Gadoxetic acid magnetic-enhanced resonance imaging in the diagnosis of cholangiocarcinoma
Riccardo Inchingolo, Cesare Maino, Marco Gatti, Eleonora Tricarico, Michele Nardella, Luigi Grazioli, Sandro Sironi, Davide Ippolito, Riccardo Faletti
Abstract The use of liver magnetic resonance imaging is increasing thanks to its multiparametric sequences that allow a better tissue characterization, and the use of hepatobiliary contrast agents. This review aims to evaluate gadoxetic acid enhanced magnetic resonance imaging in the diagnosis and staging of cholangiocarcinoma and its different clinical and radiological classifications proposed in the literature. We also analyze the epidemiology, risk factors in correlation with clinical findings and laboratory data.
Key words: Hepatobiliary contrast materials; Gadoxetic acid; Cholangiocarcinoma; Magnetic resonance imaging; Liver; Cirrhosis
INTRODUCTION
Epidemiology and risk factors
Cholangiocarcinoma (CCA) is the most widespread primary biliary tract neoplasia[1]and the second most common primary hepatic tumor, considering that hepatocellular carcinoma (HCC) is the most frequent[2,3]. CCA represents 3% of all gastrointestinal tumors, typical of elderly adults, with a peak incidence in the 7thdecade.
Recent updates showed a higher number of deaths due to CCA, in particular surpassing HCC[2], first of all, because the diagnosis of CCA occurs at an advanced stage.
The majority of cases is sporadic, however, all medical conditions that lead to chronic inflammation are considered risk factors, first of all, primary sclerosing cholangitis (PSC), viral hepatitis, biliary malformations, hepatolithiasis, bile ducts adenomas, biliary-pancreatic abnormal junction, parasite infections (O. viverrini,C. sinensis), chemical products (e.g.Thorotrast), and, finally, cirrhosis[4].
In a recent metanalysis, Bergquistet al[5]overviewed all possible risk factors for CCA: It was established a high risk for patients with choledochal cysts [Risk Ratio (RR): 36.9-47.1], cirrhosis (RR: 22.9), choledochal lithiasis (22.5-34.0) and hepatolithiasis (RR: 6.7-16.5), while the lower risk was evaluated in case of liver parasitosis (RR: 4.7), viral hepatitis (RR: 3.17-5.10), especially HCV, diabetes mellitus type II (RR: 1.89), obesity (RR: 1.56) and alcohol use (RR: 2.81). PSC represents a high-risk factor in developing CCA, with a range from 7% to 14%[6-8]. IBD is suggested to be a risk factor for CCA (RR: 1.72-3.95).
Clinical findings and laboratory data
In the early onset, patients with CCA are asymptomatic or manifest no typical symptoms. The most common clinical symptom in patients with extrahepatic CCA is jaundice. Other non-specific symptoms can be present such as weight loss, abdominal pain, night sweats, dark urine, fatigue, vomiting, pruritus, increase of cholestasisrelated lab parameters (Alanine transaminase, Aspartate transaminase, Gammaglutamyltransferase, Bilirubin), increased prothrombin time, reduction in fat-soluble vitamins[9].
The protein CA 19-9 can be elevated in the majority of patients with CCA. In particular, the value of CA 19-9 higher than 100 U/mL in PSC patients has very good sensitivity and specificity for the diagnosis of CCA (89% and 86%, respectively). However, according to literature data and clinical experience, CA 19-9 values alone are not sufficient in the diagnosis of CCA. Besides, in patients without PSC, a CA 19-9 value higher than 1000 U/mL has been linked to metastatic and unresectable CCA[10]. On the other hand, Carcinoembryonic Antigen (Carcinoembryonic antigen) and CA 125 are non-specific markers.
Classification
There are many classifications of CCA based on clinical, radiologic and pathological features.
CCA can be anatomically differentiated in intrahepatic and extrahepatic forms. Depending on anatomical location it’s possible to recognize different types of CCA[11]: (1) Intrahepatic cholangiocarcinoma (iCCA), involving the periphery of the secondorder bile ducts; (2) Extrahepatic cholangiocarcinoma: Perihilar cholangiocarcinoma (pCCA), involving the hepatic duct (left or right) or the junction; and (3) Distal cholangiocarcinoma (dCCA) involving the common bile duct.
The “Liver Cancer Study Group of Japan” classi cation is considered the most complete one because it's based on appearance, biological behaviour, growth characteristics, clinical prognosis, and imaging characterization[12]. The “Liver Cancer Study Group of Japan” classification permits also to stage the disease, evaluating tumor size, vascular, and/or serosal invasion (Table 1).
