Luminescent Properties of LuGG∶Ce Nanophosphor Synthesized via Co-Precipitation Method
, , , ,,
(1.The Key Laboratory of Photonic Devices and Materials, Anhui Institute of Optics and Fine Mechanics, Chinese Academy of Sciences, Hefei 230031, China; 2.School of Science and Engineering of Mathematics and Physics, Anhui University of Technology, Maanshan 243032, China)
Abstract:Ce3+ doped Lu3Ga5O12 (LuGG∶Ce) nanophosphor was synthesized via co-precipitation method. Its structured parameters were obtained by Rietveld refinement method. The morphology of the as-synthesized nanophosphor was determined with Scanning Electron Microscope (SEM). Under 365 nm excitation, the emission spectrum exhibits an asymmetry broad band with central wavelength at 438 nm and this broad band can be deconvolved into two peaks with wavelength centers at around 426 nm and 470 nm, respectively. The chromaticity coordinate of LuGG∶Ce is (0.176 9, 0.180 3), which corresponds to blue light emission. The results show LuGG∶Ce is suitable for realizing blue light emission under UV excitation and thus has potential applications in the fields of UV-excited white light emitting diodes(LEDs).
Key words:LuGG∶Ce; nanophosphor; co-precipitation method; UV excitation; white LED; luminescent property
0 Introduction
Ce3+plays a significant role in the rare earth ions. The materials which are doped by Ce3+have been widely used in the fields of solid-state lighting and scintillators owing to the parity allowed electric dipole transition of 5d→4f[1-3]. In the field of scintillators, a number of crystals activated by Ce3+, such as garnets[4-7], lead halide perovskites[8], oxyorthosilicates[9], and orthophosphates[10], have been proven to be potential fast and efficient scintillators. Among these, Ce-doped garnet (such as Lu3Al5O12(LuAG)[11]and Y3Al5O12(YAG)[12]) crystals exhibit reasonable light yield and very fast decay character and thus have been widely practical applications. Lu3Ga5O12(LuGG) host is of considerably higher physical density and effective atomic numberZvalue with respect to LuAG and YAG crystals, which can enhance the scintillator′s absorbing ability of ionizing radiation and reduce the amount of scintillator material needed in the detectors[13-14]. However, up to now, the scintillation and luminescent properties of LuGG∶Ce is rarely reported to our knowledge. Recently, a LuGG single crystal with high optical quality has been grown successfully by our group with Czochralski method and its scintillator properties will be discussed in our future works.
In the field of solid-state lighting, Ce3+-activated phosphors commonly used as blue or yellow light emitting phosphors because of the 4f1configuration of Ce3+in solids exhibits efficient broad band emission and the emission wavelength is highly relied on the host composition, lattice symmetry and crystal structure[15]. For example, YAG∶Ce emits yellow light under blue light excitation and thus has been widely used to gain white light for white light emitting diodes (LEDs) applications by combining the blue light of GaN chips[16]. However, the correlated colour temperature (CCT) of the realized white light varies with the input power and the color rendering index is poor[1,17-18]. Nowadays, a more appropriate approach for realizing white light has been aroused to meet the optimum requirements of white LEDs, namely, using tricolor phosphors (blue, green and red) excited by UV or blue light[19]. Therefore, much effort has been made to explore efficient UV or blue light excited blue, green or red phosphors.
In this study, co-precipitation method was applied to synthesize the Ce-doped LuGG nanophosphor. By using this method, the starting materials can be mixed sufficiently and reactivated highly, and also the sintering temperature is lower and sintering time is shorter than that of the conventional solid state reaction method. Meanwhile, the crystal structure, morphology as well as luminescent properties of LuGG∶Ce nanophosphor are also investigated.
