A Novel Nanoliposome Signal Amplification Platform for Sensitive Personal Glucose Meter Based Point-of-Care Detection of Ricin
MEN Chen ,LI Yuan-fang,HUANG Cheng-zhi,2,ZHEN Shu-jun*
(1.Key Laboratory of Luminescence Analysis and Molecular Sensing(Southwest University),Ministry of Education,College of Chemistry and Chemical Engineering,Southwest University,Chongqing 400715,China;2.Key Laboratory of Luminescent and Real-Time Analytical Chemistry(Southwest University),Chongqing Science and Technology Bureau,College of Pharmaceutical Sciences,Southwest University,Chongqing 400715,China)
Abstract:Ricin is one of the most deadly toxins known,which could be used as a biological weapon causing potential environmental and food pollution.However,common methods for ricin detection are time-consuming and limited due to their complicate and professional laboratory operations.To overcome these limitations of the common detection methods and achieve a simple,rapid and sensitive non-laboratory detection of ricin,herein,a novel method was presented for the point-of-care(POC) detection of ricin B-chain(RTB) by using amyloglucosidase loaded nanoliposome(ALN) to amplify the personal glucose meter(PGM) signal.Initially,a hybrid probe was constructed by hybridizing an aptamer on magnetic beads(MBs) with a blocker on ALN,combining the functions of target recognition,magnetic separation and signal amplification together.Then,in the presence of RTB,the blocker-linked ALN was released from the surface of MBs after magnetic separation with the formation of RTB-aptamer complex.Subsequently,as-obtained ALN supernatant was lysed with Triton X-100 to liberate amyloglucosidase to hydrolyze amylose,generating a great number of glucose.Finally,the glucose was quantitatively read out by the PGM in 10 s.Therefore,RTB can be detected by using the enhancement of PGM value.The linear range for the detection was from 0.3 μg/mL to 30 μg/mL and the limit of detection(LOD) was 67 ng/mL.Results showed that the developed method remarkably improved the sensitivity and selectivity for RTB detection,and exhibited a great potential in POC detection of other targets.
Key words:point-of-care testing;nanoliposome;ricin B-chain(RTB);personal glucose meter(PGM)
Ricin,a natural toxic protein extracted from castor beans,is one of the most deadly toxins known[1].It is formed by A chain and B chain,which are connected by a disulfide bond.One ricin can inactivate 1 500 ribosomes per minute with a very low lethal dose limit(LD50) in humans,which is 5-10 μg/kg by inhalation and 1-20 mg/kg or 8 castor beans through ingestion[1],as well as the time to death is typically 60-90 h when reached to its lethal dose[2].As yet,there is no specific effective antidote for ricin poisoning or prevention[3].Due to the features of extremely strong toxicity,easy access and high stability[4],ricin can be used as a biological weapon and causes potential environmental and food pollution[5].Moreover,ricin is a highly promising anticancer drug and biological pesticide[6].Therefore,it is of great significance to realize a simple,convenient and sensitive detection of ricin for public health and national security[7].
At present,common approaches for ricin detection include enzyme-linked immunosorbent assay(ELISA)[8-9],mass spectrometric analysis[10-11],electrochemiluminescence assay[12],surface-enhanced Raman spectroscopy(SERS)[2,13]and atomic force microscopy(AFM) recognition imaging[14].Recently,our group has developed a series of new fluorescence and fluorescence anisotropy methods for ricin detection[15-17].These methods can realize good performance in sensitivity and selectivity.However,most of these methods are time-consuming,and possess complicate operation procedures and depend on expensive and sophisticated laboratory facilities.
Compared with the above traditional laboratory-based testing,point-of-care testing(POCT) is a rapid,portable,and user-friendly platform,which has unique advantages as follows:(1) Easy to be operated by non-laboratory professionally personnel or patients themselves;(2) Less analysis time;(3) Portable and simple POC devices;(4) Convenience of use[18].Therefore,it has been employed for improved healthcare,especially in home,developing countries and resource-limited areas[19-20].With the development of POCT technology,a lot of POC devices are devoted to exploring in on-site detection[21],such as pressure meter[22-23],pH meter[24],the pregnancy test paper[25]and glucose strips etc.
