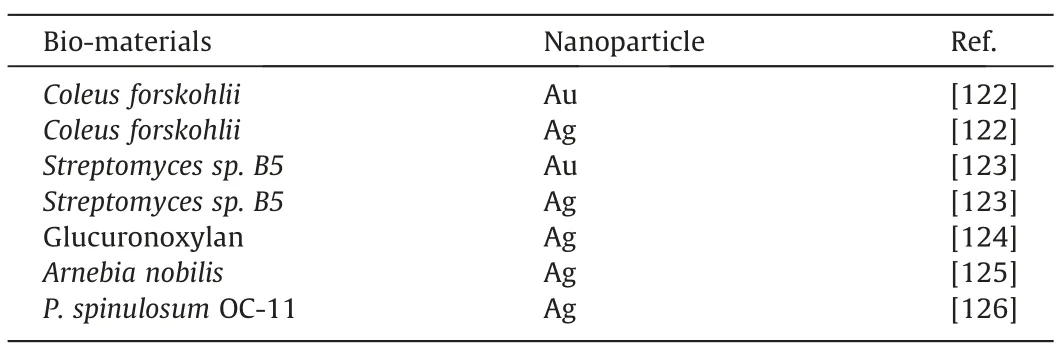
Table 7 List of Bio-synthesized Noble Metal Nanoparticle utilized as Wound healing Agents
3.3.Anticancer drug
Another important application of bio-synthesized noble metal NPs is as anticancer drugs.The effectiveness of these metals as anticancer drugs is generally evaluated by their cytotoxicity on targeted cancer cell lines based on cell viability percentage based on MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide,a tetrazole) assay or 50% inhibition concentration (IC50%).Ramesh&Armash[127]bio-synthesized AuNPs withDiospyros ferresplant extract to be used for cell viability test on cancerous cervical human cell line (HeLa).It was found that 0.05 cm3of AuNPs were enough to control the cancerous cell,based on IC50%measurement.The cytotoxicity of AuNPs on HeLa were also investigated on spherical AuNPs bio-synthesized withElecttaria Cardomomumtea extract [128].The IC50%measurement was determined to be 0.042 cm3,which was comparable to that of Ramesh &Armash’s work[127].The amphiphilic nature of AuNPs enabled them to penetrate the cancer cell easily.Hence,the energy status in tumors can be reduced,and the hypoxia status of cancer cells can be altered[127].Besides,the cytotoxicity effect could also be due to the direct action by Au+when it was released by AuNPs,which hindered mitochondrial functions in stress pathways and apoptosis [129],enabling AuNPs as a potential anticancer drug.Meanwhile,Kumaret al.[130]bio-synthesized AuNPs fromDelftia sp.strainKCM-006 microorganism loaded with Resveratrol drug.This was done to test the cytotoxicity of AuNPs and Resveratrol-loaded AuNPs on both cancerous human lung cells (A549 cell line) and MRC-5 cell line derived from healthy human lung tissue.It was found that AuNPs and Resveratrol-loaded AuNPs had no cytotoxicity effect on MRC-5 cell line,which indicated that AuNPs will not damage healthy lung cells.Meanwhile,it was found that while AuNPs did not affect the cell viability of A549 cell line,Resveratrol-loaded AuNPs was found to be more effective in decreasing the cell viability of A549 cell line as compared to that of pure Resveratrol drug.Consequently,the IC50%of Resveratrol-loaded AuNPs was determined to be 0.0143 cm3,which was much lower than that of the Resveratrol drug (0.039 cm3).This demonstrated that AuNPs alone could not treat lung cancer,but instead can be utilized as reinforcement for Resveratrol drug as additional cytotoxicity in A549 cancer cell line.Kumaret al.[130]also determined that the Resveratrol drug released from Resveratrol-loaded AuNPs was as high as 95%under acidic conditions of pH 5.2,but was only 26%at pH 7.4 by 8 h.This result is important because pH-dependent drug release is essential for cancer treatment due to different pH environments between healthy and cancerous cells.As the Resveratrol-loaded AuNPs enters from the healthy pH environment of 7.4 to the acidic environment (4.5–6.5) of cancerous cells,the endocytosis process is induced,which triggered the rapid release of Resveratrol drug from AuNPs into the cancerous cell [131].This enabled cytotoxicity on A549 cancer cell line.Therefore,in addition to cytotoxicity reinforcement on the Resveratrol drug,AuNPs could be used as an effective Resveratrol drug carrier.
