Atherosclerosis, its risk factors, and cognitive impairment in older adults
Yun-Li XING, Michael A Chen, Ying SUN, Moni B Neradilek, Xi-Ting WU, Dai ZHANG,Wei HUANG, Yining CUI, Qi-Qi YANG, Hong-Wei LI,4, Xue-Qiao ZHAO
1Department of Geriatricsand Gerontology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
2Division of Cardiology, University of Washington,Seattle,WA, USA
3The Mountain-Whisper-Light Statistics, Seattle,WA,USA
4Cardiovascular Center, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Abstract Objective To examine the association of atherosclerotic cardiovascular disease (ASCVD) and its risk factors with cognitive impairment in older adults. Methods Six hundred and fourteen subjects, aged ≥ 65 years, from one center (2016–2018) underwent clinical, laboratory assessments and the Montreal Cognitive Assessment (MoCA). Using regression analysis, the relationship between ASCVD and its risk factors was evaluated in subjects with and without cognitive impairment (MoCA score < 26). Results Older age (β = -1.3 per 5 years,95% CI: -1.7 to -0.9, P < 0.001), history of stroke (β = -1.6, 95% CI: -3.0 to -0.3, P = 0.01), and myocardial infarction (MI; β = -2.2, 95%CI: -3.6 to -0.8, P = 0.003) were independently associated with lower MoCA scores, whereas more education (β = 1.5 per 3 years, 95% CI:1.1 to 1.9, P < 0.001), higher body mass index (BMI; β = 0.5 per 3 kg/m2, 95% CI: 0.0 to 1.0, P = 0.04), higher estimated glomerular filtration rate (eGFR; β = 0.8 per 15 U, 95% CI: 0.1 to 1.4, P = 0.03), left ventricular ejection fraction (LVEF; β = 0.4 per 5%, 95% CI: 0 to 0.8, P= 0.04) and statin use (β = 1.3, 95% CI: 0.3 to 2.3, P = 0.01) were associated with a higher MoCA score. Cognitive impairment was independently associated with older age (OR = 1.51 per 5 yrs, 95% CI: 1.28 to 1.79, P < 0.001), less education (OR = 0.55 per 3 years, 95% CI:0.45 to 0.68, P < 0.001), lower BMI (OR = 0.78 per 3 kg/m2, 95% CI: 0.62 to 0.98, P = 0.03) and higher levels of high sensitivity c-reactive protein (hsCRP; OR = 1.08 per 1 mg/L, 95% CI: 1.02 to 1.15, P = 0.01). Conclusions Beyond age, cognitive impairment was associated with prior MI/stroke, higher hsCRP, statin use, less education, lower eGFR, BMI and LVEF.
J Geriatr Cardiol 2020; 17: 434-440. doi:10.11909/j.issn.1671-5411.2020.07.006
Keywords: Aging; Atherosclerosis; Cognitive impairment; Correlation; Older adults; Risk factors
1 Introduction
Atherosclerotic cardiovascular disease (ASCVD), which includes coronary, cerebrovascular and peripheral arterial disease and its risk factors increase with age.[1]They all reach high levels of prevalence in older adults, and result in a great deal of morbidity and mortality. For example, coronary artery disease is the leading cause of health loss worldwide, with a prevalence that rises steeply with advancing age. The same is true for the other ASCVD diagnoses.[2]Cognitive impairment, including mild cognitive impairment and dementia generally affects older adults.Dementia is present in nearly 50 million people worldwide,and is expected to triple by 2050. The condition is highly burdensome, with costs in the U.S. alone that in 2015 was estimated to be $818 billion.[3]
Cardiovascular health status has been found to be associated with cognitive impairment in individuals with and without diagnosed ASCVD.[4]ASCVD as well as its risk factors such as diabetes, hypertension, and smoking are well established risk factors for dementia.[3]However, the impact of ASCVD and its risk factors on cognition in older adults has not been completely defined. We therefore investigated the association of ASCVD and its risk factors with cognition in a group of older adults who were receiving risk factor management.