The most successful POC device is commercial personal glucose meter(PGM),which is ‘pocket’ size,affordable,simple operation,and widely accessible to the public[19,26].Traditionally,PGM has been only used for detection of glucose in blood.To abroad the application of PGM,Lu and other groups have constructed a series of novel methods to detect diverse non-glucose targets by using functional DNAs to achieve transformation of targets into glucose[27-30].However,these methods need to conjugate invertase with aptamer covalently with multiple steps,which is time-consuming and may result in the loss of enzyme activity[31].In addition,the sensitivities of these methods also need to be improved because one target can only be transformed into a small amount of glucose by one invertase.Therefore,it is highly desirable to develop a powerfully effective method which not only can protect the activity of enzyme but also improve detection sensitivity.
Nanoliposome is an artificial microscopic vesicles composing of a bilayer of phospholipids[32].One hand,signal molecule or enzyme can be loaded into the interior of nanoliposome gently,which is time-saving and can protect the activity of enzyme[33].On the other hand,owing to large cavity volume,one nanoliposome can encapsulate a large number of enzyme molecules[34],which can achieve the transformation of one target into large amount of glucose.Therefore,Yang group has successfully utilized nanoliposome to improve the sensitivity of PGM for the detection of disease biomarker[31].
Inspired by these above reports and based on our previous works[15-17],herein,we construct a novel POCT method by using PGM as signal readout device,aptamer as recognition element,nanoliposome as signal amplifier for RTB detection,which involves three procedures:(1) The preparation of a MBs-DNA-ALN hybrid probe by the hybridization of aptamers on the surface of magnetic beads(MBs) and blockers on the surface of amyloglucosidase loaded nanoliposome(ALN);(2) The strong binding of RTB with aptamer resulting in the release of nanoliposomes;(3) The released nanoliposomes were broken by Triton X-100 and liberating amyloglucosidases,which can hydrolyze amylose to produce glucose,and the glucose can be quantitatively read out by the PGM in 10 s.Therefore,the RTB can be detected sensitively by the significant enhancement of PGM signal.
1 Experimental
1.1 Chemicals and apparatus
All the DNA oligonucleotides(Table 1) designed in this experiment were purchased from Sangon Biotech(Shanghai,China).RTB was obtained from Vector Laboratories.Streptavidin-coated magnetic beads(MBs,with the size of 1 μm and std dev < 0.5 mm),amyloglucosidase,amylose,cholesterol and lysozyme were purchased from Sigma Aldrich(St.Louis,MO).L-α-phosphatidylcholine(PC) and horseradish peroxidase(HRP) were purchased from Aladdin(Shanghai,China).1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethyleneglycol)-2000](DSPE-PEG2000) was purchased from Pengshuo Biotechnology Co.,Ltd(Shanghai,China).Albumin fraction V(AFr),pepsase and ELISA kit was purchased from Xiya Reagent(Chengdu,China).All other chemicals were of analytical-reagent grade.Millipore Milli-Q water(18.2 MΩ·cm) was used in all experiments.
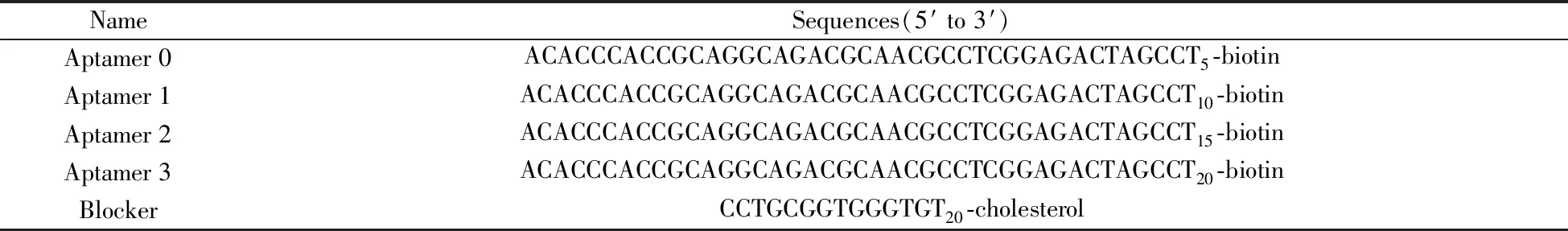
Table 1 Sequences of oligonucleotides
The PGM was purchased from Sinocare(Hunan,China).Solution blending and magnetic separation was operated by a vortex mixer Vortex-6 and a Four-dimensional rotary mixer BE-1100(Kylin-Bell,China),respectively.A thermomixer C was used for controlling temperature.A mini-extruder(Avestin Inc.,Canada) was utilized for preparation of nanoliposome.Zeta-sizer Nano-ZS instrument(Malvern,UK) was used for measuring dynamic light scattering data and zeta potential of samples.