1 Experimental
1.1 Synthesis of Ce3+ activated LuGG
The starting materials used for LuGG∶Ce nanophosphor preparation were metal Ga and Lu2O3with 4N purity, analytical grade Ce(NO3)3solution with Ce3+concentration 0.1 mol/L, and nitric acid solution and ammonia with analytical grade. Firstly, Lu2O3, metal Ga and Ce(NO3)3were weighed accurately at the stoichiometric molar ratio of Lu3+∶Ce3+∶Ga3+=2.997∶0.003∶5. Then Lu2O3and metal Ga were dissolved in HNO3solution, respectively, and the three ionic nitrate solutions were mixed fully. Subsequently, the mixed nitrate solution and aqueous ammonia were dropped synchronously into the quartz beaker with small volume aqueous ammonia, and the dropping rate was adjusted continuously to keep the pH value in the range of 8 to 10 in order to make Lu3+, Ga3+and Ce3+ions precipitated completely[20]. Then the obtained gelatinoids was precipitated by a centrifuge, filtered and washed with distilled water for several times. The gelatinous precipitate was dried at 100 ℃ for 24 h and then the precursor powder was obtained. Lastly, the precursor powder was ground thoroughly in an agate mortar and sintered in a platinum crucible at 1 200 ℃ for 3 h in air atmosphere, and then polycrystalline LuGG∶Ce nanophosphor was obtained.
1.2 Characterization techniques
To identify the generated phase and analyze the structure of the polycrystalline nanophosphor, powder X-ray diffraction (XRD) experiment was carried out using a Philips X’pert PRO X-ray diffractometer equipped with Cu Kαradiation. The XRD data was collected in the 2θrange of 15° to 80° and a scan step of 0.02° was applied (scanning rate: 10.83(°)/min). The morphology of the sintered LuGG∶Ce nanophosphor was observed by scanning electron microscope (SEM) (FEI SIRION200). Samples were press into a Cu holder to perform the excitation and emission spectra measurements using the Fluorolog-3-Tan spectrometer with spectral interval of 1 nm. The excitation source used to generate the excitation and emission spectra were a continuous Xenon arc lamp. The detectors were photomultipliers whose dark count rate was less than 5 counts per second. All the experiments were carried out at room temperature.
2 Results and discussion
2.1 X-ray diffraction, structure refined and SEM results
XRD patterns of LuGG∶Ce nanophosphor are shown in Fig.1(a). All the diffraction peaks are sharp and well indexed with those from ICSD#023850 (Pure LuGG), indicating that the LuGG∶Ce nanophosphor tends to completely crystallization and its structure belongs to the space group ofIa3d, and the structure of the LuGG host was unaffected by the doped Ce3+. A very low-intense additional diffraction with 2θapproximately at 30° was appeared, which could be attributed to the slightly overstoichiometry of Lu2O3. The structure parameters were refined by Rietveld refinement method using the general structure analysis software (GSAS)[19-20]and the initial parameter values are used referred to[13]. The refined results are shown in Fig.1(b) and Table 1. The lattice parameters are fitted to bea=b=c=1.221 5 nm, which is slightly larger than the 1.218 8 nm of LuGG (ICSD #023850). This could be explained by the ionic radius of Ce3+is larger than that of Lu3+and thus partially substituting the Lu3+with Ce3+may enlarge the lattice parameters. The residual factorRpand weighted residual varianceRwpare 6.7% and 9.1%, respectively, which means that the refined results are reliable. The unit cell structure and atom coordinated environments of LuGG∶Ce are shown in Fig.1(c), (d) and (e).
The particle sizes (d) of the as-synthesized LuGG∶Ce nanophosphor can be estimated by Scherrer′s formula:d=0.89λ/Bcosθ, whereλis the X-ray wavelength of Cu Kαradiation (0.154 nm),Bis the full width at half maximum (FWHM) of the respective diffraction peak, andθis the diffraction angle. According to Scherrer′s formula, the average particle size of the as-synthesized LuGG∶Ce nanophosphor was calculated to be about 75 nm. In order to investigate the surface morphologies of the synthesized phosphor, the SEM characterization was carried out. Fig.1(f) presents the SEM image of the as-synthesized LuGG∶Ce nanophosphor. It is obviously that the phosphor distributes homogeneously with regular morphology, and has slightly aggregation. It is further noted that the particle sizes of LuGG∶Ce nanophosphor sintered at 1 200 ℃ are between 60 nm and 80 nm, which is in agreement with the average size obtained by Scherrer′s formula.