2 Methods
2.1 Study population
We studied a total of 1455 inpatients, ≥ 65 years of age,who received medical care or routine physicals at the Geriatric Medicine Department of the Beijing Friendship Hospital between January 2016 and January 2018. As shown in Figure 1, of these 1455 subjects, 565 were excluded because they had not undergone a cognitive assessment, 45 were excluded for receiving antidementia treatment, 21 and 37 were excluded for acute myocardial infarction (AMI) and for stroke within six months, respectively, and 173 were excluded for known inflammatory conditions associated with a hsCRP > 20 mg/L. Finally, 614 participants were included in this analysis to assess the effect of ASCVD and its risk factors on cognition among older adults.
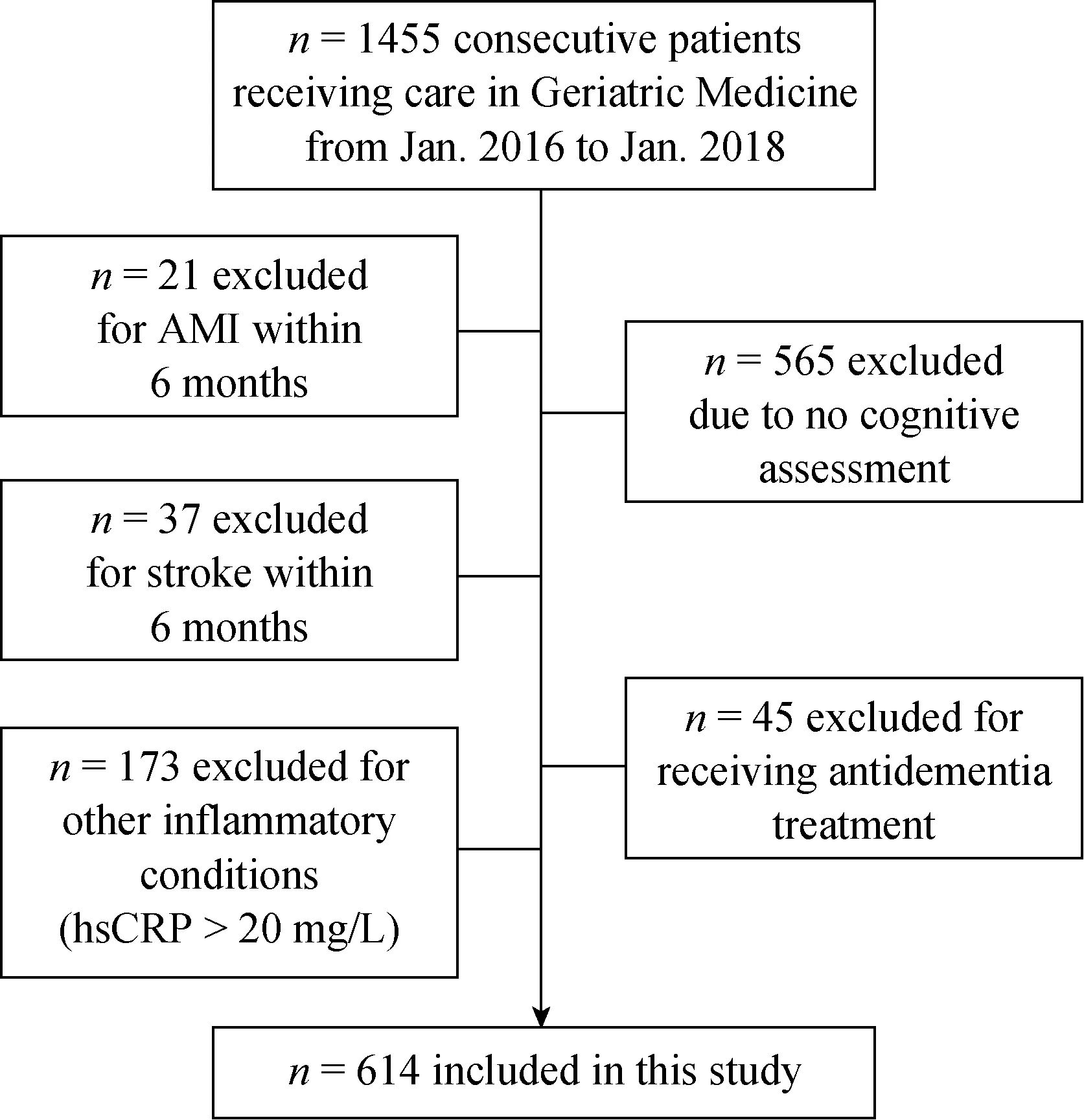
Figure 1. Patient recruitment. 1455 patients ≥ 65 years of age who received medical care or routine physicals at the Geriatric Medicine Department of the Beijing Friendship Hospital between January 2016 and January 2018 were screened. Of these, a number were excluded based for pre-specified criteria, yielding 614 participants who were included in the analysis to assess the effect of ASCVD and its risk factors on cognition among older adults.