1.2 Preparation of amyloglucosidase-loaded nanoliposome(ALN)
The preparation of amyloglucosidase-loaded nanoliposome(ALN) was according to the literature report with slight modifications[35],which contains four steps.First,L-α-phosphatidylcholine(PC),cholesterol,and DSPE-PEG2000 with a molar ratio of 70∶10∶20,were dissolved in 1 mL chloroform.Then,a thin layer was formed after the evaporation of chloroform under reduced pressure at 40 ℃ for 30 min.Second,to ensure complete removing any residual organic solvent traces,the film was treated in vacuum for 2 h at 25 ℃.Third,the obtained thin film was hydrated with 1 mL of 15 mg/mL amyloglucosidase in PBS with whirling until the solution became cloudy.Subsequently,the vesicular solution was passed through a 200 nm polycarbonate film to produce homogeneous and uniform nanoliposome suspensions.Finally,nanoliposome solution was dialyzed with 300 kDa MWCO dialysis membrane(SpectrumLabs,Rancho Dominguez,CA,USA) at 4 ℃ in PBS with stirring for 24 h to remove untrapped enzyme and stored at 4 ℃ for further use.
1.3 Preparation of the MBs-DNA-ALN hybrid probe
The aptamers were immobilized on MBs through streptavidin-biotin binding.Initially,the streptavidin coated MBs were rinsed and resuspended in 100 μL PBS.Subsequently,2 μmol/L biotinylated aptamer was added and incubated for 30 min at room temperature.Then,the supernatant was removed by magnetic separation and the MBs were washed three times with PBST(1×PBS and 0.05% Tween-20,pH 7.4) and three times with PBS.Finally,the MBs solution was resuspended in a final volume of 100 μL PBS and stored at 4 ℃ for further use.
The blockers were conjugated on the surfaces of ALN by using cholesterol molecules modified blocker inserting into the lipid bilayer of ALN.In brief,3 μmol/L cholesterol modified blocker and 6.7 mg/mL ALN were mixed in HEPES buffer(100 mmol/L NaCl,10 mmol/L HEPES,pH 7.4) and incubated at 25 ℃ for 1 h with gentle shaking.
The MBs-DNA-ALN probe was performed by the hybridization of aptamer-functionalized magnetic bead(MBs-aptamer) and blocker-functionalized amyloglucosidase-loaded nanoliposome(blocker-ALN) for 1 h at 37 ℃.Subsequently,the probe was washed three times with PBS to remove nonhybridized components and then resuspended in a final volume of 100 μL PBS.Finally,the obtained hybrid probe was stored at 4 ℃ for further use.
1.4 Procedure for RTB sensing
First,10 μL hybrid probe solution and 10 μL RTB with different concentrations were mixed together and incubated at 37 ℃ for 50 min with gentle shaking.Then,the nanoliposomes supernatant was obtained after magnetic separation.Subsequently,3% amylose solution(1% Triton X-100,10 mmol/L PBS,pH 4.5 for the most suitable pH of amyloglucosidase) was mixed with the supernatant.After incubation for 1 h at 50 ℃,nanoliposomes were lysed by Triton X-100 and released amyloglucosidase to catalyze hydrolysis of amylose,producing a certain number of glucose.Finally,the glucose was determined by PGM.Thus,different concentrations of RTB can be detected through the enhancement of PGM signal rapidly.
2 Results and discussion
2.1 The principle for the detection of RTB
The principle of the proposed method for RTB detection is shown in Fig.1,which involves three procedures:(1) The preparation of a MBs-DNA-ALN hybrid probe by the hybridization of aptamers on the surface of MBs and blockers on the surface of nanoliposomes;(2) The strong binding of RTB with aptamer resulting in the release of nanoliposomes;(3) The released nanoliposomes were broken by Triton X-100 and liberating amyloglucosidases,which can hydrolyze amylose to generate glucose.The glucose could be directly measured by a portable PGM.As one RTB could liberate large amount of amyloglucosidase to hydrolyze amylose to produce glucose,the RTB can be detected sensitively by the significantly enhanced PGM signal.
