Measuring frailty in patients with severe aortic stenosis: a comparison of the edmonton frail scale with modified fried frailty assessment in patients undergoing transcatheter aortic valve replacement
Francisco J Romeo, Maximiliano Smietniansky, Mariela Cal, Cristian Garmendia, Juan M Valle Raleigh,Ignacio M. Seropian, Mariano Falconi, Pablo Oberti, Vadim Kotowicz, Carla R. Agatiello,Daniel H Berrocal
1Division of Interventional Cardiology,Hospital Italiano de Buenos Aires, Buenos Aires, Argentina
2Division of Internal Medicine and Geriatrics, Hospital Italiano de Buenos Aires,Buenos Aires, Argentina
3Division Cardiology, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina
4Division of Cardiovascular Surgery,Hospital Italiano de Buenos Aires, Buenos Aires,Argentina
Keywords: Aortic stenosis; Frailty; Transcatheter aortic valve replacement
Frailty is generally defined as a clinical syndrome of decreased physiologic reserve which drives to increased vulnerability and susceptibility to different stressors together with poor recovery to homeostasis.[1]The relevance of frailty status in a wide range of prospective cohorts is mostly related to an increasing burden in both mortality, hospital readmissions, disability, and falls.[2,3]
In aortic stenosis (AS) population, the spectrum of frail patients significantly differs according to which frailty scale is used. Scales such as Rockwood Frailty Index,[4]Canadian Study of Health and Aging,[5]Katz ADL,[6]6 min walk test,[7]up and go,[8]gait speed[9]have been described for improving risk stratification in transcatheter aortic valve replacement (TAVR) patients alone or in combination. More than 25 subjective or objective frailty markers have been developed but because of a lack of consensus, there is variability among studies and confusion about which marker to use.
The Modified Fried Frailty Assessment (MFFA) has been widely validated in different clinical scenarios and, in TAVR, has shown incremental prognostic value for functional decline including discharge to a rehabilitation facility after TAVR[10]and 1-year mortality.[4–11]
However, the assessment involves five domains and requires special equipment, though hard to apply in clinical practice for cardiologists involved in the care of valvular patients.
The Edmonton Frail Scale (EFS), a simple frail assessment that involves 10 questions and one physical assessment (‘timed up and go’) were recently described in a small cohort of TAVR patients identifying patients likely to experience longer hospital stays.[12]The aim of this clinical experience sought to compare the prevalence of frailty using the two scales, and agreement between them.
A retrospective analysis of 128 patients who underwent TAVR at an Argentinean academic hospital from July 2014 to July 2018 was performed. All patients met criteria for severe AS defined by the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.[13]
Ours is a tertiary care academic medical center in which all possible pre-TAVR candidates are discussed by a multidisciplinary heart valve team (cardiac surgeons, interventional cardiologists, cardiologists specialized in multimodality imaging and geriatricians) on a weekly basis.
Final decisions regarding TAVR, surgical aortic valve replacement (SAVR) or futility patients are integrated into a core clinical discussion with frailty assessments, pre-TAVR multislice computed tomography (MSCT) measurements,cardiac biomarkers, and echocardiographic data.
Patient comorbidities were collected using the definitions provided by the STS data collection system.[14]
Frailty status was assessed by seven trained geriatricians using a MFFA which incorporated five domains of frailty:unintentional weight loss, exhaustion, muscle weakness,slowness while walking and low levels of activity. Each domain was scored individually in a binary fashion as normal or summed to generate a frailty score. We classified patients with a frailty score of ≥ 3/5 as frail, whereas those with a score of 1-2/5 were classified as pre-frail, and those with a score of 0/5 were classified as non-frail.[1]
Each frailty domain was scored by commonly accepted methods of the MFFA. Patients with self-reported unintentional weight loss (5% of body weight lost unintentionally in the prior year) were considered as “shrinking”.
We measured 5-m gait speed (the time required for the patient to complete a 5-m walk test), and we defined slowness as a 5-m gait speed slower than the twentieth percentile from a cohort of over 5,000 community-dwelling adults >65 years stratified by gender and height.[1]Grip strength for defining sarcopenia (i.e “weakness”) was not evaluated due to the risk of syncope in this population. However, we used the SARC-F, a questionnaire with five questions, which has high specificity, albeit low sensitivity, to identify patients with sarcopenia.[15–17]
Finally, we assessed functional performance using the Katz Index of Independence in Activities of Daily Living(ADL) scale which is a patient-reported 6-point scale measuring dependence with 6 ADLs (bathing, dressing, hygiene,mobility, continence, and feeding). Congruous with previous reports using the Katz ADL scale, we defined declined functional performance as the presence of any disability(any score < 6).
