Engineering organoid microfluidic system for biomedical and health engineering:A review
Yifan Xing,Junyu Liu,Xiaojie Guo,Haipeng Liu,Wen Zeng,Yi Wang,Chong Zhang,2,Yuan Lu,Dong He,Shaohua Ma,Yonghong He,Xin-Hui Xing,2,,5,*
1 Key Laboratory for Industrial Biocatalysis,Ministry of Education of China,Department of Chemical Engineering,Tsinghua University,Beijing 100084,China
2 Centre for Synthetic and Systems Biology,Tsinghua University,Beijing 100084,China
3 School of Chemical Engineering and Light Industry,Guangdong University of Technology,Guangzhou 510006,China
4 Institute of Biopharmaceutical and Health Engineering,Tsinghua Shenzhen International Graduate School,Shenzhen 518055,China
5 Institute of Biomedical Health Technology and Engineering,Shenzhen Bay Laboratory,Shenzhen 518055,China
ABSTRACT In recent years,organoid technology, i.e., in vitro three-dimensional (3D) tissue culture,has attracted increasing attention in biomedical engineering.Organoids are cell complexes induced by differentiation of stem cells or organ-progenitor cells in vitro using 3D culture technology.They can replicate the key structural and functional characteristics of the target organs in vivo.With the opening up of this new field of health engineering,there is a need for engineering-system approaches to the production,control,and quantitative analysis of organoids and their microenvironment.Traditional organoid technology has limitations,including lack of physical and chemical microenvironment control,high heterogeneity,complex manual operation,imperfect nutritional supply system,and lack of feasible online analytical technology for the organoids.The introduction of microfluidic chip technology into organoids has overcome many of these limitations and greatly expanded the scope of applications.Engineering organoid microfluidic system has become an interdisciplinary field in biomedical and health engineering.In this review,we summarize the development and culture system of organoids,discuss how microfluidic technology has been used to solve the main technical challenges in organoid research and development,and point out new opportunities and prospects for applications of organoid microfluidic system in drug development and screening,food safety,precision medicine,and other biomedical and health engineering fields.
Keywords:Organoids Stem cell Culture system Microfluidics Biomedical engineering Human health
1.Introduction
Thein vitromodeling of human organ structure and function is a key component of the modern approach to human health maintenance,complex diseases cognition,diagnosis,and precision treatment,providing an accurate and efficient alternative to animal experiments.In recent years,in vitrothree-dimensional(3D)tissue culture has in particular become a major focus of biomedical and health engineering research.Nature Methodshailed organoid technology,in which the formation of 3D tissue models is induced directly from stem cells,as the method of the year for 2017 [1].In 2019,Sciencepublished a special issue on organoids,explaining how they are opening up new frontiers in biomedical research[2–6].
Human organoids are micro-organs derived from pluripotent stem cells or organ-progenitor cells and displaying embryo specificity.They contain at least one cell type of the target organ,which can self-assemble and be cultured for long periodsin vitro.Therefore,organoids are considered excellent biomedical models that can be used to study both the development and the disorders of human tissues and organs,as well as to evaluate and screen drugs and medical compounds.
However,the current organoid system still has a number of drawbacks:lack of precise physical and chemical control of the microenvironment,high heterogeneity and variability,complex and imprecise manual operation,an imperfect nutritional supply system,and the lack of online analytical technology to monitor interactions among organoids limit the application of the method in high-throughput screening of drugs and bioactive or toxic compounds.The recent introduction of microfluidic technology,however,has greatly ameliorated these problems.The “organoids-ona-chip”method uses specially designed microfluidic chips for organoid culture.This approach,when combined with advanced methods from cell biology,biomaterials,and general engineering,permits the creation of organoid microfluidic system that can simulate and explore human biology and physiology in an unprecedented way,while solving the technical problems that prevent the practical application of organoids to drug development,toxicity testing and understanding of disease mechanisms.
The development and application of organoid microfluidic system in China and abroad are still in the exploratory stage,and in many ways the research has just begun.The existing literature contains no all-around review of current research progress on this interdisciplinary frontier.The present review systematically introduces the history and limitations of conventional organoid culture system,the technical problems solved by the introduction of microfluidics,and the prospects for future engineering development.
2.Development of Organoids
2.1.Brief introduction of organoids
Organoids are 3D cell complexes formed by inducing differentiation of stem cells or organ-progenitor cells throughin vitro3D culture technology.They are similar in structure and function to target organs or tissues.Organoids have stable phenotypic and genetic characteristics and can be cultured and cryopreserved for long periodsin vitro.In the process of organoid formation,cell sorting and space-specific cell-lineage typing are reproducedin vivo[7].As early as 2009,Satoet al.[8]demonstrated that 3D epithelial small intestinal organoids can be formed by single small intestinal stem cells labeled with leucine-rich repeat-containing G-proteincoupled receptor 5 (Lgr5).In the presence of Matrigel,they grew up in serum-free conditions with R-spondin,Lgr5,epidermal growth factor (EGF),and bone morphogenetic protein (BMP)[9,10].Under these culture conditions,stem cells could differentiate into specific villus-like and crypt-like structures containing all major epithelial cell types with high polarity.Further studies showed that transplanting colon organoids culturedin vitrointo dextran sulfate-induced acute-colitis mouse model could repair the damaged colonic epithelium,indicating that the expansion of single adult colon stem cellsin vitrowere feasible for colon stem cell therapy [11].Researchers have even applied gene editing methods such as CRISPR-Cas9 to human intestinal organoids to simulate colon cancer[12].This technology created an era of organoid culture,and a wide variety of mouse and human epidermoid organoids have been cultured,including models of the colon[13,14],liver [15],lung [16,17],pancreas [18],prostate [19,20],stomach [21],fallopian tube [22],taste bud [23],salivary gland[24],esophagus [25],endometrium [26]and breast [27].The historical development of organoid research is shown in Fig.1.