Intrahepatic CCA presents three patterns of growth: Mass forming (MF-CCA), periductal infiltrating (PI-CCA), and intraductal growing (IG-CCA) (Figure 1)[11,13].
MF is the most common, accounting for 65% of iCCA, presenting as a mass, usually large and characterized by central necrosis or scarring. The typical presentation is a solitary mass lesion, more frequently located in the right lobe (35%), followed by the left lobe (22%). Finally, 12% is centrally located, and 31% is multifocal[11].
Accounting for 6% of iCAA, PI-CCA develops lengthways within the wall of the bile duct and spreads along the portal tracts. The affected ducts show wall thickening and progressive narrowing[14].
IG-CCA, accounting for 4% of iCAA, develops as a polypoid or papillary mass within the bile duct lumen. A partial biliary obstruction can be found out, due to a large amount of mucin yielded[12,13].
A combined form, composed by PI and MF types, represents a 25% of iCCA[11].
According to the site of involvement and histologic features, iCCA is classified into two types: Perihilar large duct, and peripheral small duct. This new classification, in particular taking into account location, clinical and genetic factors, has a potential role in predicting prognosis[14].
Extrahepatic CCA can be further subdivided into perihilar form and distal form, according to Bismuth-Corlette classification (Table 2), in particular, useful to assess longitudinal spread. The American Joint Committee on Cancer defined classification to evaluate radial spread because perihilar and distal forms differ in presentation, natural history, and management.
Pathological findings
CCA has been classified into two wide groups: (1) The classical one, represented by adenocarcinoma, subdivided in by well, moderately, and poorly differentiated; and (2) The variant forms (e.g., adenosquamous, squamous carcinomas). However, a newer histological classification has been introduced in clinical practice[15,16].
This most recent classification divides CCA into the conventional type (ductal), the ductular type, the intraductal type, and rare variants. Moreover, in the case of iCCA, the ductal type can be subdivided, according to bile duct involvement, into small and large type. While ductular type tumors are present exclusively in iCCA, the intraductal type can be seen in iCCA, pCCA, or dCCA. Additionally, ductular and small bile duct CCA may be grouped as the small duct type[16].
Many authors demonstrated that histologic data represent an important prognostic factor of iCCA. Shamiset al[17]classified iCCA into type 1 and type 2, according to on mucin production and immunophenotype, establishing a link between histopathology and prognosis[17].
Management
To date, liver resection and liver transplantation are the only management options available for CCA. Imaging plays a fundamental role in the preoperative staging to establish the best treatment approach for each patient.
The pathologic staging system, developed for ductal CCA, has a limited value in the assessment of extra-hepatic CCA. Moreover, tumour node metastases classification does not correlate with resectability in patients with extrahepatic CCA while, conversely, the Memorial Sloan-Kettering staging system permits to determine which patients are suitable for resection and the prognosis (Table 3).
MAGNETIC RESONANCE PROTOCOL
Nowadays magnetic resonance imaging (MRI), due to its high intrinsic contrast resolution, in particular in the evaluation of biliary tree, is considered the best imaging technique to diagnose and stage CCA. The diagnostic potential of magnetic resonance cholangiopancreatography (MRCP) is now almost as good as ERCP, eliminating the need for most invasive studies[18].
An optimal protocol for CCA evaluation should include conventional T1 in- and out-of-phase imaging, T2-weighted sequences in axial and coronal planes, diffusionweighted imaging, completed with contrast-enhanced sequences. MRI should be performed on a high-field scanner (1.5T or 3T)[19]. According to clinical practice, the dynamic sequences should be performed by using standardized time points or withbolus-triggering technique.
