Pretreatment levels of circulating endothelial cells on efficacy of first-line therapy in patients with advanced non-small cell lung cancer
1People's Hospital of Guangxi Zhuang Autonomous Region,clinical cancer center,chemotherapy No.1 department,Nanning 530021,China;2People's Hospital of Guangxi Zhuang Autonomous Region,Scientific research department,Nanning 530021,China.
Abstract
Key words:Circulating endothelial cells,Non-small-cell lung cancer,First-line therapy,Efficacy
Background
According to the latest cancer statistics released by China’s National Cancer Center in January 2019,the malignant tumor has become a major public health problem that seriously threatens the health of the Chinese population.Lung cancer is the tumor with the highest morbidity and mortality rates in China,rated as 57.26/100,000 and 45.87/100,000 in 2015,respectively [1].About 80% of lung cancers are nonsmall cell lung cancer (NSCLC),and 75% of patients would have entered the middle to late stage at the time of diagnosis [2,3].With more thorough studies on NSCLC,new therapeutic drugs are emerging.However,the overall prognosis is poor,and the 5-year overall survival rate is <10% [4].For patients with advanced lung cancer,there is a high cost of treatment involved,and yet poor prognosis.Therefore,a reliable and convenient indicator for predicting the efficacy is urgently needed in order to guide individual therapy for NSCLC patients [5].
Circulating endothelial cells (CECs) are heterogeneous cell populations,which can reproduce and differentiate in peripheral blood.Some of them are derived from existing exfoliated endothelial cells of the vascular wall,and others are differentiated bone marrow-derived endothelial progenitor cells or endothelial progenitor cells in systemic circulation [6].Peripheral blood CECs are rarely detected in healthy people but could increase in patients in a variety of tumor scenarios.Also,the CECs level is indeed higher in patients with progressive conditions than those with stable conditions,and the levels have been shown to drop after chemotherapy or tumor resection surgeries [7–9].Thus,the changes in levels during pretreatment and posttreatment of CECs may predict the efficacy of tumor treatments.The purpose of this study was to explore whether peripheral blood CECs levels could be used as an indicator to predict treatment efficacy in patients with advanced NSCLC.
We prospectively analyzed the 45 patients with inoperable locally advanced and progressive stage III and IV NSCLC who met the inclusion criteria and received the first-line therapy at the chemotherapy ward of the Clinical Oncology Center of the People’s Hospital of Guangxi Zhuang Autonomous Region,from January 2019 to January 2020.The associations of pretreatment CECs level with patient's gender,age,physical condition,tumor stage,pathological type,epidermal growth factor receptor (EGFR) mutation status,and efficacy were then clarified.
Material and methods
Ethical statement
This study was approved by the ethics committee of People’s Hospital of Guangxi Zhuang Autonomous Region,clinical cancer center,chemotherapy No.1 department,Nanning (Supplementary Material).Informed consents were obtained from all participants involved in this study.
Subjects
The patients (n = 45) who were newly diagnosed with the inoperable stage III and IV NSCLC in our hospital from January 2019 to January 2020 were enrolled in this study.Inclusion criteria were as follows:1.patients diagnosed with NSCLC by cytology or histopathology;2.patients classified as inoperable stage III-IV,according to the 7th edition of international classification for lung cancer published by the Union for International Cancer Control in 2009;3.patients receiving no chemotherapy or targeted therapy;4.patients with complete assessable imaging data;5.patients with performance status (PS) score ≤ 2 according to the Eastern Cooperative Oncology Group (ECOG) Performance Scale;6.patients with a life expectancy of more than 6 months.Exclusion criteria were as follows:1.patients with a history of severe lung diseases and heart diseases,such as acute coronary syndrome;2.patients have a combination of NSCLC with second tumors or hematological malignancies;3.patients with acute and chronic infections;4.patients with autoimmune diseases,such as systemic lupus erythematosus.