In vitrocell culture models are promising tools for understanding human development and disease progression;they also provide rapid and cost-effective results for drug discovery and screening.Two-dimensional (2D) cells have been commonly used to date.It is impossible for such cells to intrinsically model 3D problems in the human body,such as those related to the structural development of nerve tissue or to nerve degeneration [29].However,3D organoids culturedin vitrocontain a variety of cell types,breaking through the simple physical contact between cells to form a closer cell-to-cell biological interaction,and making possible mutual influence,induction,and feedback between cells[30].In addition,cells in organoids are very similar toin vivocomplexes,and show significant improvement in cell number,morphology,proliferation,and differentiation [31].
Although animal models have been widely used in biomedical research and can approximately simulate human physiology,their value is often reduced by observational issues,confounding variables,and limits on access or availability.Because of the significant biological differences between humans and animals,such models may be unable to predict human responses accurately and in a physiologically relevant manner [32,33].In recent years,organoid models have made up for many of the above shortcomings.Furthermore,organoids can be used in individualized precision medicine,where stem cells or organ-progenitor cells from patients are utilized in cell and gene therapy or the transplantation and repair of tissues and organs,as well as in studying pathogenesis at a deep level.
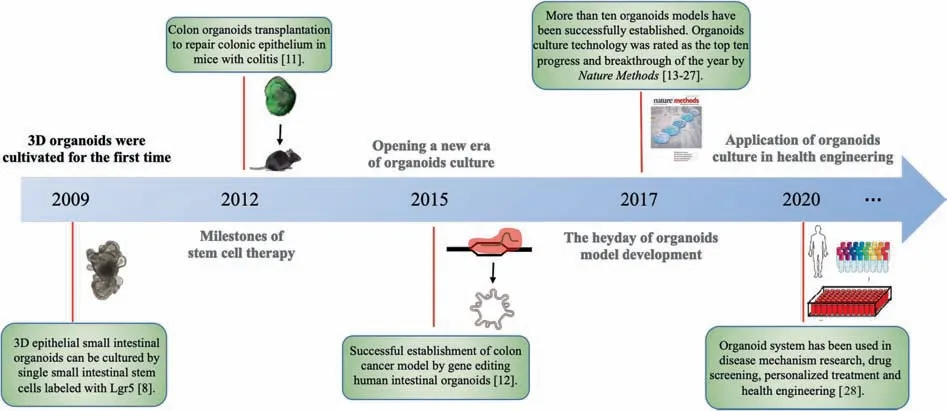
Fig.1.Timeline of the development of organoid research.
2.2.Organoid culture system
The main purpose of organoid culturein vitrois to understand the physical and chemical microenvironments of stem cellsin vivo;such knowledge can be used to induce the proliferation and differentiation of stem cells into specific organs or tissuesin vitro.The culture microenvironment is composed of cell growth regulators and the extracellular matrix(ECM),which together constitute the material basis for organoid regeneration and differentiation.Cell growth regulators (cytokines or other small molecules)include EGF,Noggin,and R-spondin;they can maintain the selfrenewal,proliferation,and differentiation of stem cells.Depending on the organoid,additional regulatory factors in the medium,such as gastrin,nicotinamide,TGF-β inhibitor,and p38 inhibitor,are often indispensable [14].The ECM simulates important aspects of thein vivogrowth microenvironment of stem cells,including the fact that such cells grow in 3D space.Matrigel,the most commonly used ECM,is a commercially available gel protein mixture derived from Engelbreth–Holm–Swarm mouse sarcoma.It consists of thousands of proteins including laminin,collagen IV,nidogen/entactin,proteoglycan;it also includes growth factors,such as proteins or steroid hormones as intercellular-signaling molecules,and nonprotein molecules[34].Matrigel can replace feeder cells in traditional culture system,effectively providing a 3D environment for stem cell proliferation and differentiation;it can also promote cell aggregation and generate cell arrangement polarity in 3D culture[35,36].The main process of organoid culture is the embedding of stem cells (or tissue fragments containing stem cells) into the ECM,adding an appropriate culture medium after curing.Organoids will become similar to the target organ form after several days of culture.
2.3.Human organoids originated from different cell types
Clevers [37]divided organoids into two main types based on their initiation from the two main types of stem cells:one type comes from pluripotent embryonic stem cells (ESCs) or their synthetic induced pluripotent stem cells (iPSCs),the other from adult stem cells(ASCs)from organs(see Table 1).Both types of organoids exploit the almost infinite expansion potential of stem cells in culture,and both display what Clevers called “an uncanny capacity”to self-organize into structures that partly mimic the physiological function and differentiation characteristics of tissuesin vivo[34].In recent years,growth factor cocktails capable of simulating a variety of organ stem cell niches have been developed.