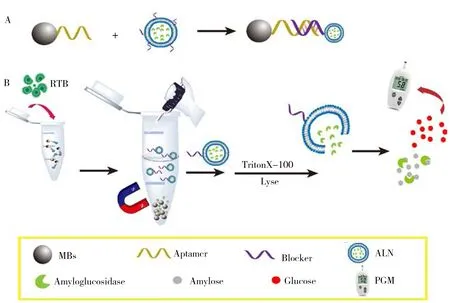
Fig.1 Schematic illustration of coupling nanoliposome with PGM for RTB detection
2.2 Characterization of the amyloglucosidase-loaded nanoliposome(ALN)
Characterization results of nanoliposome indicated that,the average size of nanoliposome was approximately 220 nm(Fig.2A) and the zeta potential was (-11.4 ± 1.3) mV(Table 2).These results provided intuitionistic evidences for the successful preparation of nanoliposome.Meanwhile,the nanoliposome rapidly released enzyme when Triton X-100 was added,and PGM signal reached the maximum within 15 min(Fig.2B).In addition,the experimental result indicated that the addition of Triton X-100 had no effect on the activity of amyloglucosidase(Fig.2C).

Table 2 Characterizations of amyloglucosidase-free nanoliposome(AFN) and ALN
2.3 Calculation of the number of amyloglucosidases in ALN
The encapsulation efficiency(EE) in the ALN and the loading capacity of amyloglucosidase molecules per ALN are important parameters in the assay.The former was determined by using the ratio of the total number of entrapped amyloglucosidase to the total added enzyme in the preparation of ALN.The total number of amyloglucosidase was estimated to be 6.2 mg according to a standard calibration curve of PGM signal with amount of amyloglucosidase(Fig.2D).Therefore,the EE was calculated to be 41.5%.The latter was calculated based on the previous literature report[35].As a result,loading capacity was calculated to be 4 100 amyloglucosidase molecules/nanoliposome,which indicated that ALN prepared in this work has a high loading ability to achieve effective signal amplification.
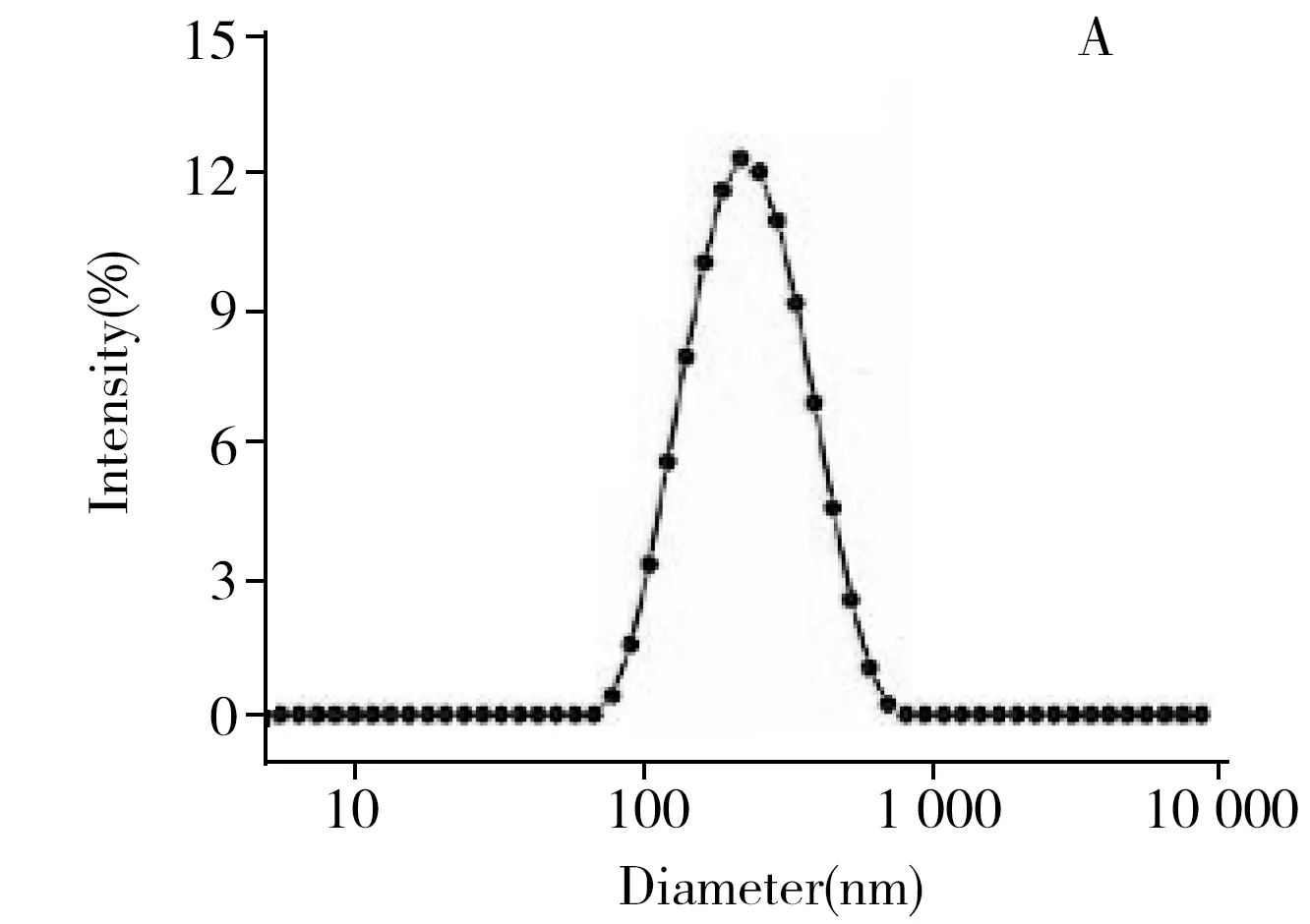
Fig.2 Size distribution of amyloglucosidase-loaded nanoliposome(ALN)(A),kinetics of amyloglucosidase releasing from nanoliposome in response to the addition of Triton X-100(B),effect of Triton X-100 on amyloglucosidase activity(C),and standard curve of PGM signal with amount of amyloglucosidase(D)experimental condition:1% Triton X-100,30 mg/mL amylose,reaction is performed at 40 ℃ for 40 min