The study was approved by the ethics committee of the Beijing Friendship Hospital affiliated with Capital Medical University and in accordance with the Declaration of Helsinki. Although the patients were not involved in the initial study design, all study participants received informed consent and were willing to support of the study data collection.We plan to share the study results with all participants once the manuscript is published.
2.2 ASCVD and risk assessment
Demographic characteristics including years of education,medical history, clinical information and concurrent medications were collected at the in-hospital setting. Vital signs were also measured.
History of MI or stroke was confirmed by medical record review. Hypertension was defined as systolic blood pressure(SBP) ≥ 140 mmHg, diastolic BP (DBP) ≥ 90 mmHg or ongoing therapy for hypertension. Type-2 diabetes was defined as glycosylated hemoglobin (HbA1c) ≥ 6.5%, a nonfasting plasma glucose concentration ≥ 200 mg/dL, fasting plasma glucose concentration ≥ 126 mg/dL, 2-h plasma glucose concentration ≥ 200 mg/dL from a 75 g oral glucose tolerance test, or if the patient was treated with oral hypoglycemic medications or insulin. Atrial fibrillation (AF)included paroxysmal or persistent AF confirmed by ECG.In addition, ICD-9 or ICD-10 codes were used to help to identify and confirm these chronic conditions.
2.3 Laboratory measurements
Lipids including total cholesterol (TC), triglyceride (TG),low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C), apolipoprotein (apo)A1, apoB and Lp(a) were measured using standard methods of the central laboratory of the Beijing Friendship Hospital.Fasting glucose, HbA1C, liver and renal function labs, and hsCRP were also measured.
2.4 Echocardiography
All subjects received an echocardiograpic (Echo) examination to assess cardiac structure and function. Echocardiography was performed routinely by a cardiologist with over 20 years of scanning experience utilizing the same standard protocol to assess left atrial diameter (LA), left ventricular end-systolic diameter (LVESD), left ventricular end-diastolic diameter (LVEDD), interventricular septal thickness (IVST) and left ventricular ejection fraction (LVEF).
2.5 Cognitive function assessment
The Montreal Cognitive Assessment (MoCA),[5]appropirately translated, was used to evaluate the cognitive function in the following domains: executive functions (5 points),name (3 points), memory (5 points), concentration (6 points),language (3 points), conceptual thinking (2 points), and orientation (6 points). A full score is 30 points. The higher scores represent better cognitive performance. To counterbalance the effect of lower education, one point was added to the final score for the individuals with < 12 years of education. Cognitive impairment was defined as the score of < 26.