2.2 Theoretical density of LuGG∶Ce
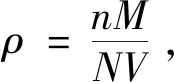
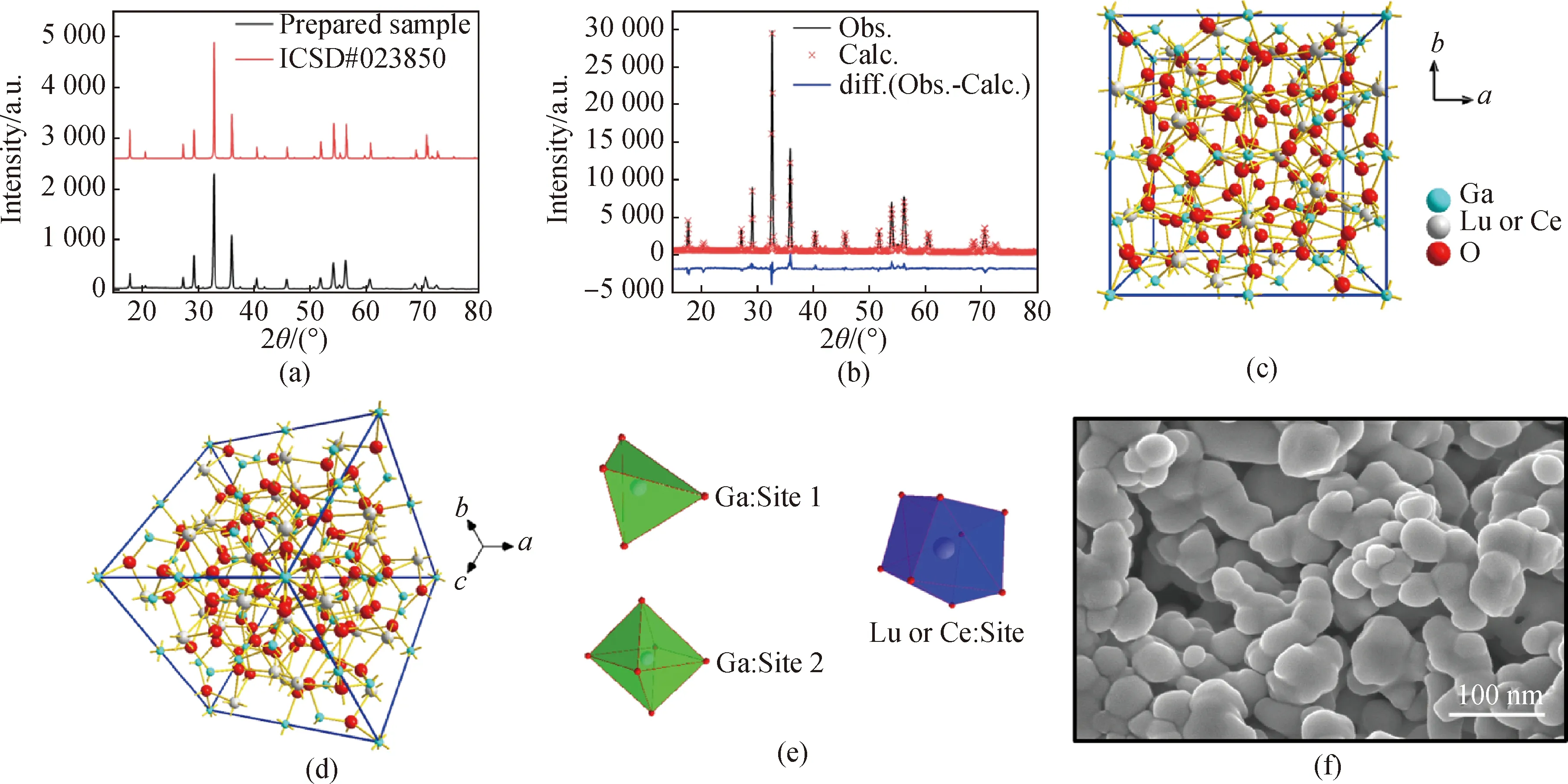
Fig.1 (a) XRD patterns of the synthesized LuGG∶Ce nanophosphor; (b) XRD Rietveld refinement for the LuGG∶Cenanophosphor (Obs.: the experimental data; Calc.: the calculated pattern; diff.: the curve of the difference between Obs. and Calc.);(c) unit cell of LuGG∶Ce: view along [001] orientation; (d) unit cell of LuGG∶Ce: view along [111] orientation; (e) atoms coordination environments in LuGG∶Ce unit cell; (f) SEM image of LuGG∶Ce nanophosphor
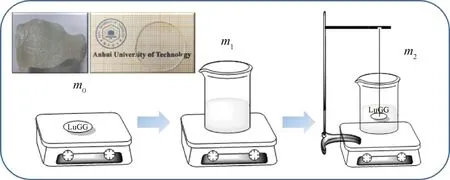
Fig.2 Schematic diagram of the LuGG crystal density measurement (insert: photograph of LuGG crystal)
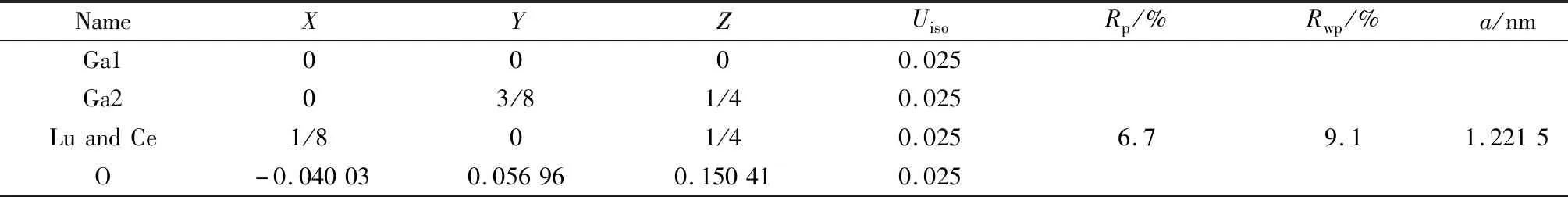
Table 1 Crystal structure parameters of LuGG∶Ce obtained by Rietveld refinement
2.3 Luminescent properties
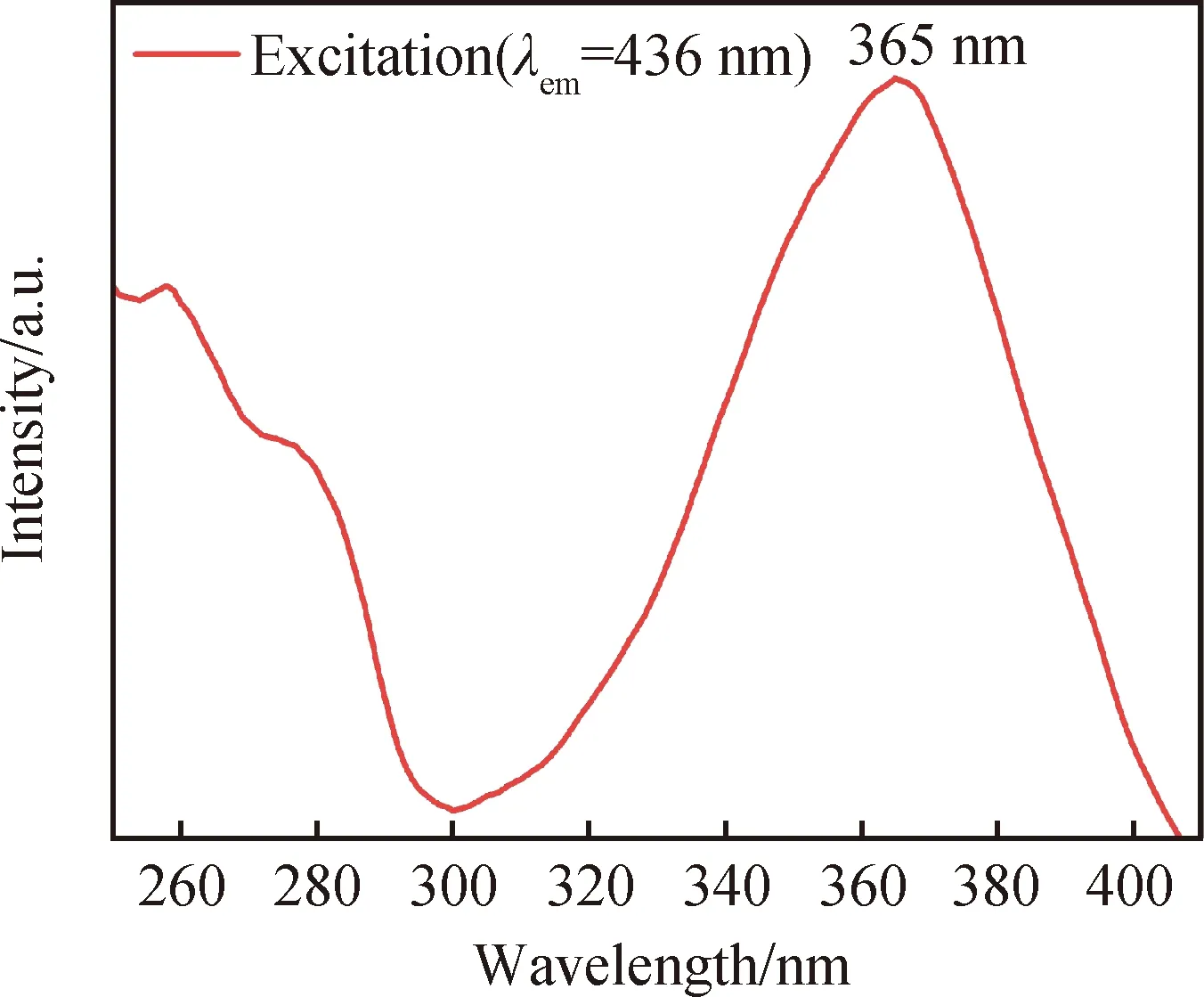
Fig.3 Excitation spectrum of LuGG∶Ce nanophosphor