As previously mentioned, the EFS comprised 10 questions and one physical assessment (“timed up and go”) performed by the same team of geriatricians. The EFS assesses nine domains of frailty (cognition, general health status,functional independence, social support, medication usage,nutrition, mood, continence, functional performance).[18,19]We classified patients with a frailty score of ≥ 8/17 as frail,whereas those with a score of 6-7/17 were classified as pre-frail, and those with a score below 6 were classified as non-frail. Of note, the EFS was validated in the hands of non-specialists who had no formal training in geriatric care and the administration requires few minutes.[18]
Activities Daily Living (ADL) and Independent Activities Daily Living (IADL) were measured by interviewing patients, relatives or caregivers.[20]The Timed Up and Go test (TUG) is a mobility assessment that evaluates patients’pace, balance, and timing in a walking exercise.[8]
Continuous variables are presented as median [interquartile range (IQR)] and compared usingt-test or Wilcoxon rank sum test when appropriate. Categorical variables were summarized as counts (frequency percentages). Categorical data were compared with the Chi2test or Fisher’s exact test when appropriate. Frailty scales were primarily analyzed in their continuous form and secondarily in their dichotomous form based on a priori cutoffs.
To test the agreement between measures, Cohen kappa statistics and standard errors are reported for the frail and pre-frail classifications. The interpretation of kappa values was based on the suggestions by Viera and Garrett. All of the analyses were considered significant at a two-tailedP-value of ≤ 0.05. All statistical tests were performed using statistical software SPSS 23.0 for Microsoft (SPSS Inc;IBM, Chicago, IL).
The median (IQR) age and BMI of participants was 84(80-87) years and 27 (24–32) kg/m2, respectively. Patients who died at 1-year follow-up were more anemic at baseline and presented higher surgical risk scores (P≤ 0.05) (Table 1).
As reported in Table 2, we did not find differences in either of the frailty scales according to alive or deceased patients at follow-up. Interestingly, the median (IQR) Mini-Cog score was 3 (1–4), and approximately 35% (45/130) of the study participants were classified as having cognitive impairment. Moreover, the median (IQR) TUG was 13.4(11–17) s and gait speed 0.62 (0.48–0.79) m/s indicating a state of moderate muscular performance with subsequent risk of both health decline, ADL difficulty, and falls.
As shown in Figure 1, according to MFFA, 54/130 (40%)patients were frail, 49/130 (38%) patients were pre-frail and 28/130 (22%) were non-frail. Thirty-day outcomes were very similar between the frail and non-frail groups according to MFFA. Ten (7%) patients died within 30 days of undergoing TAVR. Of these, seven were non-frail (9% of the non-frail group) and three were frail (5% of the frail group).
Median (IQR) length of stay (LOS) of the surviving to discharge patients (120 patients) was five days; IQR 3–7 days post procedure. Non-frail median LOS was five days and the frail group was four; (P= NS).
As shown in Figure 2, according to EFS, 39/130 (30%)patients were frail, 44/130 (34%) patients were vulnerable and 46/130 (36%) patients were non-frail. Thirty-day outcomes were very similar between the frail and non-frail groups according to EFS. From the total of 10 patients who died within 30-days after TAVR, eight patients were classified as non-frail (8% of the non-frail group) and two were frail(5% of the frail group).
Finally, according to EFS, the median (IQR) LOS of the surviving to discharge patients was 5 days for non-frail and 4 days for the frail group, respectively (P= NS). There was fair to moderate agreement between methods for determining which participants were frail [0.40 (0.084),P= 0.001].
In this single-center experience of elderly patients undergoing TAVR, the prevalence of frailty was 40% according to MFFA, and 30% according to the EFS. Of note, the EFS was validated in the hands of non-specialists who had no formal training in geriatric care. Thus, the EFS has the potentialas a practical and clinically meaningful measure of frailty in a variety of settings including pre-TAVR assessment.