2.3.1.Organoids from ESCs and iPSCs
ESCs are derived from the inner cell mass of the blastocyst.They have multi-layered differentiation ability and can proliferate infinitely and differentiate in multiple directionsin vitro[90].Like ESCs,iPSCs also have the ability to perform multi-directional differentiation.The difference is that iPSCs come from reprogramming somatic cells,and their main reprogramming method is the overexpression of multiple potential factors.The inventor of this technology,Shinya Yamanaka,was awarded the Nobel Prize in Physiology or Medicine in 2012[91].Since its advent,human iPSC technology has developed rapidly.Because of its human origin,easy access,scalability,and ability to generate almost any cell types required,this technology has been widely used in disease modeling and screening of candidate drugs [92].Gene editing and 3D organoid technology can further enhance the iPSC platform.
Stimulating the special signal pathways of ESCs and iPSCs can induce them to differentiate into different germ layers.Endoderm-derived organoids include the stomach [77],thyroid[93],liver [70],small intestine [56],and lung [66].In the process of intestinal development,the Nodal signaling pathway in TGF-β superfamily members can promote the development of projejunum into the endoderm [94],which further develops into the primitive intestinal tube including the foregut,midgut,and hindgut [95].Using these developmental characteristics,researchers[40,56]first used Nodal’s equivalent,activin A,to activate the TGF-β signaling pathway in stem cells to induce differentiation into the endoderm.After the addition of fibroblast growth factor(FGF4) and Wnt3A,the cells differentiated into the hindgut and formed hindgut globular cell bodies.Noguchiet al.[77]used mouse ESCs to culture organoids with the characteristics of gastric antrum and gastric somatic cells in adult mice.By inducing the expression ofBarx1and simultaneously regulating the Sonic Hedgehog (SHH) and Wnt signaling pathways,it was possible to establish gastric organoids with peristaltic functions,able to secrete pepsin and gastric acid.
The first example of a derived-mesoderm organoid was kidney organoid,reported in 2015.The signaling pathways of glycogen synthase kinase 3 beta(GSK3β)and FGF of human iPSCs were regulated and induced to differentiate into mesoderm,and then into renal organoids.This type of organoid had the morphology and segmentation of human embryonic nephrons,such as the middle renal duct,renal tubule,and glomerulus[83].Thus,renal organoid provided an excellent 3D model for the study of the origin and development of human kidney and related diseases,and had great clinical significance.Ectoderm-derived organoids include the brain[96],pituitary[97],inner ear[98]and retina[99].By adding different growth factors,human ESCs and iPSCs could be cultured in neural medium in three dimensions.This produced organoid which was similar to the brains of 9–10 week old embryos:they had some main areas of the human brain in the early development stage,and also had recognizable characteristics of the dorsal cortex and ventral forebrain[96].It is now possible to construct a human cerebral-cortical neural networkin vitrowith human iPSCs that can simulate the development and function of human intracortical networks,indicating that the physiological mechanism of human forebrain neural networks can be studied by constructing brain organoidsin vitro[100].
2.3.2.Organoids from ASCs
ASCs have the potential for self-renewal and multi-directional differentiation.In the process of tissue self-renewal or damage repair,the stem cell culture microenvironment can be simulated to control the proliferation and differentiation of ASCs so as to form organoids.The Wnt signaling pathway plays an important role in the regulation of epithelial type ASCs [101].Lgr5 is a protein encoded by the target gene of the Wnt signaling pathway,which plays a regulatory role by maintaining the stemness of Lgr5 positive (Lgr5+) stem cells and promoting the proliferation of stem cells [55].As a ligand of Lgr5,R-spondin can strengthen the function of the Wnt signaling pathway,activate Wnt target genes,and promote the proliferation and differentiation of stem cells.The activators of the Wnt signaling pathway,such as Wnt3A and R-spondin,are the key components in adult stem cell culture.The BMP signaling pathway,as a negative regulatory signal of the Wnt signaling pathway,can inhibit the regeneration and proliferation of stem cells [102];as an inhibitor of BMP,Noggin can antagonize BMP and promote crypt proliferation [103].By adding these growth factors needed for the growth and development of different organoids,organoids derived from digestive tract epithelium are established successively,such as small intestine [104],colon [14],liver [69],pancreas [18],stomach [74]and gallbladder[105].Organoids of epithelial tissues from non-digestive tract sources,such as the lung[106],prostate[20]and breast[107],have also been developed.
The discovery of organoid developmental signaling pathways lays the foundation for organoids derived from ASCs development.For example,Sato’s and other early classic organoid culture system were derived from adult mouse intestinal stem cells[8].During the culture period,the stem cells first proliferated to form a saccular spheroid structure,then transformed into a crypt-like bud structure,and within two weeks gradually formed a mini-gut containing the intestinal lumen.The intestinal organoid had the same distinct zonal crypt-villi structure as the normal intestinal epithelium,including all kinds of functional intestinal epithelial cells,e.g.,Paneth cells,intestinal epithelial cells,intestinal endocrine cells,and goblet cells.The proportion of different cell varieties in the organoid was the same as that in normal intestinal epithelium,and the phenotype and genetic properties of the organoid cells were stable,with no change in expression profile found after long-term continuous subculture.