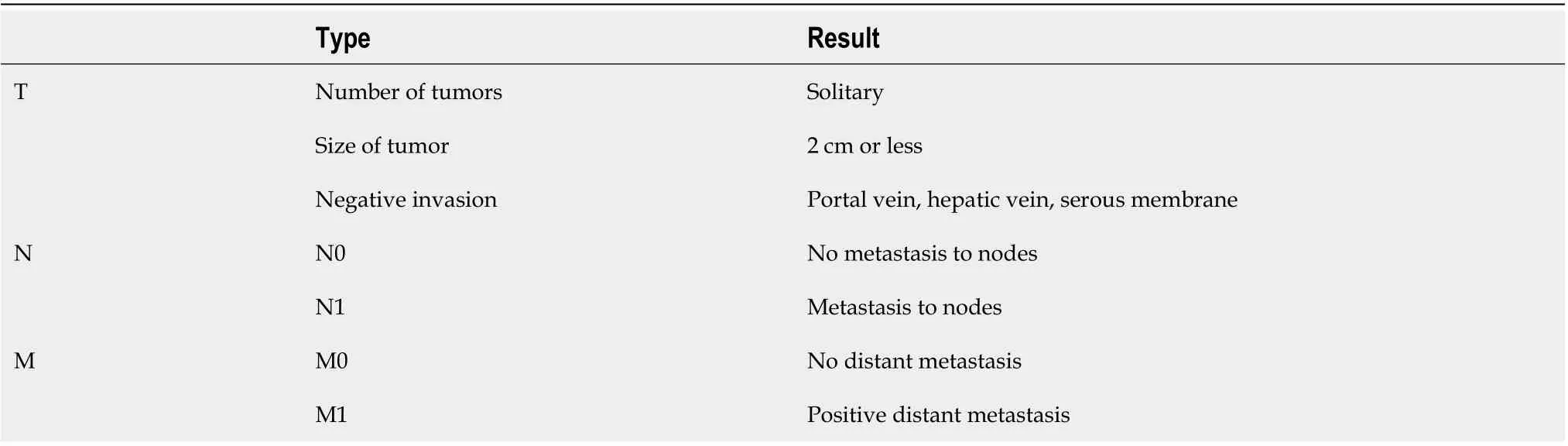
Table 1 Liver cancer study group of Japan tumour node metastases staging for intrahepatic cholangiocarcinoma
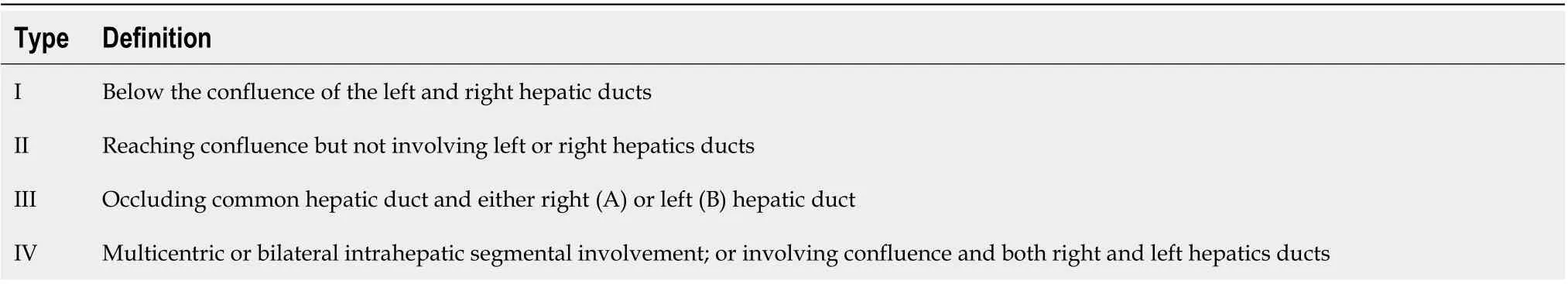
Table 2 Bismuth-Corlette classification system for perihilar cholangiocarcinoma
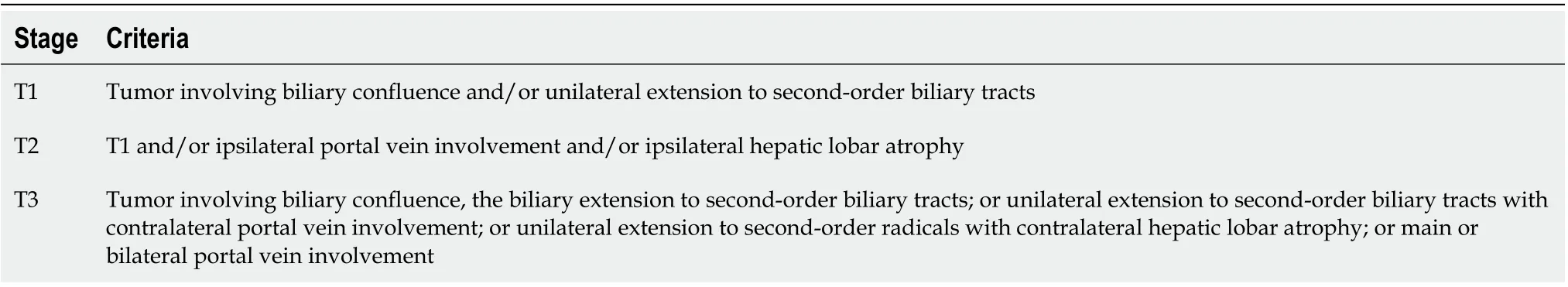
Table 3 Memorial Sloan Kettering T stage for hilar cholangiocarcinoma
MRCP
According to literature and clinical practice, MR study should be completed with MRCP sequences that nowadays are considered the non-invasive reference for biliary system assessment. MRCP is a non-contrast MR technique in which the contrast between bile and adjacent tissues, showing long and short T2 relaxation time respectively, is accentuated by using heavily T2-weighted sequences. MRCP sequences should be aligned to the common bile duct in the head of the pancreas, by using axial T2-weighted images. The patient is asked to breathe regularly during acquisition, which takes between 3-5 min to acquire.