Data collection
Patient’s demographics and clinical data were collected and recorded at diagnosis and includes age,gender,ECOG score,tumor stage,pathological type,gene mutation detection for EGFR;treatment information for tumor such as the treatment plan,efficacy assessment after 4 cycles of chemotherapy or 3 months of tyrosine kinase inhibitors therapy,and imaging data during treatment;the results of a routine blood test,serum carcinoembryonic antigen (CEA) and serum CECs within 1 week before the treatment.
Research method
Pretreatment peripheral blood (5 mL) sample was collected from each patient using EDTA anticoagulant blood collection tube,stored at 4 ℃,and tested within 6 hours.Two aliquots of the blood sample were obtained.About 1.5 mL of mononuclear cells were extracted by lymphocyte isolation and then added 3 times volume of PBS before being centrifuged at 1300 rpm for 5 min (r = 20 cm).After removal of the supernatant,the cells were resuspended in PBS and 100–200 μL of cell suspension was used for testing.Each of the 4 test tubes for flow cytometry (FCM) was added with 50 μL of prepared cell suspension,and then added CD45-PE-Cy5 2 μL,CD146-PE 10 μL,CD106-FITC 10 μL.IgG-APC was used as a control group FCM antibody,respectively.Then the samples were mixed by shaking and incubated at room temperature in the dark for 15 min.The 500 μL of a hemolytic agent was added and mixed by shaking and incubated at room temperature in the dark for another 10 min.Flow cytometer (BD Biosciences #660346,USA) was used for detection within 15 min after complete hemolysis.
Efficacy assessment
According to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) [19],the short-term efficacy is divided into the complete response (CR):the disappearance of all target lesions and the shortest diameter of all pathological lymph nodes (including target and non-target nodules) must be reduced to 10 mm or less;partial response (PR):at least a 30% decrease in the sum of the diameter of target lesions,taking as reference the baseline sum diameter;stable disease (SD):neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for progressive disease (PD),taking as reference the smallest sum longest diameter (LD) since the treatment started;PD:at least a 20% increase in the sum of LD of target lesions,taking as reference the smallest sum LD recorded since the treatment started,or the appearance of one or more new lesions,and at least an increase of 5 mm in the absolute value of sum diameter.The enhanced CT with a slice thickness of 5 mm was used for the efficacy assessment.