2.4 Feasibility study of RTB detection
To validate the feasibility of the proposed ALN-PGM strategy,the PGM signal in the absence and presence of RTB were compared.As show in Fig.3A,in the absence of RTB,a negligible weak PGM signal was measured because the ALN was not released from the surface of the MBs and consequently,resulting in releasing few amyloglucosidase to hydrolyze hydrolysis amylose to produce few glucose.After incubation with RTB,the PGM signal has a very significant enhancement and can reach to 25 mmol/L.The result successfully proved that the MBs-DNA-ALN probe can bind with RTB and lead to the release of ALN for further production of massive glucose by the entrapped amyloglucosidase,which can be quantitatively read out by the PGM.
In addition,KI/I2solution was used to further confirm the mechanism of RTB detection.As shown in Fig.3B,in the presence of RTB,the consumption of a large amount of amylose by the released amyloglucosidase brings about rapid color fading of blue amylose-KI/I2solution to colorless.In contrast,no obvious color change could be observed in the absence of RTB.All of these results indicated that the proposed strategy could be used for the quantitative detection of RTB.
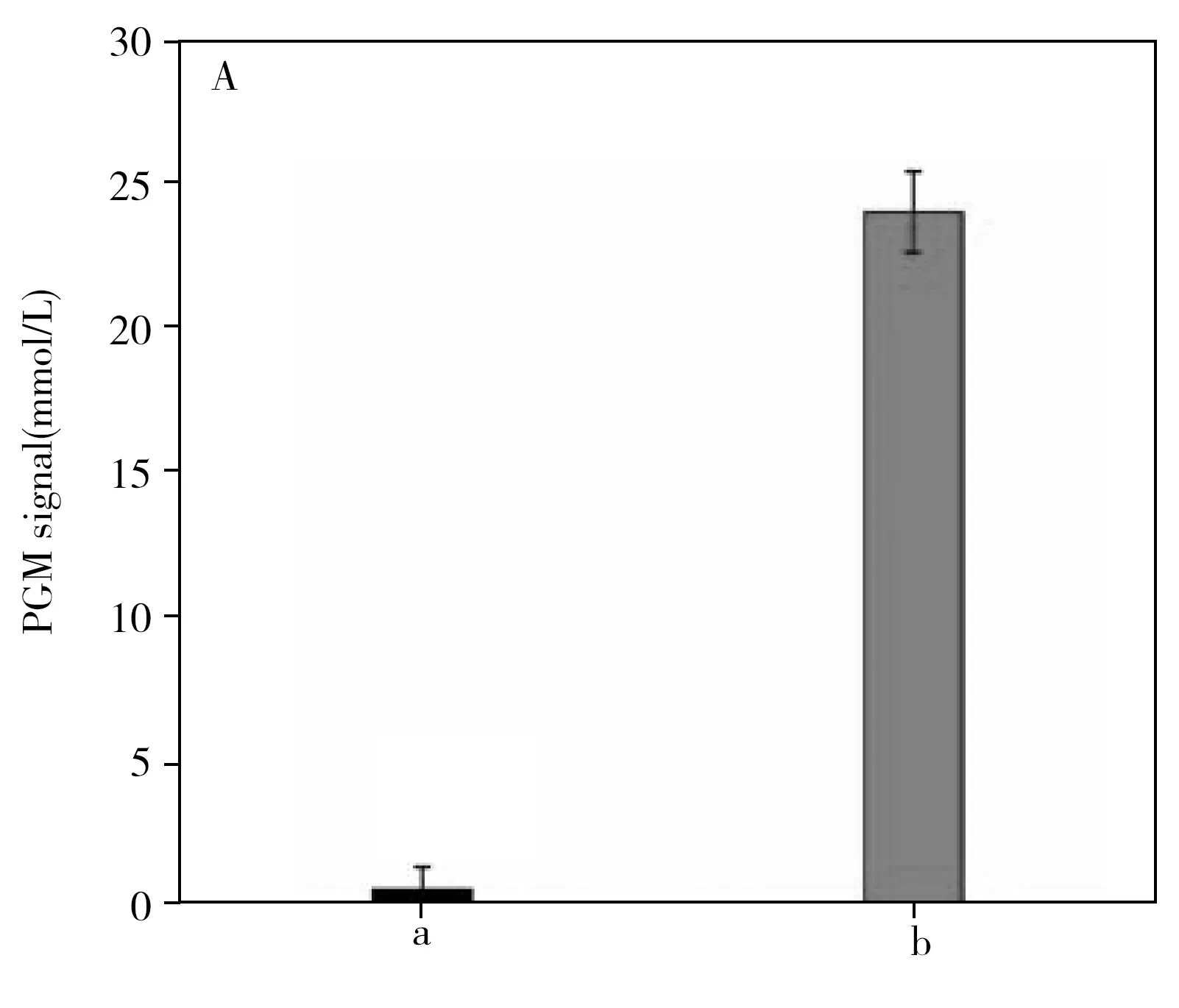
Fig.3 PGM signal responses of the proposed method under different assay conditions(A),and study of the feasibility of the sensing system by a KI/I2 solution(B)a:in the absence of RTB;b:in the presence of RTB
2.5 Optimization of experimental conditions