Thin multi-slice MRCP allows to obtain a high-resolution visualization of the biliary tree by 3D-images in particular with multiplanar reconstructions (MPR) and maximum intensity projection reformation can be generated to better assess the hepatic biliary tree and pancreatic ducts.
As a general recommendation, patients should fast for at least 4 h to reduce gastrointestinal peristalsis and gastric secretions. Moreover, this approach allows for obtaining a gallbladder distention. Negative contrast agents permit to reduce fluid signal in the stomach and duodenum, such as blueberry juice or iron oxide[20].
MR imaging findings and contrast agents
On T1-weighted images, CCA shows a hypo-to isointense signal, while on T2-weighted images the signal is slight to high hyperintense, thus reflecting tumor components, in particular fibrosis, mucin, and necrosis.
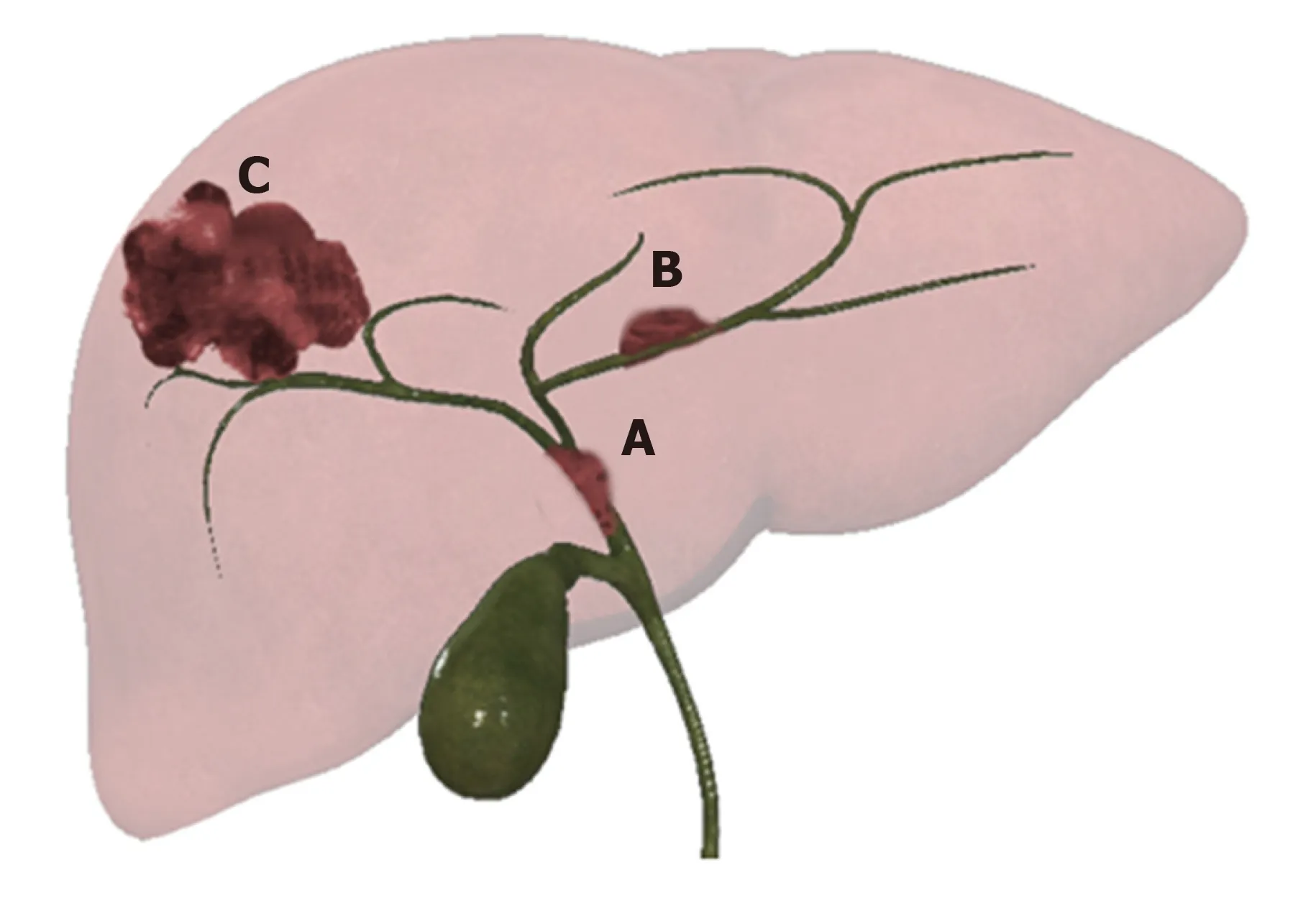
Figure 1 Graphic representation of 3 different patterns of growth of intrahepatic cholangiocarcinoma. A: Intraductal growingcholangiocarcinoma; B: Periductal infiltrating-cholangiocarcinoma; and C: Mass forming-cholangiocarcinoma.