Bio-synthesized AgNPs have also demonstrated cytotoxicity on the HeLa cell line.Al-Sheddiet al.[132]bio-synthesized AgNPs of 33 nm in size fromNepeta deflersianaextract.The optimum concentration was determined to be 10 μg·cm-3,which successfully decreased the cell viability by 90%viaMTT assay.Biosynthesized AgNPs also demonstrated cytotoxicity on ovarian cancer cells.For example,Moldovanet al.[133],managed to biosynthesize spherical AgNPs of 25 nm in size withLigustrum Ovalifoliumand found that it had cytotoxicity effects on both A2780 and A2780-Cis(cisplatin-resistant)human ovarian cancer cell line.The IC50%on both A2780 and A2780-Cis cell lines by AgNPs were determined to be 7 and 14 μg·cm-3,respectively.Meanwhile,Sathishkumaret al.[134]bio-synthesized AgNPs withDendrophthoe falcata(L.f) Ettingsh,to evaluate the cytotoxicity on MCF-7 breast cancer cell.It was determined that IC50%on the MCF-7 breast cancer cell line was 5 μg·cm-3of AgNPs concentration.The cytotoxicity of bio-synthesized AgNPs on breast cancer was also investigated by Syedet al.[85]but on a different cell line.In this work,Syedet al.[85]bio-synthesized spherical AgNPs with 5–25 nm in size fromHumicola sp.Fungus.It was found that the cell viability of DA-MB-231 human breast carcinoma cell line could decrease to 42.18% by utilizing AgNPs of concentration 1000 μg·cm-3.The cytotoxicity by AgNPs can be induced via the uptake of NP through clathrin-dependent endocytosis and macrocytosis [135].Besides that,AgNPs can induce oxidative stress by increasing the Reactive Oxygen Species (ROS) generation,which in return,triggers the mitochondrial membrane damage and cancer cell cycle alteration[132].
Al-radidi [136]bio-synthesized spherical PtNPs of size 1.3–2.6 nm with two different types of dates,namely Ajwa and Barni.It was found that not only did they have cytotoxicity on MCF-7 breast cancer line,but also on colon cancer cell HCT-116 cell line and Hepatocellular Carcinoma (HePG-2) cell line.The IC50%by PtNPs synthesized from Ajwa on MCF-7,HCT-116,and HePG-2 cell lines were determined to be 289,94.2,and 118 μg·cm-3respectively.Meanwhile,the IC50%by PtNPs synthesized from Barni on MCF-7,HCT-116,and HePG-2 cell lines were determined to be 189,175,and 91 μg·cm-3respectively.The potential of biosynthesized PtNPs to treat breast cancer was also investigated by Rokadeet al.[137],who utilizedGloriosa Superbafor the biosynthesis of spherical PtNPs of 0.8–3 nm in size.The cell viability on MCF-7 breast cancer cell line was determined to decrease to 49.65%based on MTT assay.The high cytotoxicity by PtNPs on cancer cells was due to the interaction with cellular functional groups of cellular proteins,nitrogen bases,and phosphate groups of DNA,all of which contribute to the death of cancerous cells [137,138].