Trivalent Ce3+ion has only one electron in the 4f orbit, and its 5d state is strongly affected by crystal field of the host. Because the Ce3+ions substitute for Lu3+at sites of D2symmetry in LuGG host, all the degeneracy except Kramer′s degeneracy is removed and the excited 5d states are split into five sub-bands in maximal[21]. Fig.3 shows the excitation spectrum of LuGG∶Ce nanophosphor for emission monitored at 436 nm. It can be seen that the excitation spectrum consists of two parts: one is below 300 nm, there are two weak bands with wavelength centers at around 250 nm and 280 nm, respectively; the other one is from 300 nm to 400 nm, which is a broad and strong band with wavelength center at around 365 nm (larger than the 340 nm of YAG∶Ce[22]). All the two excitation bands can be ascribed to the optically allowed 5d04f1→5d14f0transitions.
The emission spectrum of LuGG∶Ce under the excitation of 365 nm is shown in Fig.4(a). It can be seen that the emission spectrum exhibits an asymmetry emission band with wavelength center located at around 438 nm, which is due to the transition occurred from lowest 5d level to 4f ground state of Ce3+. Additionally, this broad emission band also can be deconvolved into two peaks with wavelength centers at around 426 nm (23 474 cm-1) and 470 nm (21 277 cm-1), respectively. The deviation of this two deconvolved bands (the energy difference between the two spin-orbital split states2F7/2and2F5/2) is 2 197 cm-1which is accordance with the theoretical energy difference of2F7/2and2F5/2of Ce3+and close to the 2 000 cm-1of Ce∶YAG reported in the literature[23]. The schematic of the energy transfer mechanism in LuGG∶Ce nanophosphor is shown in Fig.4(b).
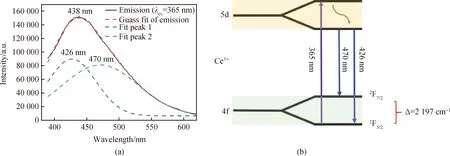
Fig.4 (a) Emission spectra of LuGG∶Ce nanophosphor; (b) schematic of the energy transfer mechanism in LuGG∶Ce nanophosphor
The CIE chromaticity coordinate for LuGG∶Ce emission under excitation at 365 nm is shown in Fig.5. The chromaticity coordinate for LuGG∶Ce is (0.176 9, 0.180 3), which is corresponding to blue emission, suggesting that LuGG∶Ce is suitable for realizing blue light emission using UV excitation and thus promising for white LEDs application under UV excitation.