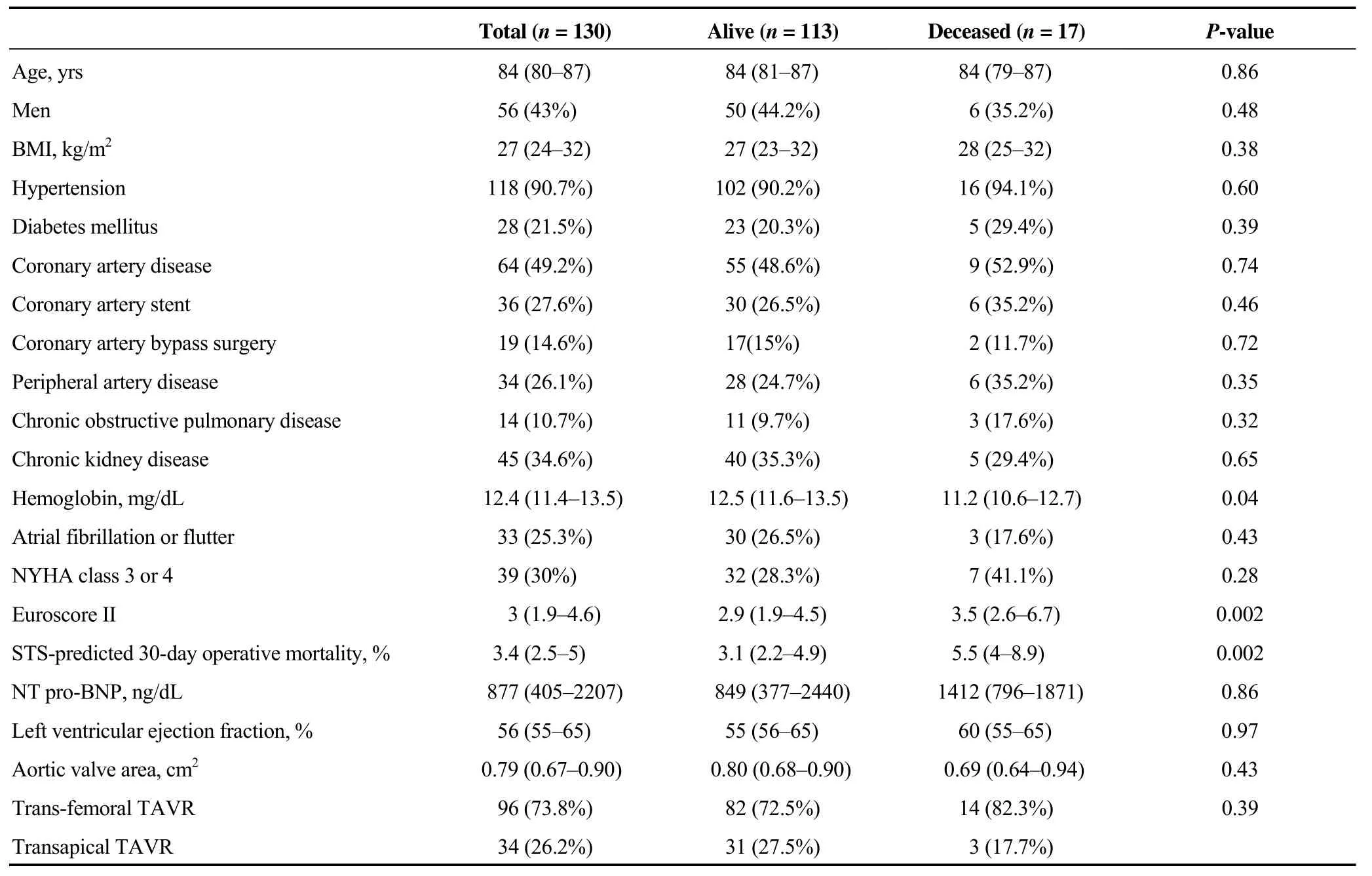
Table 1. Baseline characteristics.