Additionally,Rokadeet al.[137]also bio-synthesized spherical PdNPs of 5–8 nm in size withGloriosa Superba.The cell viability on MCF-7 breast cancer cell line was determined to decrease to 36.26% based on MTT assay.Aziziet al.[139]bio-synthesized spherical PdNPs with 6–18 nm in size by usingCamelia Sinesisto be used as cytotoxicity comparisons on MOLT-4 leukemia cell line with conventional anti-drugs such as Cisplatin and Doxorubicin.The IC50%value achievable by PdNPs was determined to be 0.006 μmol·dm-3,which was significantly lower than that of Cisplatin (0.013 μmol·dm-3) and Doxorubicin (2.133 μmol·dm-3),clearly demonstrated the effectiveness of PdNPs as an anticancer drug as compared to that of Cisplatin and Doxorubicin.Likewise,Shanthiet al.[140]also demonstrated the ability of PdNPs biosynthesized bySyzygium aromaticumto treat cervical cancer cells.The bio-synthesized PdNPs with size ranging 20–25 nm demonstrated IC50%value of 45 μg·cm-3on the HeLa cell line.Moreover,Shanthiet al.[140]also demonstrated that the bio-synthesized PdNPs did not affect the cell viability of a healthy H8 cell line,proving PdNPs as an excellent anticancer drug without harming the healthy