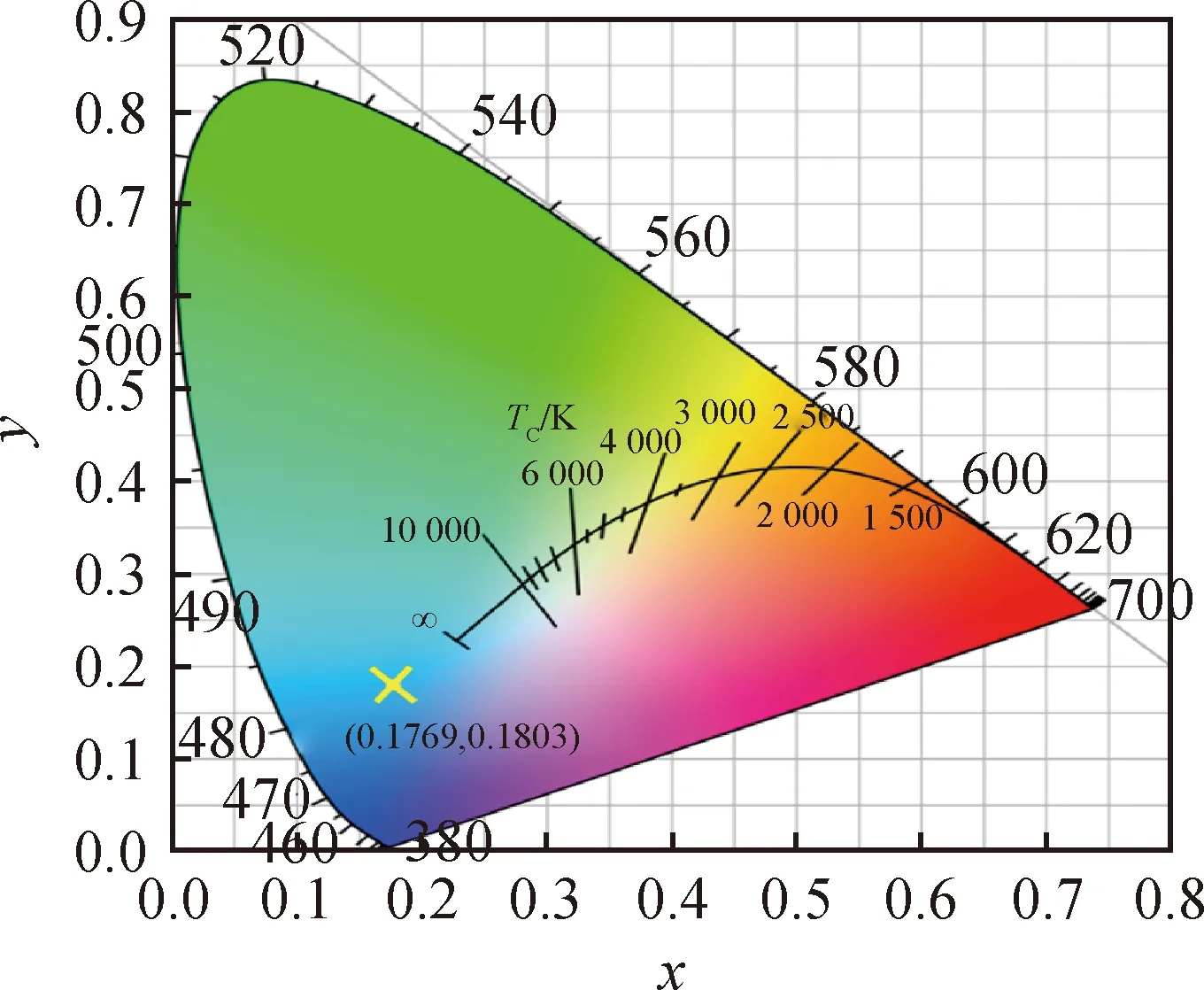
Fig.5 Chromaticity coordinate of LuGG∶Ce nanophosphor
3 Conclusion
Using the metal Ga, Lu2O3and Ce(NO3)3as starting materials and aqueous ammonia as precipitator, LuGG∶Ce nanophosphor was synthesized via co-precipitation method. By Retvield refinement method, the lattice parameter of the as-prepared LuGG∶Ce is obtained as 1.221 5 nm. The excitation bands are ascribed to the electron transitions from 4f ground state to different excited 5d levels of Ce3+. Under 365 nm excitation, broad emission band with central wavelength at 438 nm is observed and the chromaticity coordinate for this emission band is (0.176 9, 0.180 3), which corresponds to blue emission. The results provide the structural and luminescent properties of LuGG∶Ce, which are essential for further investigation of this material for potential application in the fields of UV-excited white LEDs and scintillators.