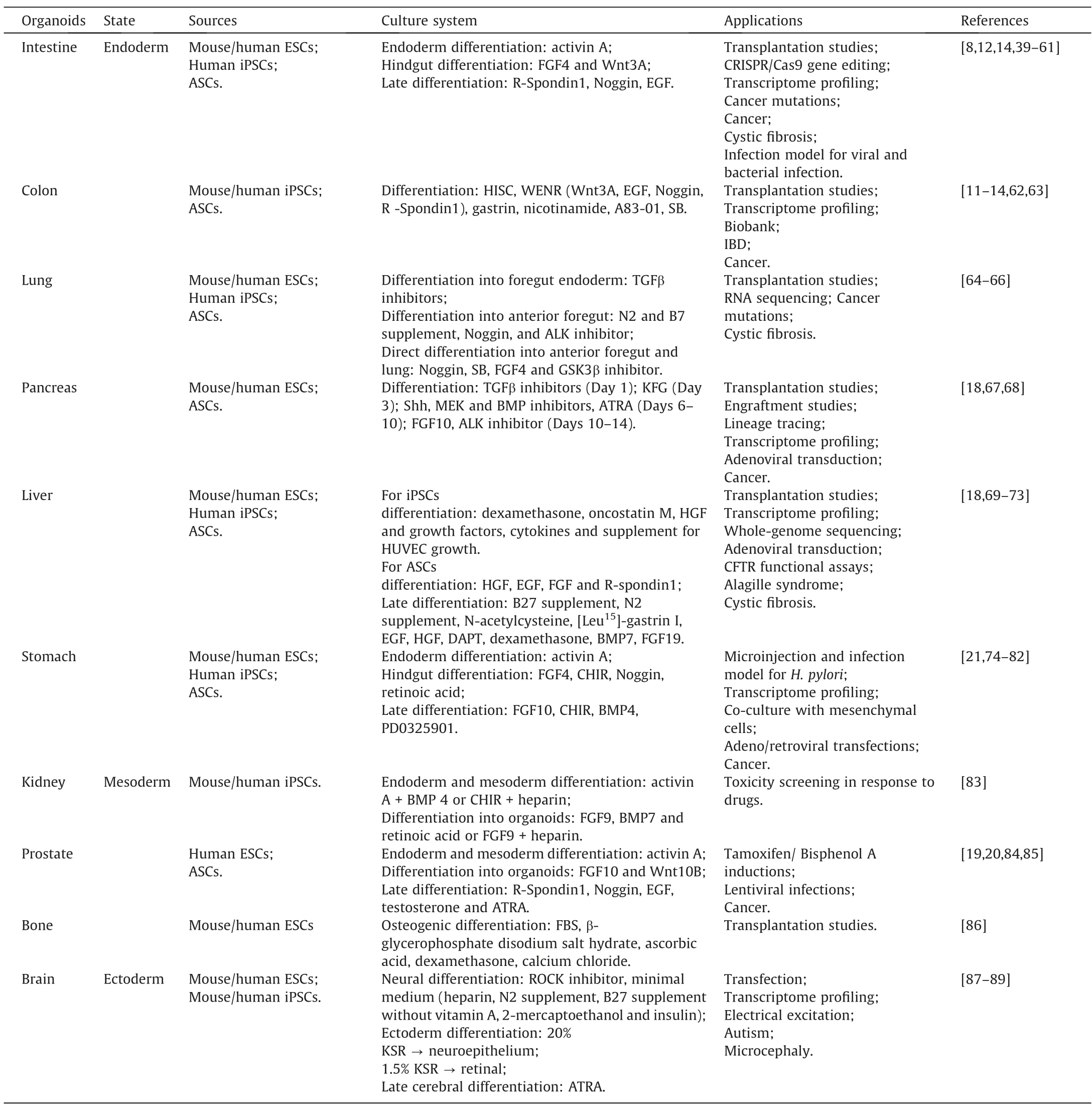
Table 1 Organoids originating from various kinds of cells,and their applications [7,34,38]
Researchers have cultivated many kinds of organoids from tissues of the digestive system.The lingual papilla contains Lgr5+cells that grow into taste buds after culture;calcium imaging technology has shown that they respond to taste stimulation [108].Barker and Stangeet al.[74,75]used the R-spondin system to culture Lgr5+stem cells of the pyloric gland and main cells of the gastric region expressing the tumor necrosis factor receptor superfamily Troy were cultured into gastric organoid models.After acute injury,the pathway for the formation and reconstruction of the cystic duct and pancreatic lobule were activated,and Lgr5+cells appeared near the bile and pancreatic ducts [67,69].Huchet al.[69]successfully obtained human gallbladder organoids from human bile duct cells by optimizing the conditions of a mouse liver organoid culture system.These organoids can express markers of bile duct cells,maintain the stability of their genome during long-term culture,and be induced to differentiate into functional hepatocyte lines.Then,under similar culture conditions,they used human pancreatic duct tissue to develop organoid models with budding cystic structure.Transplantation of these organoids under the renal capsule can form functional pancreatic tissue [67].Some researchers have also successfully cultivated human prostate organoids;exon sequencing showed that the culture system could maintain genetic stability well [19].