human cell.Like PtNPs,the high cytotoxicity by PdNPs was due to its interaction with cellular functional groups[137,138].Also,PdNPs can induce the formation of free radicals,lactate dehydrogenase leakage and cell-cycle disturbance,reinforcing PdNPs as potential alternative anticancer drug.Table 8 displays the bio-synthesized noble metal nanoparticle which were used as anticancer drug.
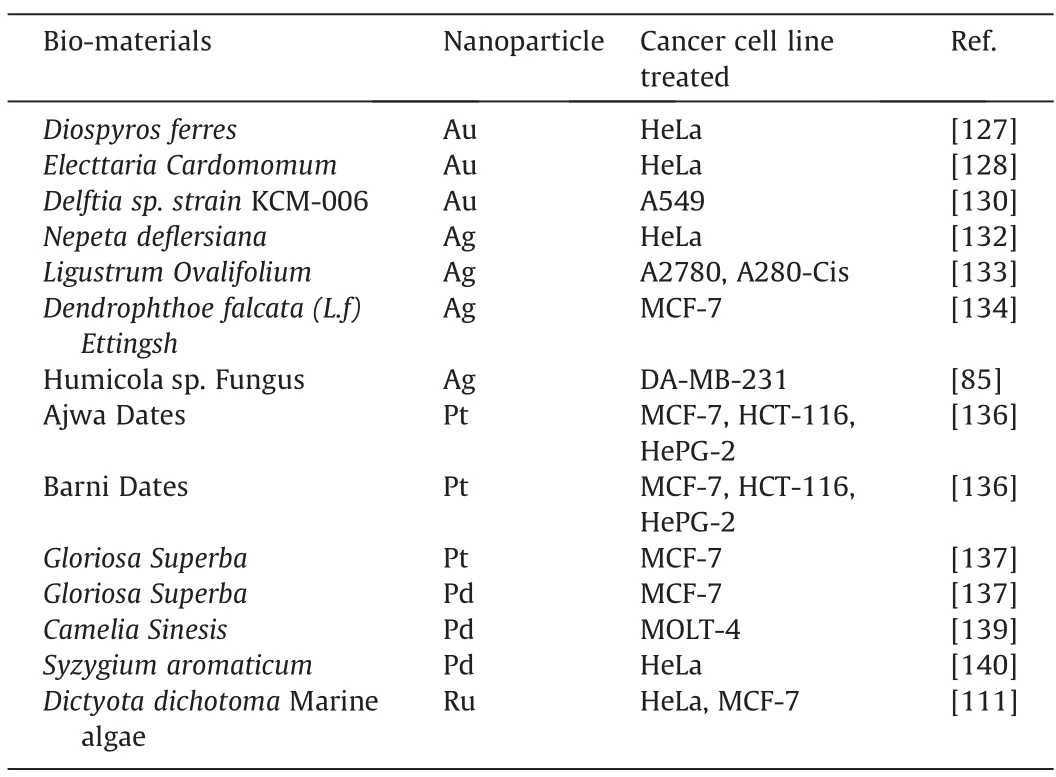
Table 8 Lists of Bio-synthesized Noble Metal Nanoparticle utilized as Anticancer Drug
3.4.Bio-imaging
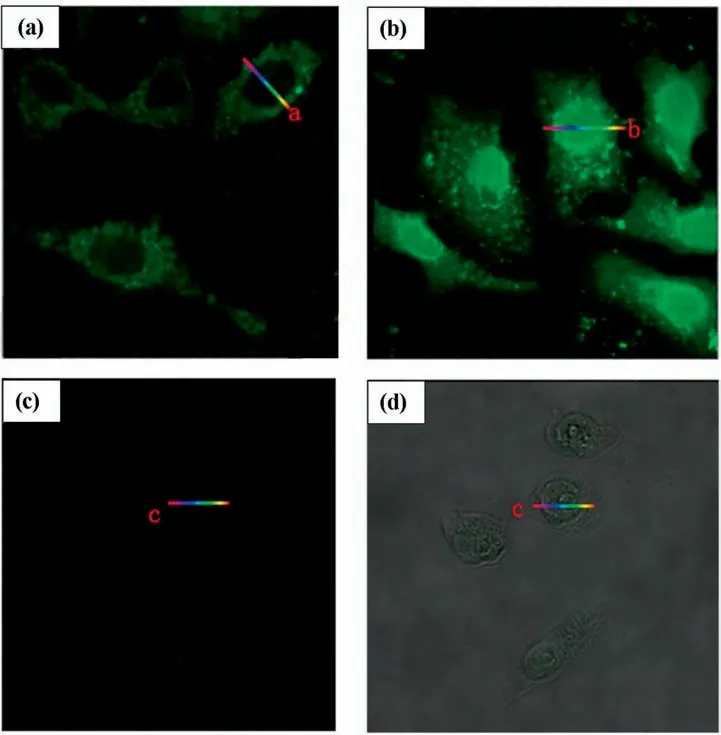
Fig.9.Laser confocal fluorescence micrographs of HepG2 Cell line(a and b)and L02 control cell line(c and d)incubated with HAuCl4solutions.a:after 24 h incubation;b,c and d:after 48 h incubation (This figure is reprinted with permission from Wang et al. [142].Copyright (2013),Nature).
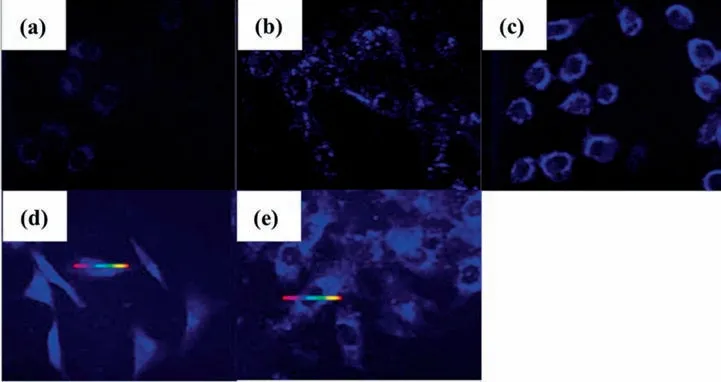
Fig.10.Laser confocal fluorescence micrographs of (a) L02 Cell line;(b) HCT-16 Cell line;(c) HeLa Cell line;(d) HepG2 Cell line;(e) A549 Cell line incubated with H2PtCl6solution (This figure is reprinted with permission from Chen et al. [144].Copyright (2015),American Chemical Society).