In order to achieve the best sensing performance,the concentration of blocker,aptamer and MBs,the spacer number of aptamer,the time of RTB incubation and hydrolysis by amylose were optimized.When the concentration of blocker is high,more blockers will linked to one nanoliposome,which will produce a large steric effect for the linking of nanoliposome through the hybridization of blocker and aptamer and significantly reduce the production of glucose.Therefore,we firstly optimized the concentration of blocker.As shown in Fig.4A,when the blocker concentration ranged from 1.5 μmol/L to 3.0 μmol/L,ΔPGM(ΔPGM is the change of the PGM signal after the addition of RTB) increases continuously,and as the concentration increases beyond 3.0 μmol/L,the ΔPGM decreases continuously.Therefore,3.0 μmol/L is the optimal concentration of the blocker.Similarly,the high concentration of aptamer can increase the number of nanoliposome on the surface of MBs,but excessive aptamer can also bring large steric hindrance and reduce the detection sensitivity.As shown in Fig.4B,ΔPGM increased gradually when the concentration of aptamer increased from 0.5 μmol/L to 2.0 μmol/L and decreased when it was greater than 2.0 μmol/L.Therefore,2.0 μmol/L was selected as the optimal concentration of aptamer.The number of aptamer spacer also plays a role in regulating the distance between the aptamer and MBs.When the number of spacer is less,the distance is short and the steric hindrance is large,and RTB is not easy to react with aptamer to generate signals.When the number of the T bases of spacer was 10,ΔPGM signal achieved maximum.Therefore,the number of the T bases of spacer was selected as 10(Fig.4C).
In addition,effect of concentration of MBs on ΔPGM was also studied.As shown in Fig.4D,when the concentration of MBs was from 0.5 mg/mL to 1.5 mg/mL,ΔPGM increased continuously.When it exceeded 1.5 mg/mL,ΔPGM was constant.Therefore,1.5 mg/mL was an optimized concentration of MBs.
Furthermore,the reaction time of MBs-DNA-ALN probe with RTB and the hydrolysis of amylose were also investigated.The optimal time were 50 min and 1 h(Fig.4E,4F),respectively.