2.6 Statistical analysis
Key variables had up to 22% missing values (BMI,HbA1C, Lp(a), ApoB and ApoA1 had the most missing values). Furthermore, the number of subjects with any missing value compounded in the multivariate analyses. To avoid the impact of excluding subjects with missing values on decreased sample size and on generalizability we utilized multiple imputation and retained all subjects in the analysis.Five multiple imputations were created by the random forest method.[6]Continuous variables were expressed as mean ±SD and categorical variables were expressed as number and percentage. The estimates of means, variances (SD2) and percentages were averaged across the multiply imputed data sets.P-values comparing subjects characteristics between cognitively impaired and unimpaired subjects were calculated using linear and logistic regressions (as appropriate)and the regression statistics combined across the multiply imputed data sets using Rubin’s rules.[7]Univariate and multivariate linear and logistic regressions were performed to identify factors that were associated with MoCA score and cognitive impairment, respectively (statistics were again combined across the multiply imputed data sets using Rubin’s rules). Variables statistically significant in the univariate models were entered into the multivariate models.Pearson correlation between pairs of predictors was calculated to understand inter-dependency of predictors. The ability of each variable to predict cognitive impairment was assessed by the area under the receiver operating characteristics curve (AUC) from the univariate analysis (represents single-variable prediction) and by the decrease in the AUC when a predictor was dropped from the multivariate model(represents the incremental contribution of the predictor to the multivariate model). The AUCs were averaged across the multiple imputations. A sensitivity analysis with predictive mean matching[8]an alternative method of imputation identified no material differences from the primary imputation method (results not shown). AP< 0.05 was considered statistically significant. All calculations were carried out in R (Austria, Vienna), version 3.5.0.
3 Results
3.1 Clinical characteristics, ASCVD and its risk factors
Among the 614 subjects included in this analysis, the mean age was 82 years, the average years of education received was 14, 68% were men, the average BMI was 24,32% were smokers, 79% had hypertension, 32% had diabetes, 16% had stroke and 15% had a history of MI.
Full characteristics of the study population are shown in Table 1. Notably, 57% of subjects were taking anti-platelet or anti-coagulation therapy, 61% were on a statin, and 41%were on an ACEI or ARB. Their mean systolic and diastolic blood pressure were 136 and 72 mmHg, respectively. Average eGFR and LVEF were normal (72 mL/min per 1.73 m2and 64%, respectively). Their average TC was 158 mg/dL,LDL-C was 93 mg/dL, and ApoB was 75 mg/dL.
3.2 Prevalence of cognitive impairment
Of these 614 older adults, 429 (70%) were identified as having cognitive impairment with MoCA scores < 26. As shown in Figure 2, the cognitive impairment occurred in all seven domains.
When examining the specific domains of impairment in individuals classified as being impaired not only had a significantly lower overall score (17 ± 6vs. 28 ± 1,P< 0.001),but also had significantly reduced executive function (2.8 ±1.5vs. 4.7 ± 0.6,P< 0.001), reduced ability of naming (2.3± 0.9vs. 2.9 ± 0.3,P< 0.001), more impaired memory (1.2± 1.4vs. 3.8 ± 1.1,P< 0.001), less ability to concentrate(3.4 ± 1.7vs. 5.7 ± 0.7,P< 0.001), reduced language skill(1.7 ± 1.0vs. 2.6 ± 0.6,P< 0.001), reduced conceptual thinking (1.0 ± 0.8vs. 1.8 ± 0.4,P< 0.001), and more trouble with orientation (4.8 ± 1.6vs. 5.9 ± 0.3,P< 0.001).
3.3 Associations of MoCA score and cognitive impairment
Subjects with and without cognitive impairment were compared according to demographic and clinical characteristics, ASCVD and its risk factors. As shown in Table 1,compared to subjects with normal cognitive function, subjects with cognitive impairment were significantly older (83± 6vs. 79 ± 7 years,P< 0.001), less educated (13 ± 4vs. 15± 3 years,P< 0.001), had slightly lower BMI (23 ± 4vs. 24± 4 kg/m2,P= 0.025), twice the rate of prior MI (18%vs.9%,P= 0.005), significantly lower eGFR (69 ± 19vs. 77 ±17 mL/min per 1.73 m2,P< 0.001), significantly higher level of hsCRP (3 ± 4vs. 2 ± 3 mg/L,P< 0.001) and lower LVEF (63% ± 7%vs. 65% ± 6%,P= 0.017).
Univariate analysis identified age, years of education,BMI, history of stroke, history of MI, fasting blood sugar,creatinine, eGFR, ApoA1, hsCRP, statin use, LV end-systolic diameter and LVEF were significantly (P< 0.05) associated with MoCA scores. These variables were included in the multivariate model for MoCA score (Table 2).