The dynamic enhancement pattern after gadolinium-based contrast administration may be variable. In the arterial phase, it shows a rim enhancement while a progressive, centripetal, enhancement can be appreciable in the delayed phases, due to its intrinsic fibrotic appearance. If the tumor is predominantly fibrotic, enhancement may only be visible in the delayed phase.
Ancillary features include vascular encasement, biliary obstruction, lobar atrophy, capsular retraction, and lymphadenopathy.
Dynamic imaging can be performed with both extracellular and hepatocyte-specific contrast media. Liver post-contrast signal intensity is greater with the use of hepatocyte-specific agents compared with traditional gadolinium-based extracellular contrast agents. In this setting, CCA appears as a hypointense lesion in the hepatobiliary phase (HBP), considering the absence of functioning hepatocytes[21].
ROLE OF Gd-EOB-DTPA
Gd-EOB-DTPA (Bayer Schering Pharma, Berlin, Germany) is a gadolinium-based MRI hepatocyte-specific contrast agent. It shows a biphasic mechanism of action: First distribution in the extracellular space and then selective uptake by functioning hepatocytes and biliary excretion through the organic anionic transporting polypeptide (OATP8). In patients with preserved liver function, the hepatic uptake can be evident after 20 min and lasts for several hours after EOB-DTPA injection (i.e.the hepatobiliary phase).
This phenomenon allows evaluating both vascular features and the functional status of the nodules with a single examination. Indeed, the nodules with low or no OATP expression (i.e.CCA and most of the other hepatic lesions) show no hepatocytespecific contrast agent s uptake and appear hypointense in the HBP.
Regarding the Gd-EOB-DTPA administration, the European society of gastrointestinal and abdominal radiology consensus statement provides recommendations[22]. The approved dose is 0.025 mmol/kg with a flow-rate of 1-2 mL/s followed by a saline flush. Dynamic sequences should be obtained by using a bolus triggering technique. Optimal multiphasic dynamic contrast-enhanced imaging, with fewer artifacts, is feasible using multi-arterial phase imaging[23]and in fact, its role in the evaluation of hepatic lesions particularly HCC has been well studied[24,25].
As mentioned in the first chapter, the imaging features of CCA have been classified into four different growth patterns: MF-CCA, PI-CCA, IG-CCA, and mixed type (MFCCA and PI-CCA). There is a close correlation between growth pattern and anatomical location: PI-CCA and IG-CCA are uncommon in iCCA and are usually seen in pCCA and dCCA, while the majority of CCA arising in the large perihilar bile duct shows a mixed type growth pattern.
Several characteristics of EOB-DTPA MR cholangiography allow differential diagnosis between different subtypes of cholangiocarcinoma with high accuracy[26].
MF-CCA
The vascular dynamic enhancement pattern of MF-CCA with EOB-DTPA is similar to CT contrast agents and the non-specific extracellular gadolinium-based agents (Figure 2): An arterial ring-like or band-like contrast enhancement in the early dynamic phase (arterial phase and late portal phase) with delayed progression (a progressive or concentric filling in the portal and delayed phase). This enhancement pattern is related to the fibro-cirrhotic nature of the disease with a fibrous central scar. A less frequent pattern is an HCC-like hypervascularity[27], which is more common in small lesions due to relatively less fibrous tissue.
Contrary to the extracellular contrast agents, sometimes the delayed EOB-DTPA enhancement is less evident in the transitional phase. The transitional phase is stated as the time frame between the portal venous phase and the HBP, representing the transition of the contrast media from the extracellular space into bile ducts[28]. Therefore, the lesions appear hypointense relative to the surrounding hyperintense liver parenchyma (pseudo-wash-out).