By taking advantage of the noble metal nanoparticle special fluorescence properties with size-dependent and sharp contrast in color [141],bio-synthesized noble metal nanoparticles,especially in nanoclusters form,can also be used as a bio-imaging application for cancer detection,in addition to their application as an anticancer drug.Furthermore,as discussed in Section 2,many microorganisms can be successfully utilized to bio-synthesized noble metal nanoparticles.Based on this motivation,Wanget al.[142]investigated the possibility of in-situ bio-synthesizing AuNPs with hepatic tumour cell line (HepG2) and normal hepatic cell line(L02).This was done by first injecting HepG2 and L02 cells into rats,which was then followed by injecting HAuCl4precursor into the targeted cells and were incubated for 48 h.The fluorescence images generated were evaluated with laser confocal fluorescence micrographs,as displayed below as Fig.9.By 48 h,a clear green fluorescence image was observed on HepG2 cells,as displayed in Fig.9 (b),while a low-intensity fluorescence image was observed on L02 cells,as displayed in Fig.9 (d).The fluorescence observed is a clear indication that AuNPs were spontaneously formed insitu upon the reduction of HAuCl4by HepG2 cell line.To explain this phenomenon,Wanget al.compared the H2O2generated by these cells,and it was found that H2O2generated by HepG2 cells was 25% higher than that of L02 cell,indicating that ROS production growth on tumour cell like HepG2 cell line is larger than that of normal cell like L02 cell [143].Since ROS in the cell was generatedviathe reduction of dioxygen,the cells will provide Au3+ion with an alternative electron donator to initiate anin-situbiosynthesis of fluorescent AuNPs clustersviabio-reduction in the cytoplasm[143].As the level of H2O2was low in L02 cells as compared to that of HepG2 cells,lesser AuNPs could be generatedinsituin L02 cells,resulting in dimmer fluorescent.This is displayed in Fig.9 (d),as compared to that of HepG2 cells,as displayed in Fig.9 (b).Similarly,Chenet al.[144]utilized the same method,but instead,H2PtCl6precursor was used to bein-situbiosynthesized PtNPs by healthy L02 cell.Cancerous cells of HCT-116 cell line,HeLa cell line,HepG2 cell line,and A549 cell line were also utilized toin-situbio-synthesize PtNPs.As observed in Fig.10,all cancerous cells observed clear blue fluorescent,indicating a huge amount of PtNPs was bio-synthesized by cancerous cells.Alternatively,instead of in-situ bio-synthesis,Zhanget al.[145]bio-synthesized AuNPs with bovine serum before incubated into HeLa and MCF-7 cancerous cell lines.As display in Fig.11,both the HeLa and MCF-7 cell lines could not be observed unless they are incubated with AuNPs,giving clear red fluorescent images.Similar results can be observed for AgNPs bio-synthesized withOlax scandensextract on A549 cancerous cell line [146].Either in-situ or non-in-situ bio-synthesized noble metal nanoparticles,can be used effectively as bio-images to detect cancer cells in the bodies of organism.
In addition to cancerous cell bio-imaging,noble metal nanoparticles can also be used to detect normal cellsviabio-imaging.However,as discussed above,since in-situ bio-synthesized noble metal nanoparticles could not display clear images of healthy cells,the detection of healthy cells is only feasible by using metal nanoparticles bio-synthesizedvia non-in-situmeans.Kotcherlakotaet al.[147]demonstrated this by bio-synthesizing AuNPs withZ.elegans(ZE)leaf.The bio-synthesized AuNPs,as well as the pure ZE leaves extract,were treated on CHO and SK-OV-3 cells.The fluorescence images were observed and compared under Fluorescein (FITC)and Cynine(Cy5)channel.As displayed in Fig.12,the fluorescence images can be observed for both ZE leaves-treated cells,which indicated that ZE leaf does indeed have fluorescent properties in it.However,it was also observed that both cells treated with AuNPs bio-synthesized with ZE leaf displayed much clearer fluorescence images than that treated by pure ZE leaf.This demonstrated that AuNPs could enhance the fluorescent property of ZE leaf,as the fluorescent molecules of ZE leaf were conjugated on the fluorescent molecules of AuNPs.Meanwhile,Penget al.[148]bio-synthesized PdNPs with ascorbic acid as a reducing agent and methionine bio-molecules as a sealing agent to be used for fluorescent studies on a wide range of haemoglobin cell concentration(0.5–10 μmol·dm-3).It was found that at increasing haemoglobin concentration the fluorescent intensity peak at 500 nm was found to decrease.Despite that,the peak was still observable even at 10 μmol·dm-3concentration of haemoglobin.This demonstrated the potential of bio-synthesized PdNPs for the bio-imaging of cells.Table 9 summarises the bio-synthesized noble metal nanoparticles for bio-imaging applications.