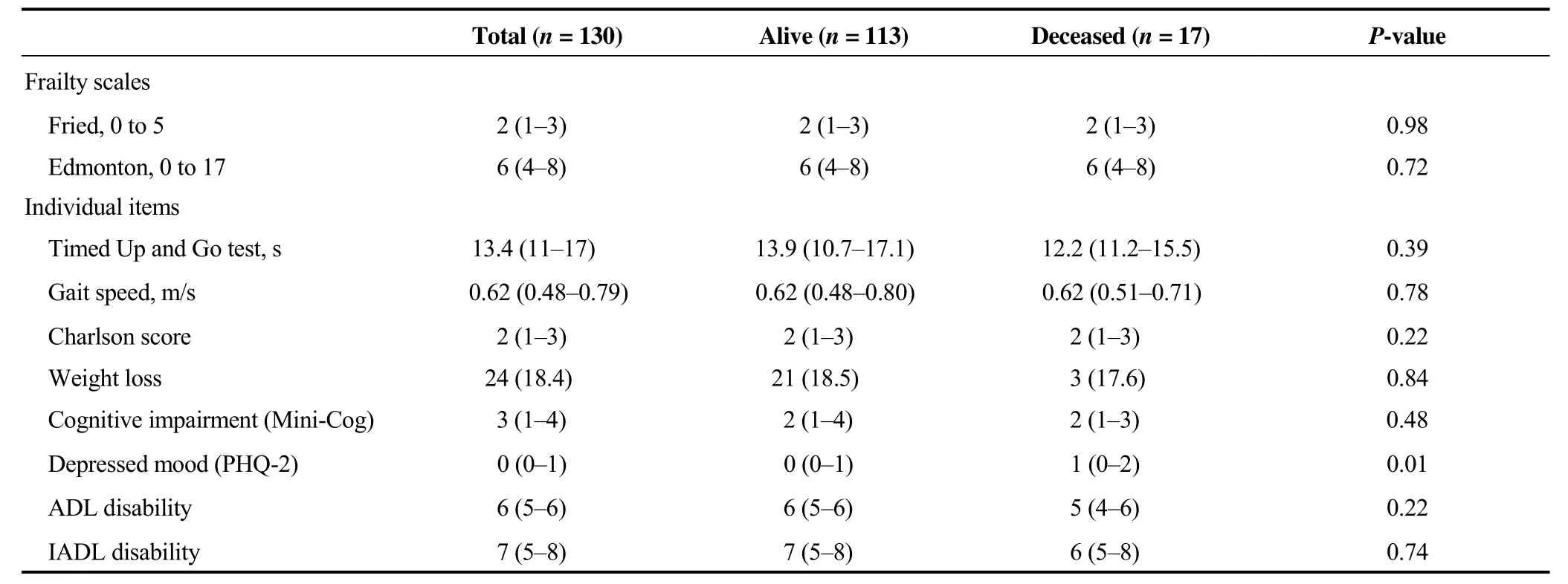
Table 2. Frailty scales and geriatric domains.
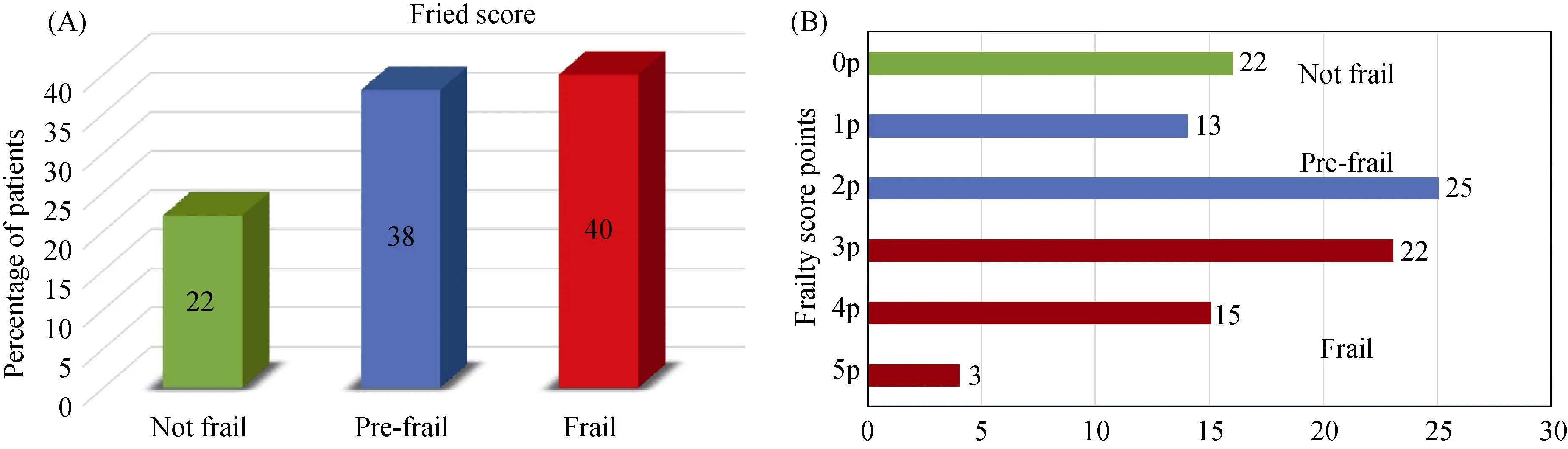
Figure 1. (A): Percentage of screened TAVR patients according to frailty status; (B): percentage of TAVR patients scoring 0 to 5 on adapted Fried’s frailty index. TAVR: transcatheter aortic valve replacement.