In the HBP phase, MF-CCA is usually heterogeneously hypointense due to the absence of OATP8 (i.e., absence of Gd-EOB-DTPA uptake). An additional feature of MF-CCA in the HBP is the presence of target sign (i.e., central hyperintensity less than surrounding parenchyma associated with a peripheral hypointense rim)[29]that reflects the presence of fibrosis. However, those with HCC-like arterial hyperenhancement seem to have less fibrosis in comparison to ones with typical rim-enhancement[30].
Other typical imaging features of MF-CCA are capsular retraction, bile duct dilatation, vascular involvement, and central scars[31]. All these features can be reliably evaluated using Gd-EOB-DTPA-enhanced MR cholangiography and in particular in a pre-surgical setting with 3D T1-weighted spoiled gradient-echo images with high flip angle (a flip angle more than 20° is recommended and 35-40° seemed to be the best) in the HBP. It revealed an improvement of diagnostic accuracy compared to the conventional magnetic resonance cholangiopancreatography with only T2 acquisition[32].
PI-CCA and IG-CCA
PI-CCA presents an irregular wall thickening with stenosis or obliteration of the involved bile ducts and upstream dilation (Figure 3). Typically this tumor enhances slowly and gradually and at the delayed phase reveals an enhancement peak[31]. In the HBP, it is difficult to distinguish PI-CCA from enhanced liver parenchyma. Due to the impaired function of biliary cells, the HBP showed almost no EOB-DTPA in the common bile duct.
IG-CCA may present a variety of appearances depending on the ductal dilation (diffuse, cystic, or minimal dilation), the amount of mucin, and the extension (focal or diffuse) (Figure 4). This growth pattern has a typical heterogeneous arterial enhancement with delayed progression[33]. In HBP the polypoid nodules were homogeneously hypointense, and Gd-EOB-DTPA cannot be excreted into the dilated bile ducts, because these ducts are excluded from the biliary tree. Thus MRCP will show only bright and dilated excluded duct.
It should be underlined that the enhancement, especially in the PI-CCA and IGCCA is better seen on MRI than on CT due to the higher contrast resolution[27]. In the HBP, the contrast between the healthy liver parenchyma and CCA may allow a more accurate assessment of tumor extent, helping to address patients to the correct management and, consequently, to evaluate prognosis[34,35].
Advantages of EOB-DTPA
The use of EOB-DTPA is also important in terms of prognosis: The presence of daughter nodules[36]and intrahepatic metastasis[37]are poor prognostic factors. The high signal intensity of liver parenchyma in the HBP allows improving lesion conspicuity and detection of satellite nodule and intrahepatic metastasis[35]. Moreover, a recent study highlighted the role of capsule penetration, (i.e., pathologically defined serosal perforation or invasion) and tumor size; the latter better define with low interobserver variability in the HBP, as prognostic factors for postoperative outcomes in MF-CCA[38].
Finally, HBP seemed to be effective in estimating regional liver functional reserve[39]and liver volume to guide surgical procedures. This technique has been reported to be also useful in the setting of surgical complications, in particular for bile leaks detection after hepatobiliary surgery[40].

Figure 2 Mass forming cholangiocarcinoma. On a background of the alcohol-related cirrhotic liver, there is a 2 cm lesion in segment VIII with capsular retraction (white arrow). The lesion is hypointense on T1 IP and OP images. A and B: Slightly hyperintense on T2 and SPAIR; C and D: DWI restriction; E: The dynamic enhancement pattern after gadoxetic acid administration is a peripheral rim of enhancement in arterial and portal phase; G and H: Progressive centripetal enhancement on the delayed phase; I: The lesion demonstrates hypointensity in the hepatobiliary phase; L: The patient underwent percutaneous liver biopsy. Biopsy specimen stained with hematoxylin and eosin respectively at 4 × and 20 ×; M and N: Showed an adenocarcinoma (orange arrow) on a background of the cirrhotic liver (orange arrow); N: The magnification better depicts the appearance of the adenocarcinoma with the tubular aspect. The immunohistochemistry confirmed the positivity for PDX1 and CK7 and negativity for CDX2 and CK20, in keeping with cholangiocarcinoma.
The use of EOB-DTPA could be useful also for differential diagnoses[22]. A small nodule (< 3 cm) in a patient at risk for HCC without typical hallmark imaging features cannot be certainly differentiated from CCA. However, a recent meta-analysis[41]identified eleven MRI features for differentiating HCC from MF-CCA such as capsular retraction, rim enhancement in the arterial phase, progressive enhancement in the delayed phases, target appearance in diffusion-weighted imaging, and, finally, bile duct dilation. These features are included in Liver Imaging Reporting and Data System (Liver imaging reporting and data system) as the LR-M category, indicating a probably or malignant lesion without specific HCC features[42].