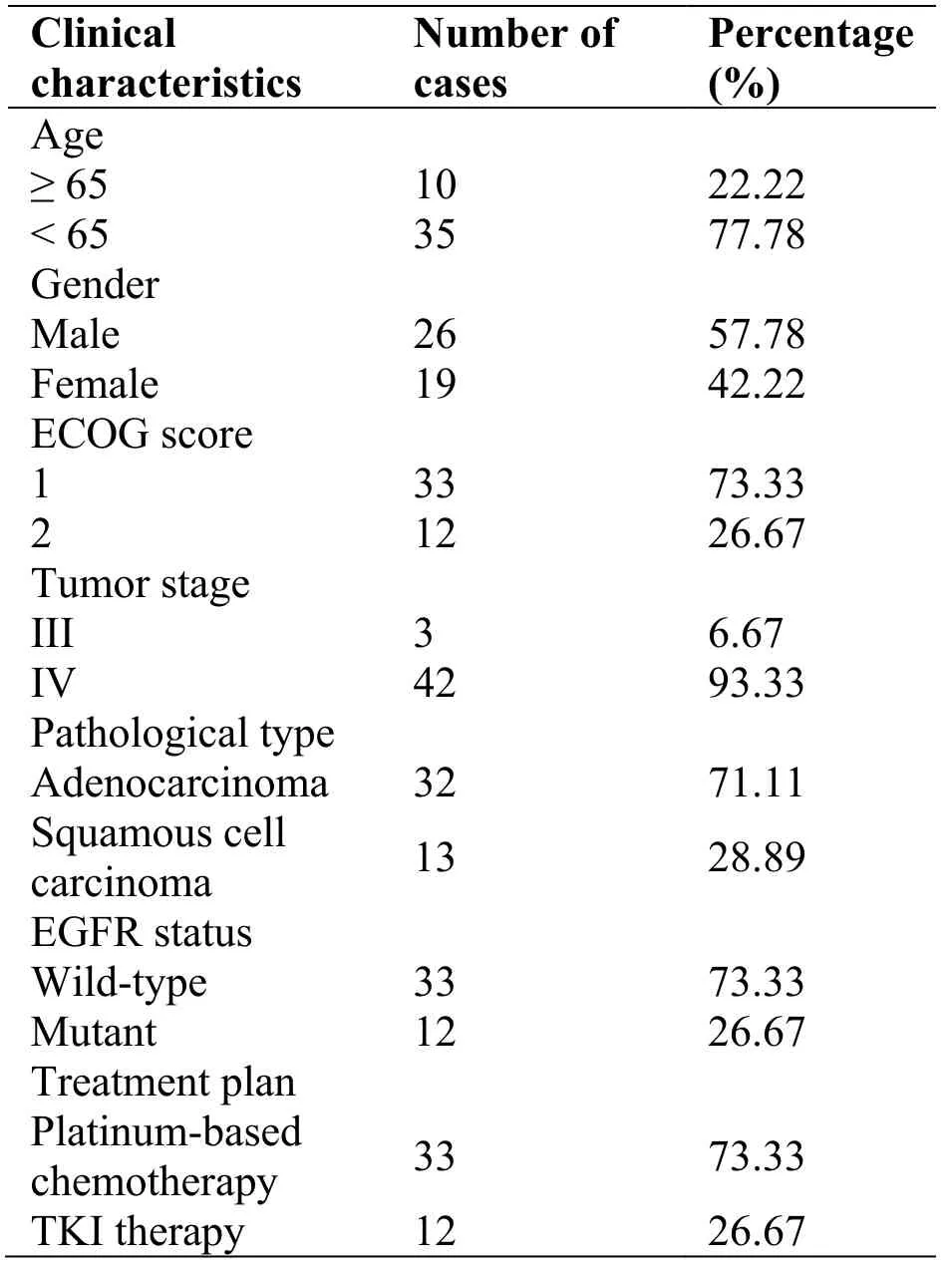
Table1 Basic clinical characteristics of patients enrolled
The subjects were divided into two groups as the response (CR + PR) and nonresponse (SD + PD).
Statistical method
Data were analyzed using the SPSS 22.0 statistical program.Since estimations were not normally distributed,the data were expressed as median and interquartile ranges.The count data were expressed as quantity (percentage).The bivariate correlation analyses were utilized to express association strength,and the Spearman’s rank correlation coefficient was employed to determine the relationship between two variables.If the measured data were still not normally distributed,the differences between groups were analyzed using the nonparametric test and Mann–Whitney U test.APvalue <0.05 was considered statistically significant.
Results
Patients meet the inclusion criteria
A total of 45 patients were enrolled in this study,and their median age was 60 years (range 36–76 years).Patients with wild-type EGFR,n = 33,underwent the platinum-based chemotherapy,while the rest of the patients,n = 12,with mutant EGFR were treated with tyrosine kinase inhibitors,as shown in Table 1.
Correlation between CECs and patients’ baseline clinical characteristics
CECs levels did not significantly correlate with patient's gender,PS score,tumor stage,pathological type,EGFR status,pretreatment CEC level,or treatment plan (P>0.05),but correlated with patient’s age (P<0.05).Since this study involved only 10 patients aged 65 or more,and 35 patients aged below 65,might be due to the small sample size.CECs significantly correlated with treatment efficacy (r = 0.474;P<0.05) (Table 2).
Differences in CECs for different clinical characteristics
There were no significant differences in CECs for different age,gender,PS score,tumor stage,pathological type,or EGFR status (P>0.05).There was a significant difference in CEC count between the response group and the nonresponse group (P<0.05).The higher the CEC count,the worse the efficacy was (Table 3).