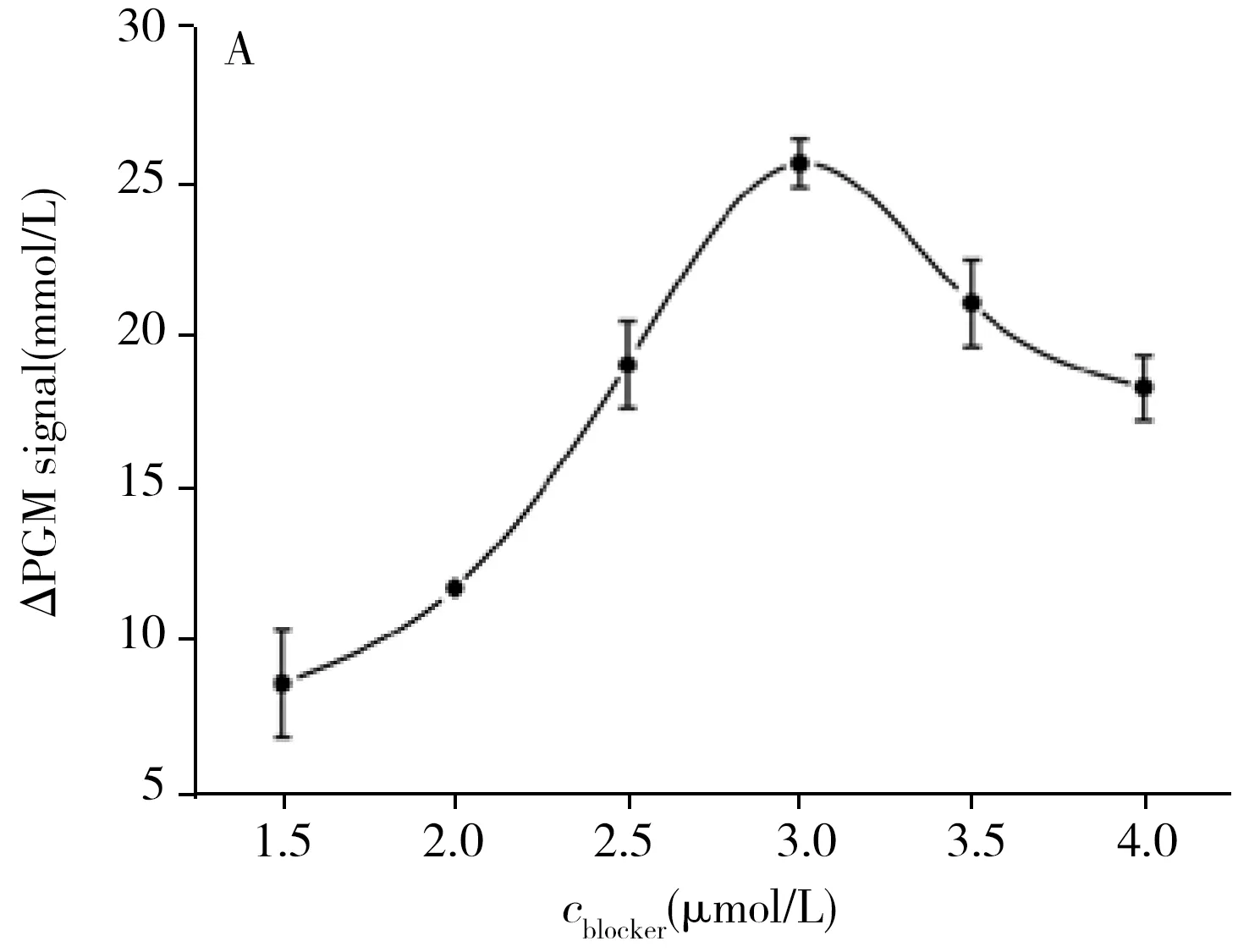
Fig.4 Effect of concentration of blocker(A) and aptamer(B),the spacer number of aptamer(C),concentration of MBs(D),reaction time of hybrid probe with RTB(E) and enzyme hydrolysis(F)experimental condition:A:2.0 μmol/L aptamer,10 T spacer number of aptamer;B:3.0 μmol/L blocker,10 T spacer number of aptamer;C:3.0 μmol/L blocker,2.0 μmol/L aptamer,all of the other conditions in A-C were:1.5 mg/mL MBs,50 min RTB reaction time,and 1 h enzyme reaction time;D:50 min RTB reaction time,1 h enzyme reaction time;E:1.5 mg/mL MBs,1 h enzyme reaction time;F:1.5 mg/mL MBs,50 min RTB reaction time.All of the other conditions in D-F were:3.0 μmol/L blocker,2.0 μmol/L aptamer,10 T spacer number of aptamer.All of RTB concentrations in A-F were 25 μg/mL
2.6 Sensitivity and selectivity of the RTB detection
To illustrate the analytical performance of the proposed method,we detected RTB with different concentrations under the optimal conditions.As shown in Fig.5A,there was a good linear relationship between ΔPGM signal and concentration of RTB in the range of 0.3-30 μg/mL.The linear regression equation was ΔPGM=0.860c+1.592(r2=0.985 0),wherecwas the concentration of RTB(μg/mL).The limit of detection(LOD,3σ) was 67 ng/mL.In addition,compared with the previously reported fluoroimmunoassay,aptamer arrays assay and ELISA for detecting RTB(Table 3),our method not only can reduce the detection time but also can improve the detection sensitivity.