Moreover, it has been recently shown[27]that the presence of thicker arterial ring enhancement, during the EOB-MRI study, associated with dot-/band-like internal enhancement could help differentiate MF-CCA carcinoma from IG-CCA and PI-CCA. Moreover, Gd-EOB-DTPA with MRCP can differentiate between the different growth patterns.
A recent meta-analysis[43]indicated that MRI with extracellular contrast agent had a sensitivity of 94% and specificity of 71% with an area under the curve of 0.92, comparable to CT in the evaluation of the resectability of hilar cholangiocarcinoma, but the use of EOB-DTPA seemed to increase both sensitivity and specificity. However, both CT and MRI show low sensitivity for nodal status while PET/CT appears to be the best technique, however, it has no clear role for evaluating surgical resectability.
Overall, MRI with EOB-DTPA provides an accurate assessment of tumor extent, biliary tree, vessels, and invasions of adjacent structures and is, therefore, a fundamental evaluation system before surgery which also allows differential diagnosis and providing prognostic information.
CONCLUSION
Cholangiocarcinoma is the second most common hepatic tumor and is often asymptomatic and can be diagnosed late. Regardless of its intra or extrahepatic presentation, it requires an imaging technique that allows evaluating both anatomical sites. MRI, strengthened by a multiparametric study, with the use of cholangiographic sequences and with the use of the hepatospecific contrast medium can allow a complete diagnostic evaluation to manage the best therapeutic option.
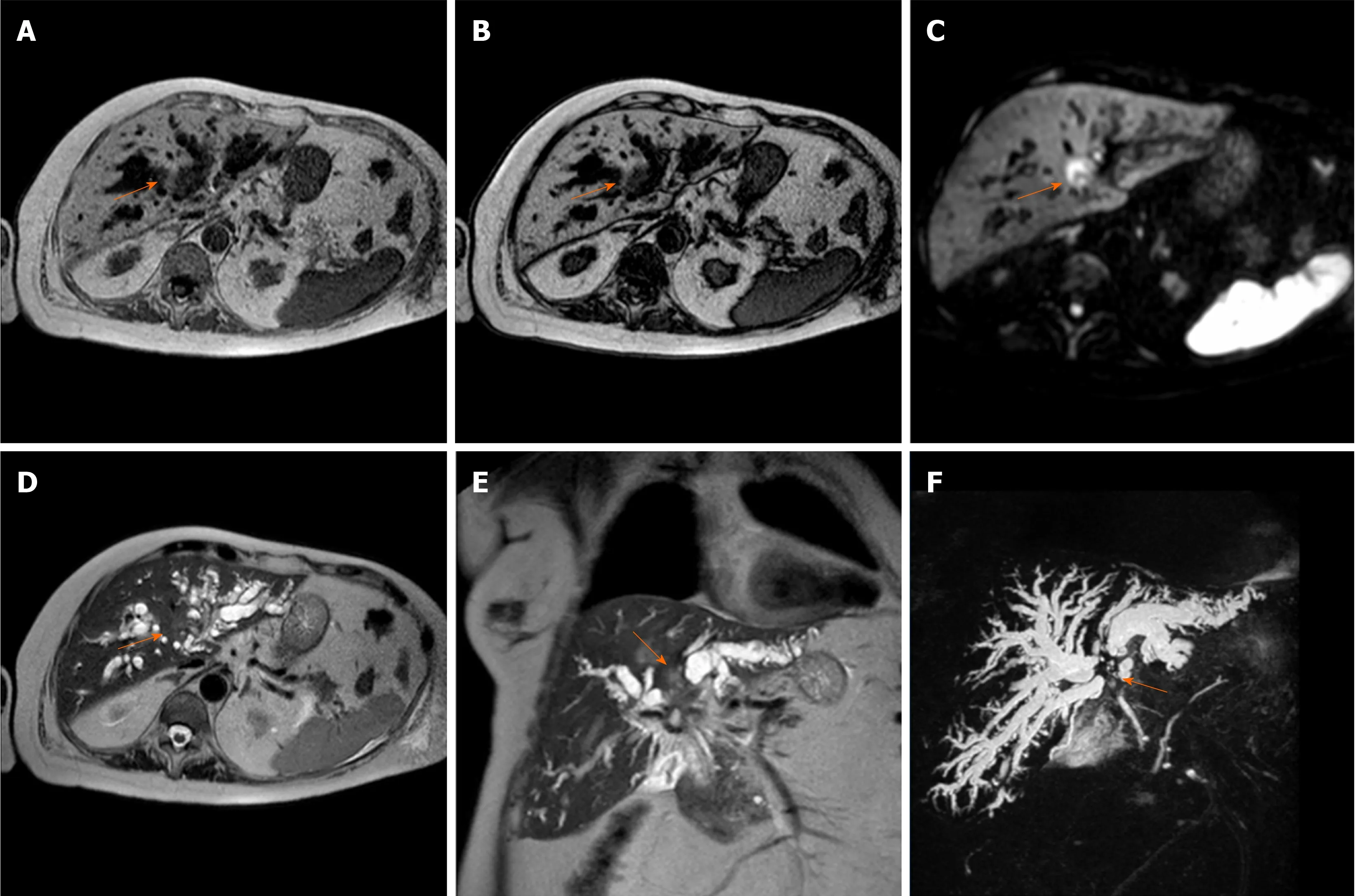
Figure 3 Perihilar cholangiocarcinoma. A case of pCCA (orange arrow), arising at the junction with the involvement also of in the right and left hepatic duct, in an 86-year-old female. The magnetic resonance cholangiopancreatography is reported. The lesion is a hypointense mass in T1-weighted images. A and B: Hyperintense in the higher b-value of diffusion-weighted images; C: Mild hyperintense on T2-weighted images; D and E: Finally the use of the 3D respiratory-triggered heavily T2-weighted FSE sequences; and F: Maximum intensity projection reconstruction is useful to detect the strictures at the junction of the biliary tree.
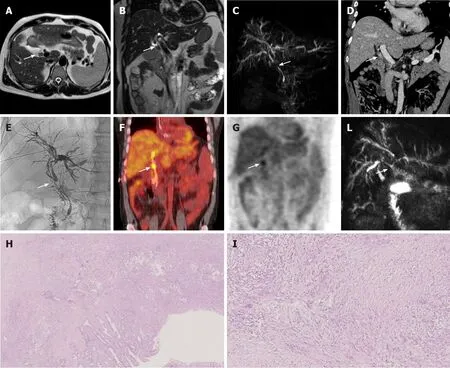