3.Engineering Organoid Microfluidic System to Solve Existing Challenges
3.1.Limitations of current organoid models
Although thein vitroorganoid model is a promising tool with great potential for high-throughput culture and drug screening,there are still some major limitations in traditional organoid research and technology:(1)At present,the most prominent problem is that the organoids have limited standardization.The current culture system usually produces organoids in plates or Petri dishes under static conditions with sequential addition of growth factors lacking physical and chemical microenvironmental control [89].Since the organoids are cultured in a 3D extracellular matrix,there is a certain edge effect in their growth without any constraint or culture scaffold.The shape,size,and biological characteristics of organoids make it hard to ensure relative uniformity.Therefore,it is difficult to reproduce the complex and dynamic microenvironment of a developing organ that provides instructive cues for organogenesis[29,109].This heterogeneity brings some difficulties to high-throughput and accurate quantitative analysis,and to use organoids for toxicity screening or high-throughput testing[6,110].Moreover,due to the differences between single cells and cell clusters,the gene and phenotype information carried by organoids are not completely representative.(2) In many cases,cells or tissues culturedin vitrohave been unable to reproduce the vascular network in the organism;this results in obstacles to nutrient metabolism,gas exchange,and waste removal that limit the growth and maturation of the organoids [89,92].For example,traditional brain organoid mimic the initial development of the human brain,but fail to show the mature tissue and complexity seen in the adult human brain [96].(3) Many current organoid models do not consider biomechanical forces (such as the shear stress of blood cells),blood flow,matrix,and immune cells [111].As a result,the growth statein vivocannot be completely simulated (4) The manual selection,transfer,and culture of organoids are cumbersome,inefficient,and have poor repeatability,making it difficult to achieve high throughput.
3.2.Organoids-on-a-chip
Researchers can now use well-defined biomaterials and microtechnologies to guide thein vitrodevelopment of organoids and make them highly controllable [112].This is possible with microfluidic chip technology,which controls and processes volumes of microfluids (usually measured in microliters or smaller)in micropipes [113].The microfluidic technology-“organoids-on-a-chip”-obviates the need to grow organoids in an uncertain 3D ECM without any spatial constraints,and at the same time,solves the problem of nutrient availability.Combined with cell biology,biomaterials,and engineering methods,organoids-on-a-chip has become a new and distinctive field in biomedical and health engineering in recent years.Miniaturized organoid models of functional biological units have been produced on chips,including models of the lung,liver,kidney,intestine,heart,bone marrow,cornea,skin,and blood–brain barrier [32].With the ability of precise microfluidic control,organoids-on-a-chip can realize parallel and controllable culture for organoids,including biochemistry,hydrodynamics,and a solid scaffold (ECM) microenvironment[110].It becomes possible to detect and monitor organoid growth and function status through offline optical and electrochemical methods,and at least partially to eliminate inter-individual culture differences [6].
3.3.Engineering organoid microfluidic system and its applications
As mentioned above,the current organoid system has limitations that restrict the biomedical application of organoids.Currently,researchers are trying to integrate microfluidic technology and organoid research to build an engineering organoid microfluidic system.This is a more systematic and general platform using organoids-on-a-chip as its core component,but also introducing more advanced technologies to provide automatic culture,highthroughput regulation and analysis,multi-organoid co-culture,and biosensor capabilities [6].The organoid microfluidic system provides a new blueprint for organoid research and applications that allows the organoid system to have more flexibility and possibilities.
3.3.1.Reducing organoid heterogeneity
Microfluidic technology replaces manual operation with micro devices and automatic control,significantly reducing the variability in organoid size and microenvironment.This includesin situdifferentiation of organoids,improvement of the properties of gel microspheres that encapsulate organoids,and screening of mature organoids.
Microarray systems have been used for thein situdifferentiation of organoids.Qin’s group designed a perfused microfluidic chip device containing micropillar-array structures to generate human hepatic organoids from iPSCs in a simple and controlled manner (Fig.2(a)).The dimensions of the micropillar array and gaps were optimized,which allowed the formation of uniform EBs in the confined space of the structure,and subsequentin situdifferentiation and 3D culture,avoiding the multiple operations required by conventional methods.Growing under these conditions,organoids could strongly express the marker gene of the liver,and showed some basic functions of the liver,including high sensitivity to toxic substances [114].Similar micro devices have also been used to differentiate brain organoidsin situ;the organoids showed specific characteristics of neuronal differentiation,brain regions,and cortical tissue [115].These cases indicated that micro-engineering technology,by setting micropillars,micropumps,and other micro devices,could generate organoids with complete function and a higher level of homogeneity,thus laying a foundation for the engineering of organoids and the construction of an organoid-based drug screening platform.
Another common way to build high-fidelity organoids in a reproducible and high-throughput manner is to use gel microspheres to encapsulate them [34].Advances in biomaterials and microfabrication technologies have made it possible to create 3D organoid models with more physiological relevance in a controllable manner [116].Hydrogel microspheres have been recognized as excellent 3D culture scaffolds and carriers for transplanted cells in tissue engineering owing to their uniform morphology,their proper permeability,and the ability to scale-up their production[117–119].A microfluidic method,the design of multi-phase fluid inlets,droplet generation,and capsule fabrication units,was used for the one-step fabrication of hybrid hydrogel capsules(complexations of Na-alginate and chitosan) that allow for 3D culture,growth,and generation of hiPSC-derived organoids in a reproducible and high-throughput manner (Fig.2(b)).The organoids contained islet-specific α-and β-like cells with high expression of specific genes and proteins,and were sensitive to glucose[120].In order to incorporate 3D aggregates from primary and iPSC-derived cells in a perfused platform (Fig.2(c)),researchers have also encapsulated the cells in a polyethylene glycol hydrogel to prevent aggregation and overgrowth once on chip [121].