Discussions
In the malignant tumor,the normal vascular endothelial cells around the tumor are considered to have the ability to undergo phenotypic changes,function,and genetic characteristics due to the longterm exposure in the tumor microenvironment.The formed tumor endothelial cells participate in tumor angiogenesis by activation [10–12] and collection of inflammatory cells [13–15],vascular endothelial-tomesenchymal transition [16–20],and other mechanisms,which are strongly associated with the generation and development of tumor vessel,resulting in the growth,invasion,and metastasis of the tumor.Therefore,the peripheral blood CECs levels in tumor patients may aid in assessing the angiogenesis of tumors,change of condition,and efficacy of the medication.With the augmentation of individualized treatment of NSCLC,it is urgent to find simple,easy,and effective biomarkers that can dynamically monitor treatment efficacy and evaluate the prognosis in NSCLC.
Hladovec method of endothelial cells estimation [21] was the earliest approach to detect CECs responses after in China.With continuous improvement,immunological techniques are mostly used to isolate and identify CECs,mainly including immunomagnetic separation and FCM.In FCM,anti-endothelial cell antibodies with different fluorescent markers are used for the rapid analysis of multiple parameters and detection of CECs subgroups.According to available literature,CECs are defined more as CD45?CD146+.In order to avoid thrombocytopenia,and so on,DNA-specific staining such as DRAQ5 was also used in some studies to identify CECs [8] further.
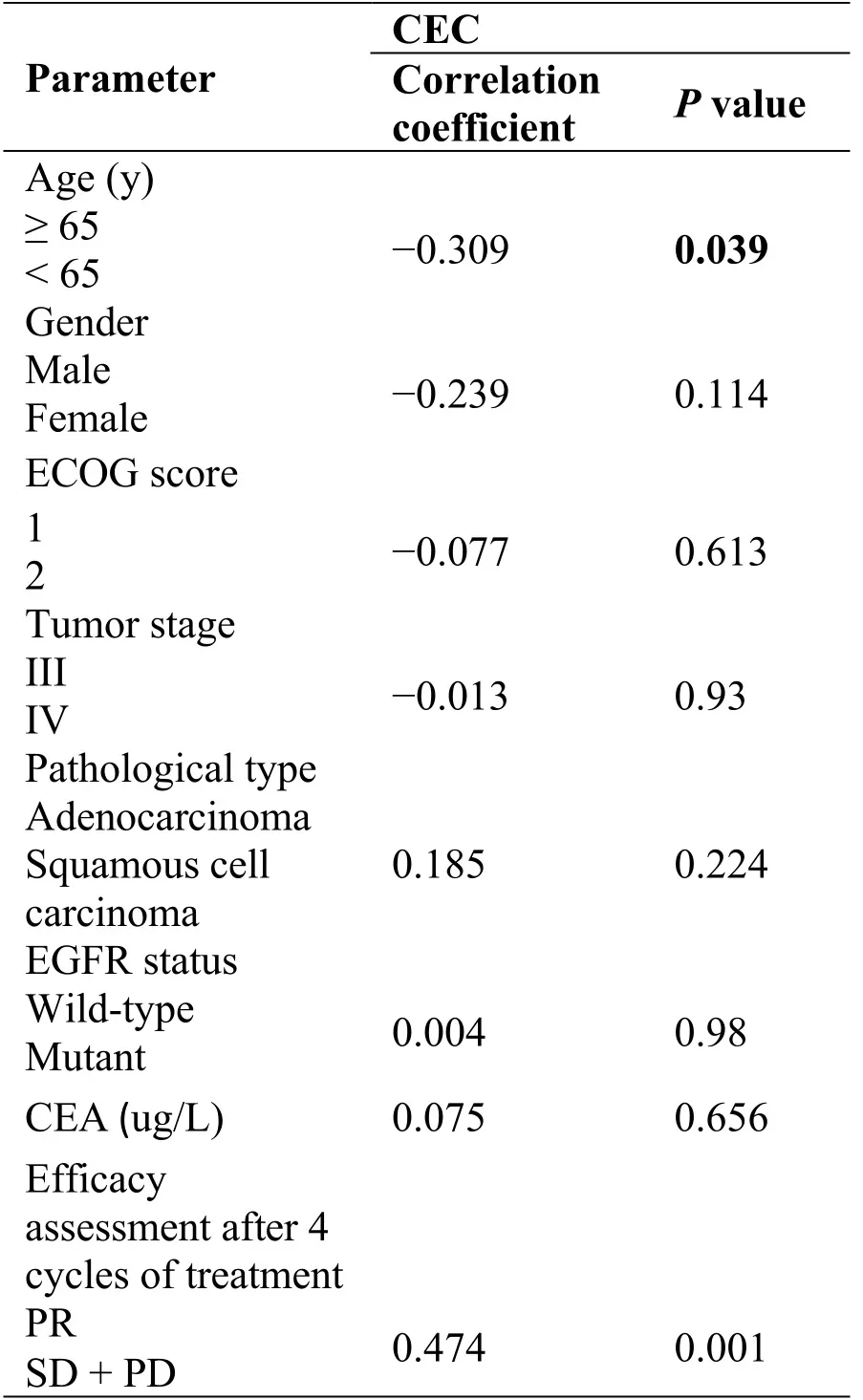
Table2 Analysis of the correlation between CECs and patients’ basic clinical characteristics
In 2006,Huang Chun et al.[22] firstly reported the dynamic change of CECs before and after treatment in NSCLC patients in a randomized,double-blind,controlled trial.They found that the treatment group treated with NP (vinorelbine and cisplatin) and endothelial inhibitors was better than the control group treated with conventional chemotherapy with NP in both short-term and long-term efficacies,showing the value of vascular-targeted therapies in the clinical treatment of NSCLC.The number of CECs had an average decrease of 0.29% ± 0.47% in the treatment group,which was 0.01% ± 0.43% in the control group (P= 0.033),suggesting that CECs may be related to the efficacy and prognosis.After further stratified analysis,the number of posttreatment CECs decreased significantly in beneficiaries of the treatment group but increased slightly in patients with PD.Yang Hong et al.