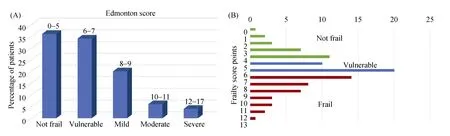
Figure 2. (A): Percentage of screened TAVR patients according to frailty status; (B): percentage of TAVR patients scoring 0 to 17 on adapted Edmonton frailty index. TAVR: transcatheter aortic valve replacement.
Despite we did not found differences in frailty prevalence between alive and deceased patients at follow-up, most of the patients were frail or pre-frail, i.e., “vulnerable”. It is important to mention that frailty is a dynamic concept that is able to be reversed with adequate intervention.[21]We believe our results may be influenced by the fact that patients with extreme frailty, severely impaired functional performance, dementia and less than a year of life expectancy were excluded from TAVR according to our comprehensive geriatric assessment.
The association of both EFS[22]and MFFA[23,24]with multidimensional geriatric conditions has already been studied showing an association with several geriatric conditions such as independence, drug assumption, mood, mental, functional and nutritional status.
In general, we found a similar distribution of cognition impairment, deficits in physical performance and comorbidities burden between alive and deceased patients at 1-year follow-up. However, deceased patients showed significantly higher rates of mood disorders. The influence of both cognitive impairment and depression on TAVR has not been conclusive in the literature.
There was a fair to moderate agreement between assessment methods in our cohort. These findings are similar to those reported by Pritchard,et al.[25]who showed agreement between MFFA and other frailty assessment method(SPPB, i.e., Short Performance Physical Battery) and also Islam,et al.[26]and Theou,et al.[27]whom both showed agreement between MFFA and CFS, i.e., Clinical Frailty Scale.Although the EFS has been compared against the clinical impression of frailty by geriatric specialists[18]appearing to be valid, reliable and feasible for routine use by non-geriatricians, there is no previous data in the literature comparing these two scales for frailty assessment neither agreement between them.
Given that physical activity expressed by the TUG and gait speed are important indicators of frailty,[28]it is not surprising that agreement between the EFS and MFFA is fair to moderate. Our findings show that for identifying frail or pre-frail older adults, either method could be used, but consideration should be provided to other aspects specifically involving motor abilities.
Unfortunately, there is still a lack of consensus for a single frailty method in clinical practice. In this direction, in order to improve understanding of how frailty impacts survival, functionality, and quality of life in TAVR recipients,a protocol has been recently published for a systematic review which will surely shed light on this issue.[29]
Our study has some limitations. Our data are derived from a retrospective, single-center experience, which limits the external validity of our findings. Second, the sample size in our report is modest, which limits our ability to detect small but important differences in outcomes between groups. In this direction, because of the lack of statistically significant differences between frail and non-frail patients with mortality,we did not perform adjusted multivariate analysis. Moreover,it is probable that frail patients had more incidence of other important outcomes such as falls, disabilities and long-term functional decline which were not analyzed in this manuscript.
Finally, the hand grip test was not performed in the vast majority of the patients due to the risk of syncope regarding their clinical status (AS). Weakness in MFFA was then punctuated by geriatricians regarding other method (SARC-F questionnaire). Although the prevalence of frailty rates where similar to those reported in the literature, this limitation could influence our results.
In conclusion, the prevalence of frailty using both MFFA and EFS is similar to that reported in the literature. A fair to moderate agreement was estimated between methods.
Acknowledgements
Many thanks to the staff in our team who participated in the patient follow-up. All authors have no conflicts of interest to disclose.
 Journal of Geriatric Cardiology2020年7期
Journal of Geriatric Cardiology2020年7期
- Journal of Geriatric Cardiology的其它文章
- Atherosclerosis, its risk factors, and cognitive impairment in older adults
- Plasma big endothelin-1 is an effective predictor for ventricular arrythmias and end-stage events in primary prevention implantable cardioverterdefibrillator indication patients
- Remote monitoring of implantable cardioverters defibrillators: a comparison of acceptance between octogenarians and younger patients
- Quality of life, physical performance and nutritional status in older patients hospitalized in a cardiology department
- Left atrial diameter and atrial fibrillation, but not elevated NT-proBNP,predict the development of pulmonary hypertension in patients with HFpEF
- Modified subintimal plaque modification improving future recanalization of chronic total occlusion percutaneous coronary intervention