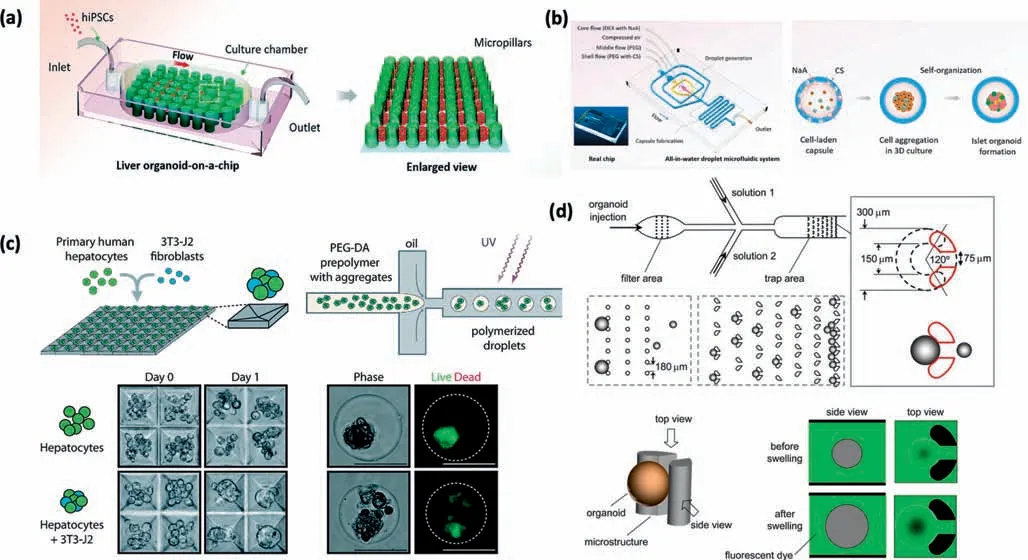
Fig.2.Engineering organoid microfluidic system to reduce organoid heterogeneity.(a) A perfused microfluidic chip device contained micropillar array structures to generate uniform size human hepatic organoids in a controlled manner.It allowed in situ differentiation and 3D culture of these organoids.(b) A novel droplet microfluidic system included multi-phase fluid inlets,droplet generation,and capsule fabrication units.It produced microdroplets in a reproducible and high-throughput manner.(c) A perfused platform incorporatded 3D aggregates from primary and iPSC-derived cells.(d) A“pinball-like”micro device with two micro column array chambers,was used to analyze enteroid swelling.The improper size organoids were filtered out by the different gaps and shapes of the micro-columns.
In addition to the above two methods,screening mature organoids is also a feasible method of reducing size heterogeneity.A“pinball-like”micro device was used to analyze enteroid swelling caused by cholera toxin.The organoids were continuously injected into two microcolumn array chambers.The gaps and shapes of the micro-columns in the two chambers were different,so that the oversized and the undersized organoids were filtered out,and those suitable for the analysis were intercepted (Fig.2(d)).The degree of swelling caused by the toxin was evaluated by quantitative analysis of organoid volume [122].This ingenious label-free device can be used for organoid-based high-throughput drug screening.
3.3.2.Improvement of microenvironment and nutrient supply
The supply of nutrients is an important factor in the formation of high-quality organoids.Organoid structures are more complex than cell lines and often have larger 3D structures (size:300 μm–1 mm).This makes it difficult for cells located in the center to get enough nutrients,leading to the apoptosis of organoid cells.
Micro-engineering technology can solve this problem to some extent.In one study,human mid-brain specific organoids were cultivated in a microfluidic system,and the oxygen and nutrient supply was controlled intelligently by a circuit system and computer algorithm.This method significantly reduced the “dead core”area[123].Microfluidic technology could also improve the nutritional supply of liver organoids.A multi-chamber microfluidic device was designed to culture liver organoids and to form a vascular network to simulate blood flow by shaking,so that it could achieve better nutrition supply.The results showed that liver organoids cultured with this device successfully formed 3D vascular structures and showed better liver function and metabolic activity[124].These cases show that micro-engineering technology can better simulate thein vivospontaneous process and satisfy the nutrient supply during organogenesis,and thus better realize biochemical microenvironmental control of organoids.
The importance of the stability of the organoid microenvironment(growth factor gradient,physical structure)in reducing organoid heterogeneity is also undeniable [6].Researchers have used microfluidics technology to control the chemical and physical microenvironment of organoids precisely,e.g.,by the use of microtubules to control the gradient of growth factors to cause spontaneous intestinal or neural organoid differentiation [125,126],or of microfluidic power to promote angiogenesis of renal organoids for a drug screening platform [127].
3.3.3.Simulation of multiple organoids interactions
With the development of organoids-on-a-chip technology,the advantages of organoids for simulatingin vivoenvironments are attracting more attention.However,the human body is a complex system with multiple tissues featuring multi-organ cooperation.Therefore,the combination of different kinds of organoids can further simulate the complex environmentin vivoand provide more reliable preclinical data.Such multi-organoid microfluidic system has been applied to efficacy verification and drug screening.