Age, years of education, BMI, history of MI, creatinine,eGFR, hsCRP and LVEF were significantly (P< 0.05) associated with cognitive impairment and were included in the multivariate model for cognitive impairment. Most pairs of variables in the multivariate models had weak correlations(rbetween -0.3 and 0.3), except age and eGFR (r= -0.36)and eGFR and creatinine was (r= -0.79). The multivariate model for MoCA score showed that older age (β = -1.3 per 5 years increase, 95% CI: -1.7 to -0.9,P< 0.001), history of stroke (β = -1.6, 95% CI: -3.0 to -0.3,P= 0.014) and history of MI (β = -2.2, 95% CI: -3.6 to -0.8,P= 0.003)were significantly and independently associated with lowerMoCA scores, while more education (β = 1.5 per 3 years,95% CI: 1.1 to 1.9,P< 0.001), larger BMI (β = 0.5, 95% CI:0.0 to 1.0,P= 0.037), higher eGFR (β = 0.8 per 15 units increase, 95% CI: 0.1 to 1.4,P= 0.030) and LVEF (β = 0.4,95% CI: 0.0 to 0.8,P= 0.044), and statin use (β = 1.3, 95%CI: 0.3 to 2.3,P= 0.011) were associated with higher MoCA scores. The presence of cognitive impairment was significantly and independently associated with older age(OR = 1.51 per 5 years increase, 95% CI: 1.28 to 1.79,P<0.001), less education (OR = 0.55 per 3 years more education, 95% CI: 0.45 to 0.68,P< 0.001), smaller BMI (OR =0.78 per 3 kg/m2, 95% CI: 0.62-0.98,P= 0.027) and higher levels of hsCRP (OR = 1.08 per 1 mg/L increase, 95% CI:1.02 to 1.15,P= 0.014).
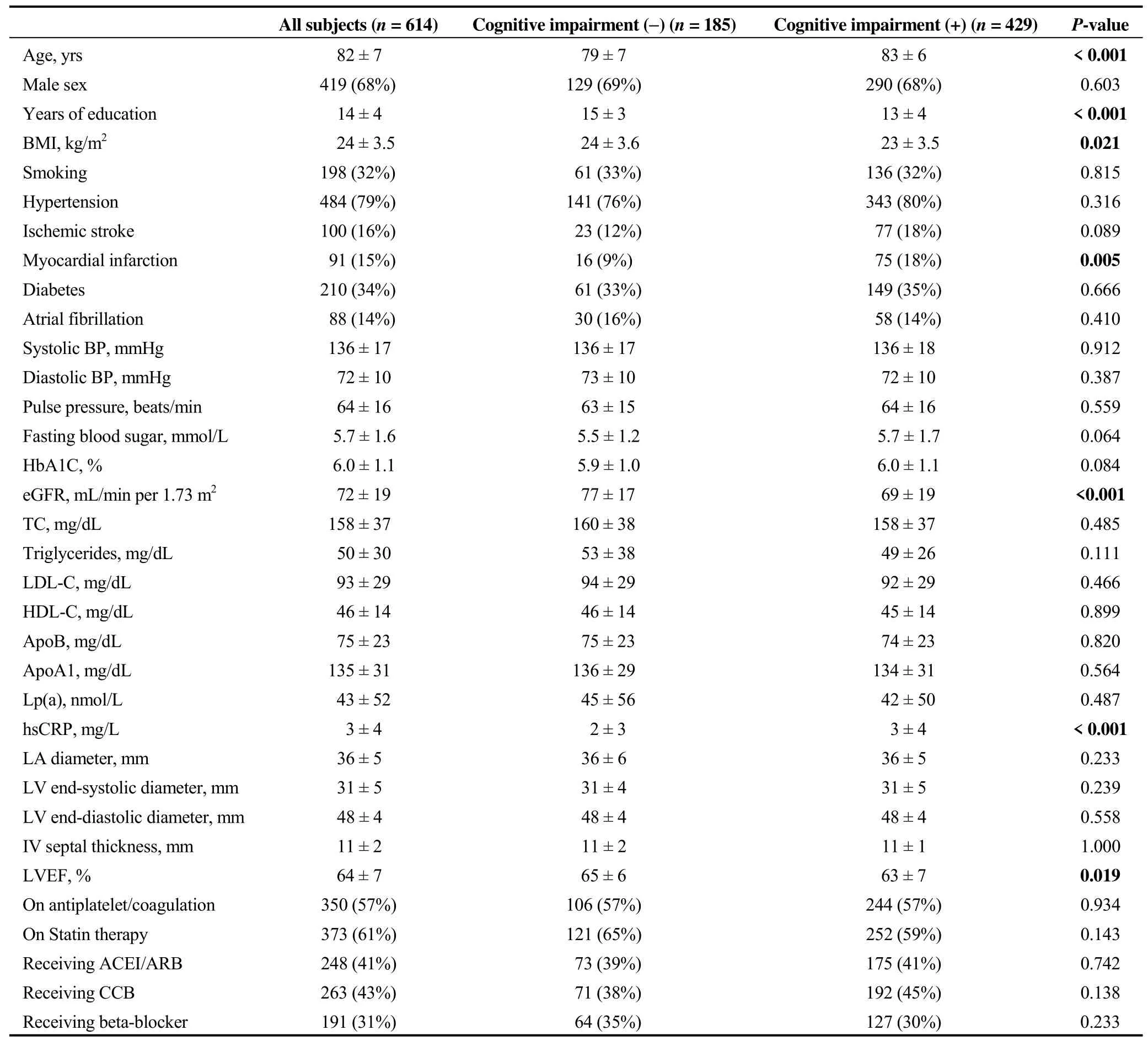
Table 1. Demographic and clinical characteristics, ASCVD and its risk factors.
3.4 Prediction of cognitive impairment
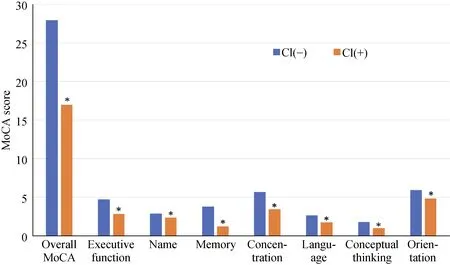