Figure 4 Distal cholangiocarcinoma. A case of distal cholangiocarcinoma (white arrow) involving the common bile duct in a 52-year-old male patients with primary sclerosing cholangitis and ulcerative colitis. The patients underwent magnetic resonance cholangiopancreatography with axial. A: Coronal; B: T2-weighted images and a maximum intensity projection reconstruction of the 3D respiratory-triggered heavily T2-weighted FSE sequences; C: Then contrast-enhanced Computed Tomography; D: For staging the disease. Moreover, a percutaneous transhepatic biliary drainage for diagnostic confirmation with tissue collection was performed; E: And finally, PET scan; F and G: It was used to resolve a diagnostic dilemma about a pulmonary nodule. The patient underwent surgery; H and I: It was reported the surgical resection specimen at histology stained with hematoxylin and eosin respectively at 2 × and 10 ×: The tumor was an adenocarcinoma, with an invasion of the wall of the bile duct for 6 mm with also perilesional papillary epithelial dysplasia, the final stage according to the VIII edition of the Union for International Cancer Control is T2, N0. L: The maximum intensity projection reconstruction of the 3D respiratory-triggered heavily T2-weighted FSE sequences, 6 mo after the surgery: The irregular dilatation of the intrahepatic biliary tract persists (in patients with primary sclerosing cholangitis) and the patency of the biliodigestive anastomosis is highlighted with the white arrow.
ACKNOWLEDGEMENTS
We wish to thank Prof. Adam Cassels for his contribution to amending and polishing the language of this manuscript.
 World Journal of Gastroenterology2020年29期
World Journal of Gastroenterology2020年29期
- World Journal of Gastroenterology的其它文章
- Rno_circ_0005139 regulates apoptosis by targeting Wnt5a in rat anorectal malformations
- Impact of mobile health and medical applications on clinical practice in gastroenterology
- Role of long noncoding RNA-mediated competing endogenous RNA regulatory network in hepatocellular carcinoma
- Multifocal gastrointestinal epithelioid angiosarcomas diagnosed by endoscopic mucosal resection: A case report
- Role of nutritional status and nutritional support in outcome of hepatitis B virus-associated acute-onchronic liver failure
- Dysregulation of microRNA in cholangiocarcinoma identified through a meta-analysis of microRNA profiling