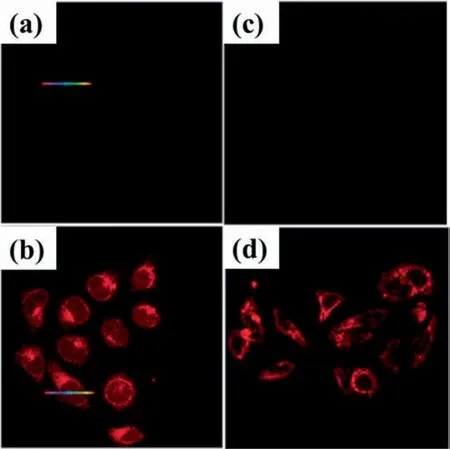
Fig.11.Laser confocal fluorescence micrographs of (a) MCF-7 Cell line without AuNPs;(b)MCF-7 Cell line with AuNPs;(c)HeLa Cell line without AuNPs;(d)HeLa Cell line with AuNPs.AuNPs was bio-synthesized with bovine serum(This figure is reprinted with permission from Zhang et al. [145].Copyright (2015),Royal Society Chemistry).
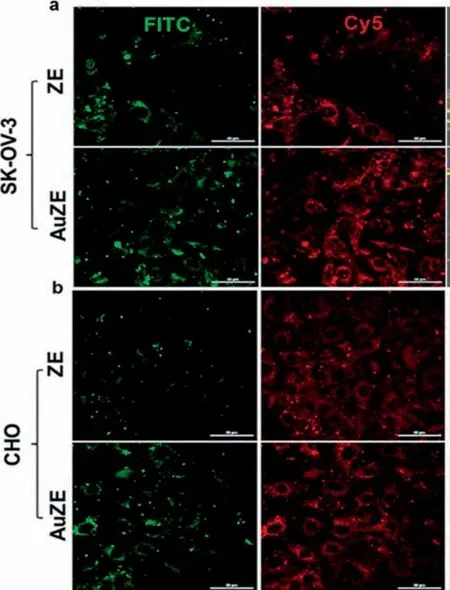
Fig.12.Fluorescent images of(a) SK-OV-3 cell and;(b) CHO cell treated with both Z.elegans (ZE) leaf and AuNPs bio-synthesized with ZE leaf (AuZE) under FITC and CHO channels (This figure is reprinted with permission from Kotcherlakota et al.[147].Copyright (2019),American Chemical Society).