Figure 2. Cognitive impairment was present in 70% of the sample, and affected all measured domains. Individuals with cognitive impairment compared to those without had a significantly lower overall score (17 ± 6 vs. 28 ± 1, P < 0.001), significantly reduced executive function (2.8 ± 1.5 vs. 4.7 ± 0.6, P < 0.001), reduced ability of naming (2.3 ± 0.9 vs. 2.9 ± 0.3, P < 0.001), more impaired memory (1.2 ± 1.4 vs. 3.8 ± 1.1, P < 0.001), less ability to concentrate (3.4 ± 1.7 vs. 5.7 ± 0.7, P < 0.001), reduced language skill (1.7 ± 1.0 vs. 2.6 ± 0.6, P <0.001), reduced conceptual thinking (1.0 ± 0.8 vs. 1.8 ± 0.4, P < 0.001), more trouble with orientation (4.8 ± 1.6 vs. 5.9 ± 0.3, P < 0.001). *P< 0.001. MoCA: montreal cognitive assessment.
Table 3 shows the ability of each variable in the model to predict cognitive impairment (either singly in the univariate analysis or incrementally in the multivariate model). The univariate AUC was 0.68 for age, 0.63 for years of education, 0.58 for BMI, 0.62 for eGFR, 0.60 for hsCRP and 0.56 for LVEF. The AUC using a full multivariate model including all 8 variables was 0.79. Modest to moderate decreases in multivariate AUC was observed by dropping each of these 6 variables: -0.025 for age, -0.040 for years of education, -0.01 for BMI, -0.002 for eGFR, -0.008 for hsCRP and -0.005 for LVEF. This analysis indicates that some of these risk factors represent similar effects on cognitive impairment (i.e., no risk factor had a uniquely independent ability to predict cognitive impairment).

Table 2. Multivariate regression for associations of MoCA score and cognitive impairment.