Researchers have constructed a liver-stomach-intestine organoids platform using micro-engineering technology.In this device,three kinds of organoids were cultured in a 4 × 8 microporous array and connected to each other through the flow of culture medium in the microchannels.Under the induction of bile acid,the expression of related enzymes and genes in the liver and intestine organoids in the system was consistent with thatin vivo,indicating that the system could successfully simulate the hepatic and intestinal circulation of bile acid metabolism [128].The establishment of this system provided a good model for the study of drug metabolism in multiple organoids.Another representative multiorganoid system was a liver-heart–lung organoids platform:liver,heart,and lung organoids were cultured in different chambers,connected to the central chamber through microtubules.The circulating power of the medium was provided by a pump.This system revealed that chemotherapeutic drugs (in this case,bleomycin)could produce cardiotoxicity through cardiopulmonary interaction,something that has not found in single-organoid studies[129].This system reflects the great potential of multi-organoid platforms in drug toxicity screening and improving drug approval efficiency.Compared with single-organoid microfluidic system,multiorganoid microfluidic system is closer to the real situationin vivo,and they have broad development prospects in preclinical drug screening and efficacy verification.However,the development of this field also faces obstacles,such as the different growth factors and culture conditions of various organoids.Therefore,a universal medium is needed as the “circulatory system”of multiorganoid system.
In addition to various iPSC-induced organoids,patient-derived organoids have also been used in disease research and drug screening.A mouse xenograft tumor model was successfully established using patient-derived colorectal-cancer organoids.This model had high sensitivity and specificity for predicting the response of patients to targeted drugs or chemotherapy.In addition,it could be used for functional genomics,simulating the behavior of cancerin vivoandin vitro,and integrating molecular pathology and early clinical trials in the decision-making process [130].A highefficiency drug screening platform based on patient-derived organoids has also been attempted [131].Patient-derived tumor cells culturedin vitrointo tumor organoids were used to screen immune cells with tumor toxicity.Lymphocytes were extracted from patients or healthy donors,and then co-cultured with tumor organoids to screen for tumor cytotoxic lymphocytes.Using this method,eleven specific antigen sites were selected from fiftyseven candidate antigen sites using tumor organoids from three patients.The selected lymphocytes were then amplifiedin vitroand transplanted back into the patient.Since the tumor organoids came from the patients themselves,this therapy had good efficacy and specificity [132].
According to a recent study,patient-derived organoids maintained the tumor diversity of the source patients [133].In fact,the genes mutated in these organoids were not present in the driver gene and drug target regions,which indicated that these patient-derived organoid models closely resembled theirin vivocounterparts and formed a reliable platform for personalized drug screening.Although the development prospects of patient-derived organoids are extremely encouraging,there are still many problems to be solved.The most prominent is the very high specificity of patient-derived organoids:it may be difficult to obtain highquality mature organoids by using normal organoid culture methods.It would be valuable to develop special organoid culture programs for different patient-derived organoids,but this is challenging.In fact,for each type of disease,it will be necessary to establish a multi-organoid microfluidic platform with pathological tissues and other organoids from the same patient to establish a new and unique patient body systemin vitro.Drug screening using this platform will be highly targeted and specific.Furthermore,multi-organoid system not only simulates the interaction of organs,but also increases the reliability and stability of the clinical data.The current applications of the organoid microfluidic system are shown in Fig.3.
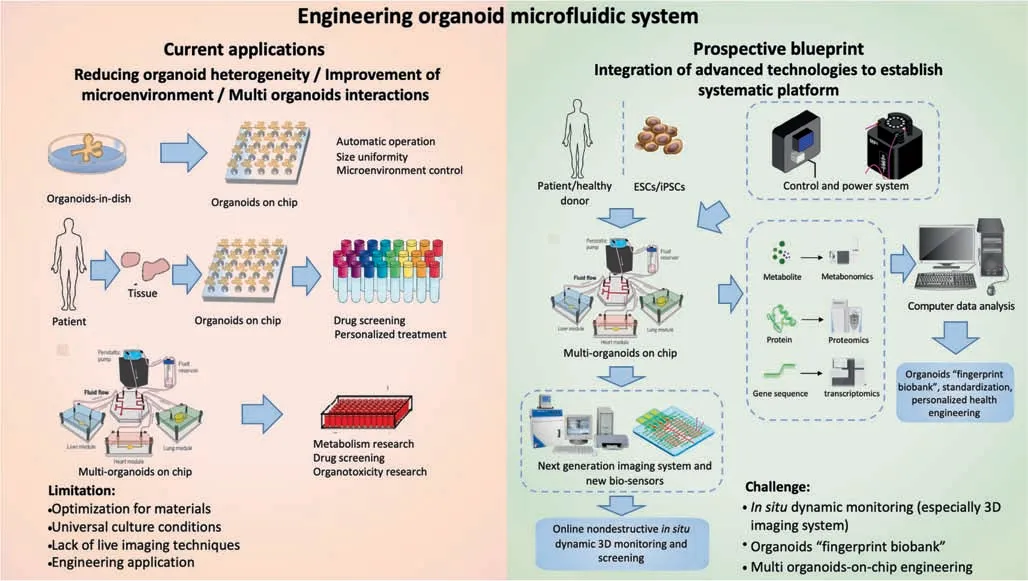
Fig.3.Overview of engineering organoid microfluidic system applications and prospects.Organoid microfluidic system has effectively solved the problems affecting current organoid models.Therefore,they can be widely used for drug screening,toxicity testing,personalized treatment,and other applications.In the future,the integration of organoid microfluidic system combined with biosensors,omics analysis,and other system will further broaden the application prospects.