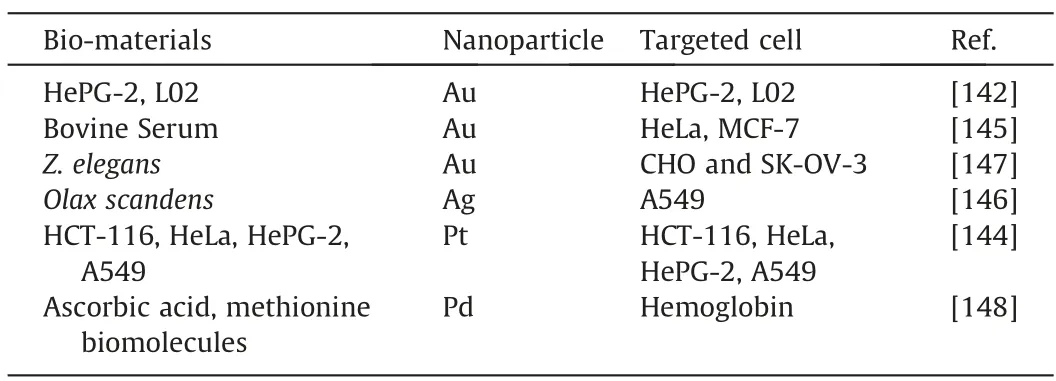
Table 9 Lists of Bio-synthesized Noble Metal Nanoparticle utilized as Bio-imaging
4.Future Perspective and Outlook
Throughout the years,various bio-materials have been successfully utilized for the bio-synthesis of noble metal nanoparticles.The roles of individual bio-molecules as bio-reducing agent,stabilizer,and capping agents on the size,shape,and uniformity of the biosynthesized noble metal nanoparticles are well documented.Based on these pieces of knowledge,it is expected research in this area will be expanded more soon involving many unexplored biomaterials for the bio-synthesis of not only noble metal nanoparticles but in general,metal nanoparticles as a whole.Meanwhile,it is also well documented that many bio-synthesized noble metal nanoparticles have demonstrated excellent and comparable performance with conventional chemicals in various biological applications.For example,the in-situ bio-synthesized noble metal nanoparticles by cancerous cell lines for bio-imaging has proven to be possible,which otherwise could not be achieved if NaBH4chemical reducing agent is utilized for the in-situ synthesis.Hence,these examples demonstrate their potential as alternative materials for their utilization in biological applications in commercial and industrial scale.
However,most of the documented bio-synthesized noble metal nanoparticles only involve Au,Ag,Pt and PdNPs.On the other hand,there are only handful of studies on other bio-synthesized noble metal nanoparticles using limited sources of biomaterials such asAspalathus Linearis[64],Gloria superbaL leaf extract[65]andDictyota dichotomaMarine algae [111].Tables 2–5 display wide lists of bio-materials that were utilized for the bio-synthesized of Au,Ag,Pd,and Pt NPs,but until now,have not been utilized for the biosynthesis of Ru,Rh,Ir and Os NPs.Therefore,this could be a potential research that future researchers can explore in the next step.
Additionally,there are also many questions remained unanswered.Take antimicrobial and wound healing biological applications for example.While it is proven that bio-synthesized noble metal nanoparticles can be used to treat various microorganisms and wound with performance comparable to treatment by other commercials chemicals,it is not known how well biosynthesized noble metal nanoparticles performed as compared to that of chemically synthesized noble metal nanoparticles.Besides,if bio-synthesized noble metal nanoparticles performed better,what are the roles of the bio-materials on the bio-synthesis of noble metal nanoparticles which lead to its excellent performance?Also,what are the toxicity,both qualitatively and quantitively,of bio-synthesized noble metal nanoparticle as compared to that of chemically synthesized noble metal nanoparticle in various biological applications?Furthermore,since noble metal nanoparticles are generally expensive,will their utilization in various biological applications be justifiable as compared to that of conventional methods? These are the key research questions that should be answered and studied intensively by future researchers to further expand the utilization of bio-synthesized noble metal nanoparticle in the biological application.More importantly,answering these research questions by future researchers in the next step of research will also justify whether bio-synthesized noble metal nanoparticles should be utilized conventionally in biological application or otherwise.Nevertheless,the current development of biological applications by bio-synthesized noble metal nanoparticles is still ongoing intensively,and will eventually reach the goal of full commercialization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No.21536010).
 Chinese Journal of Chemical Engineering2021年2期
Chinese Journal of Chemical Engineering2021年2期
- Chinese Journal of Chemical Engineering的其它文章
- Molecular design,synthesis strategies and recent advances of hydrogels for wound dressing applications
- Monoclonal antibody-based cancer therapies
- Recent advances in systemic and local delivery of ginsenosides using nanoparticles and nanofibers
- Concepts,processing,and recent developments in encapsulating essential oils
- Engineering organoid microfluidic system for biomedical and health engineering:A review
- The potential of ionic liquids in biopharmaceutical engineering