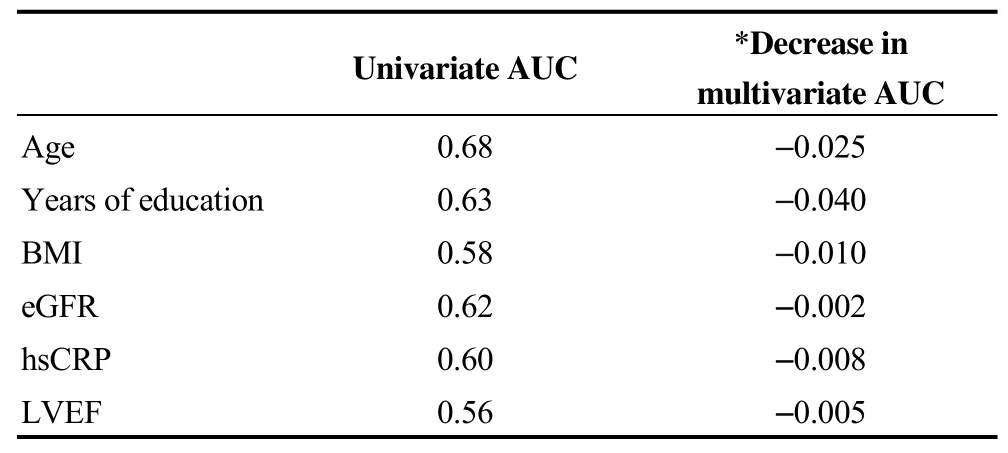
Table 3. Ability of variables to predict cognitive impairment.
4. Discussion
In this group of older adults who had a mean age of 83 years with range of 65–100 and received a reasonable effective management for ASCVD and its risk factors, we found a very high prevalence of cognitive impairment (70%). As expected, increasing age was associated with the presence(vs. absence) of cognitive impairment (defined as a MoCA score < 26) and lower MoCA scores. Beyond the impact of age, we also found that less education, history of stroke or MI, statin use, lower BMI, eGFR and LVEF, and higher hsCRP were associated with lower MoCA scores. The presence of cognitive impairment was correlated with older age, less education, lower BMI, lower eGFR and higher hsCRP. Furthermore, the predictive ability of the multivariate model in this sample (with eight predictors variables)was shown to have AUC of 0.79. The independent contribution of each variable to the multivariate model AUC was only moderate, indicating that these factors may have overlapping associations with cognitive impairment.
ASCVD and its risk factors are highly prevalent in aging population.[1]Cognitive impairment is clearly associated with increasing age.[3]Cognitive impairment has been reported in patients with a variety of cardiovascular disorders or risk factors.[9–11]In this study, we found that patients with a history of stroke or MI were more likely to have cognitive impairment. Since statins are the standard of care for patients with MI and stroke it was predictable that statin use was associated with cognitive impairment. The small but statistically significant difference in left ventricular ejection fraction in our study likely reflects the higher burden of ASCVD, especially MI, in the cognitive impairment group.
In addition, multiple factors were found to be associated with both ASCVD and cognitive impairment.[4,12–14]For example, in our population, higher levels of C-reactive protein were associated with cognitive impairment. Others have also found that inflammation is associated with ASCVD as well as with dementia.[15,16]Whether it does so by the same mechanisms has not been determined, but interestingly,chronic inflammation is also associated with the “frailty syndrome”, which by some definitions, also includes cognitive dysfunction.[17]
In this study lower eGFR was associated with lower MoCA scores. Chronic kidney disease (CKD) is associated with an increased risk of cognitive decline as well as for ASCVD, possibly due to shared risk factors for such as diabetes and hypertension. One systematic review and metaanalysis of population-based studies found that albuminuria as a marker of CKD was most consistently associated with cognitive impairment (including dementia) with weaker associations for other markers such as GFR, serum creatinine and cystatin C.[18]
At first, it may seem counterintuitive that in this study lower BMI was associated with cognitive impairment, since high BMI is a risk factor for ASCVD. However, the relationship between the two may be more complex. Although obesity (and high BMI) is a risk factor for cognitive impairment/dementia in mid-life (age 45–65 years), there is some evidence of an association between low BMI and cognitive dysfunction in late life.[3]A proposed explanation for this is that while high BMI may be a risk factor for the development of cognitive impairment much like other ASCVD risk factors, in the preclinical phase of cognitive impairment, when other cardiometabolic changes are at play,BMI falls, resulting in an reversal of the relationship.[19]
The current data showed an inverse relationship between years of education and cognitive impairment. This may be the result of what has been called “cognitive reserve”, a term has been used to describe the fact that despite some subjects having a significant burden of neuropathologic changes usually associated with Alzheimer’s disease, Lewy body and multi-infarct dementia they have little or no commensurate cognitive impairment. These patients often have achieved higher levels of education. Whether this phenomenon is due to adaptability in cognition or starting from a higher baseline is uncertain, although both may play a role.[20]