4.Conclusions and Future Prospects
4.1.Conclusions
The development of organoids has brought significant changes to health-related biological research.Organoid biomimetic system can simulate the physiological and pathological state of the human bodyin vitroto predict the response to drugs or other stimuli.Organoids have a wide range of applications in basic life science research,clinical disease simulation,and development of new drugs and bioactive compounds.The combination of microfluidic chip and organoid technologies to establish an organoid microfluidic system has gone far toward solving the current problems of organoid culture and application.This approach has reduced organoid heterogeneity byin situdifferentiation of organoids,improving the properties of the gel microspheres that encapsulate organoids,and screening mature organoids.In addition,microengineering technology can optimize the microenvironment and nutrient supply with vascular networks or other channels.Multiorganoid interactions can also be simulated effectively.Engineering organoid microfluidic system has played an extremely important role in many pharmaceutical and health engineering fields such as drug screening,food safety,and precision medicine.Combined with more advanced biotechnology,such as various sensor systems,omics analysis,and advanced biomaterials,organoid microfluidic system will find further applications and contribute to the development of the healthcare industry.
4.2.Prospects:Integration of advanced technologies to establish systematic platforms
With the rapid development of biomedical and health engineering,simple structural design of organoid microfluidic devices no longer meets the requirements of high-throughput,visual,and dynamic screening of drugs and bioactive or toxic compounds.Therefore,organoid microfluidic device research increasingly involves integration of structural design and other relevant downstream technologies.
With the help of micro-engineering technology,electrical signals can control the movement of organoid droplets and contribute to organoid automatic generation and toxicity or bioactivity testing[28].Micro devices can also be integrated with other types of sensors to form a multifunctional organoid microfluidic system from screening to evaluation.One representative study described an integrated automated sensing platform combining multiple components,such as micro bioreactors,breadboards,physical sensors,and electrochemical biosensors,that can be used for dynamic and continuous monitoring of organoid morphology,microenvironment,and biomarkers[134].In addition to multi-sensor platforms,the improvement of biomaterial embedding organoids could also help with effective control of the organoid microenvironment and collection of monitoring data [134–136].Furthermore,in situor online continuous measurement technology requires highlevel automated imaging system because of the complex 3D multicellular structure of the organoid.In order to achievein situnon-destructive dynamic 3D monitoring,the development of real-time imaging technology and equipment is urgently needed.Single-objective selective-plane illumination microscopy(soSPIM),laser scanning confocal microscopy (LSCM),and optical coherence tomography (OCT) [137–139]have been widely used in medical diagnosis because of their high signal-to-noise ratio and spatial resolution:they have potential applications in 3D organoid realtime imaging as well.The integration of omics technology into an organoid microfluidic system is another promising development direction [140].Through dynamic omics analysis of organoids (e.g.,single cell sequencing,analysis of the expression level of genes and proteins,metabolomic analysis of organoid metabolites,and proteomics technology),the whole differentiation and formation stage of organoids will be comprehensively and dynamically understood.Based on this platform,a biobank with an organoid microfluidic system could be set up,and the unique“fingerprints”of various organoids displayed.This would be of great significance for engineering and standardization [33,141].A blueprint for the development of the organoid microfluidic system is included in Fig.3.
However,the system still has many limitations that need to be solved.For example,some organoids can only simulate human organs to a limited extent;this is obvious of brain tissue,with its complex physiological structures and many cell types.The physiological and biochemical functions of some organoids are still under elucidation.Moreover,there are differences between different batches of organoids,with possible significance for application.For microfluidic system,it is still a challenge to simulate fully the growth environmentin vivoand to form mature and stable organoids;meeting this challenge will require the integration of the above-mentioned technologies.
In summary,the development opportunities and challenges of organoid microfluidic system coexist.Many future improvements in organoid microfluidic system are possible:improvement of the biomaterials of organoid microspheres;development of online and non-disruptive detection system based on 3D optical imaging;automatic microfluidic organoid culture;functional characterization;development of a computer analysis system integrated with the equipment.Overall,organoids represent a force that in the near future will revolutionize the way human development and health are understood.The combination of microfluidic chip and organoid technology will achieve high-throughput,automated screening and predictions of the response of the human body to bioactive compounds and drugs.It will find many innovative applications in various health engineering fields such as food safety,nutriceutical function analysis,and precision medicine;it will also have important uses in academic research.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Y.F.Xing and J.Y.Liu contributed equally to this work.The authors thank the Key Laboratory for Industrial Biocatalysis,Ministry of Education of China,Department of Chemical Engineering,Tsinghua University,Beijing,China.This work was supported by the Key Areas Research Development Projects of Guangdong Province (No.2019B020210001),the Tsinghua-U Tokyo Collaborative Research Fund (No.20193080052),and the Key Areas Research Development Projects of Hebei Province (No.20375502D).
 Chinese Journal of Chemical Engineering2021年2期
Chinese Journal of Chemical Engineering2021年2期
- Chinese Journal of Chemical Engineering的其它文章
- Molecular design,synthesis strategies and recent advances of hydrogels for wound dressing applications
- Monoclonal antibody-based cancer therapies
- Recent advances in systemic and local delivery of ginsenosides using nanoparticles and nanofibers
- State of arts on the bio-synthesis of noble metal nanoparticles and their biological application
- Concepts,processing,and recent developments in encapsulating essential oils
- The potential of ionic liquids in biopharmaceutical engineering