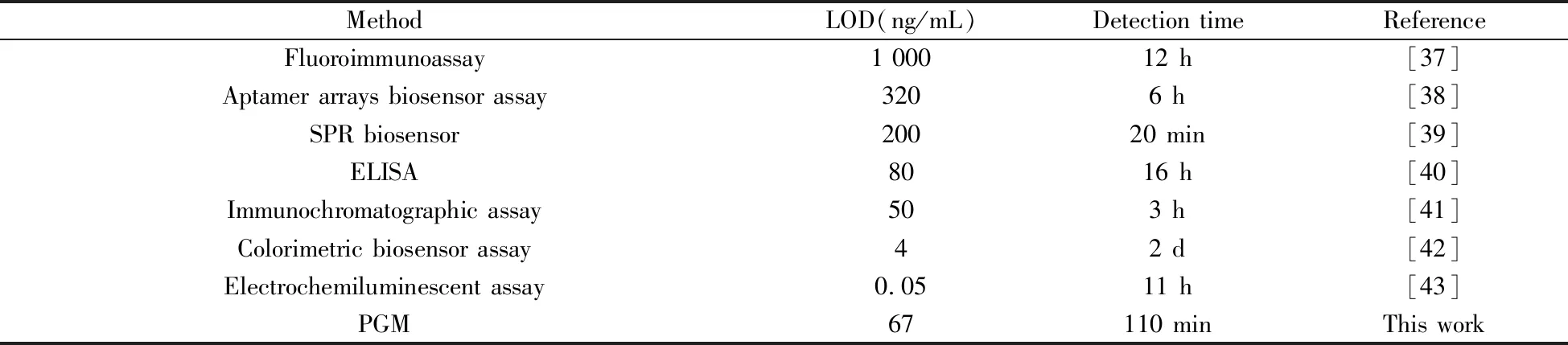
Table 3 Comparison of different methods for detecting ricin
The selectivity of our method was investigated by comparing ΔPGM mediated by RTB and various proteins,such as lysozyme,HRP,BSA,pepsase and albumin fraction V(AFr) under the same experimental conditions.As shown in Fig.5B,apparent PGM signal changes could only be produced by RTB,indicating that the proposed method possess excellent selectivity.
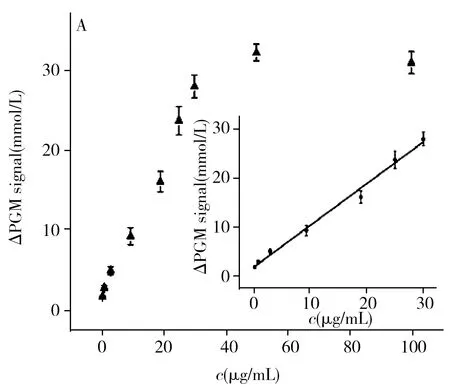
Fig.5 The linear relationship between ΔPGM signal and RTB concentration(A),selectivity of the purposed method based on nanoliposome amplification combined with PGM(B)experimental condition:A:3.0 μmol/L blocker,2.0 μmol/L aptamer,10 T spacer number of aptamer,1.5 mg/mL MBs,50 min RTB reaction time,1 h enzyme reaction time;B:concentrations of RTB,lysozyme,HRP,BSA,pepsase and AFr were all 25 μg/mL
Moreover,the ricin content in castor beans was tested as a practical application.The ricin contents of castor beans detected by our method and the standard ELISA kit were (3.60±0.14) % and (2.75±0.07) %,respectively.These results are consistent with 1%-5% content reported in the literature[36].
3 Conclusion
In summary,PGM and nanoliposome were utilized to construct a simple,low-cost,sensitive and selective sensing strategy for POCT detection of ricin successfully.The proposed method has at least three advantages:(1) PGM,which is low-cost,‘pocket’ size,simple operation,and widely accessible to the public,could replace the expensive and sophisticated laboratory-based equipment and enable POCT detection of ricin;(2) Selectivity and sensitivity of RTB detection has been remarkably improved by this method;(3) We believe the strategy has great potential in POCT of other kind of targets based on appropriate aptamers and blockers sequences.