Finally, this group of older adults was currently wellmanaged for their ASCVD and its risk factors. Overall, 79%had hypertension with an average treated BP of 136/72 mmHg; 32% had diabetes with an average HbA1C of 6%;15% had a prior MI; the average LVEF of 62%; 16% had prior stroke; 32% had a history of smoking; 14% had atrial fibrillation; 57% were on antiplatelet or anticoagulant medications; 61% were on a statin (mean LDL-C of 93 mg/dL);31% were on a β-blocker and 41% were on an ACEI/ARB.Nevertheless, we found 70% of subjects had cognitive impairment. These findings call for studies to evaluate whether aggressive ASCVD risk management implemented earlier in life would reduce incidence of cognitive impairment in later life and whether intensified ASCVD risk management would benefit older adults’ cognitive function.
This study has some limitations, first our data, while high quality, was acquired at a single center in China, which may limit the generalizability of the results. Second, this crosssectional study only enabled an analysis of associations at a single time point. The results therefore do not differentiate between differences among patients and differences within a patient (progression). The results also do not necessarily imply causation. Third, to account for patients with missing values we utilized a multiple imputation approach which assumes that the data were missing at random (i.e., missing random after controlling for available variables). Although a limitation, this approach is more robust and preferable than an analysis limited to patients with complete data (the latter would assume the data were missing completely at random).Fourth, the predictive ability of the multivariate models as measured by the AUC is descriptive and only reflects the ability to predict cognitive impairment in this study. As we do not intend to use the multivariate models for prediction in the future we did not attempt to cross-validate the AUC statistics to generalize to a more general population. In general, we would expect the AUCs to decrease in an independent sample from a similar population.
In conclusion, this study showed a relationship between ASCVD such as MI and stroke and many of its risk and related factors (age, LVEF, elevated hsCRP, eGFR, statin use, BMI) as well as educational status and cognitive impairment. Similar research with larger populations, and in other countries should be undertaken. Because this was a cross-sectional analysis it is not possible to make conclusions about the mechanistic relationship between atherosclerosis (or atherosclerosis risk factors) and cognitive impairment. One possible avenue for future research would be to evaluate the relationship by evaluating the longitudinal impact of ASCVD risk factor management on the future development of cognitive impairment.
Acknowledgments
This study was funded by grants from Beijing Healthcare Committee Fund (19-8); Research Foundation of Beijing Friendship Hospital, Capital Medical University (yyqdkt 2017-6); Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support(ZYLX201838). The sponsors had no input into the design,methods, recruitment of subjects, data collection, analysis or paper preparation.
 Journal of Geriatric Cardiology2020年7期
Journal of Geriatric Cardiology2020年7期
- Journal of Geriatric Cardiology的其它文章
- Measuring frailty in patients with severe aortic stenosis: a comparison of the edmonton frail scale with modified fried frailty assessment in patients undergoing transcatheter aortic valve replacement
- Plasma big endothelin-1 is an effective predictor for ventricular arrythmias and end-stage events in primary prevention implantable cardioverterdefibrillator indication patients
- Remote monitoring of implantable cardioverters defibrillators: a comparison of acceptance between octogenarians and younger patients
- Quality of life, physical performance and nutritional status in older patients hospitalized in a cardiology department
- Left atrial diameter and atrial fibrillation, but not elevated NT-proBNP,predict the development of pulmonary hypertension in patients with HFpEF
- Modified subintimal plaque modification improving future recanalization of chronic total occlusion percutaneous coronary intervention