[23] detected the pretreatment and posttreatment CECs in peripheral blood of 20 healthy people and 68 NSCLC patients.They found that the CECs levels in NSCLC patients were significantly higher than the values in the healthy people.After 2 cycles of endostar combined with NP regimen,the number of CECs in patients with CR + PR was lower than that in patients with SD and PD (P<0.001).After treatment,the number of CECs in patients with CR + PR and SD was lower than that before treatment (P<0.001);in patients with PD,the difference in the number of CECs before and after treatment was not statistically significant.The study suggested that endostar could negatively regulate tumor angiogenesis,improve antitumor response,and inhibit tumor growth.Thus,the combined detection of the CECs before and after treatment predicted the efficacy of endostar combined with the NP regimen for NSCLC therapy.According to the study of Vasseur A et al.[24] in metastatic breast cancer with negative HER-2,CEC count was obtained from 251 patients at baseline and from 207 patients after a 4-week treatment.No statistical difference was observed in CEC count between the baseline and 4-week treatment.In the single variable and multivariate analysis,the high baseline CEC count was associated with the short progression-free survival.The study confirmed that CEC count might be related to the prognosis of chemotherapy and bevacizumab for patients with advanced metastatic breast cancer.
Our study indicated that the level of pretreatment CECs significantly correlated with treatment efficacy(P<0.05) but irrelevant to the patient's physical condition,pathological type,tumor stage,and serum CEA level (P>0.05).Moreover,there was a significant difference in CECs count between the groups of response (PR) and nonresponse (SD + PD) (P<0.05).Further,the level of pretreatment CECs significantly correlated with the efficacy of first-line therapy for NSCLC.Therefore,compared with a low level of CECs,a high level of CECs led to poor efficacy.These results are consistent with previous studies.Therefore,CEC is an effective indicator for predicting the efficacy of first-line therapy for advanced NSCLC.CECs could be used as a potential peripheral blood marker to monitor treatment progress in malignant tumors.
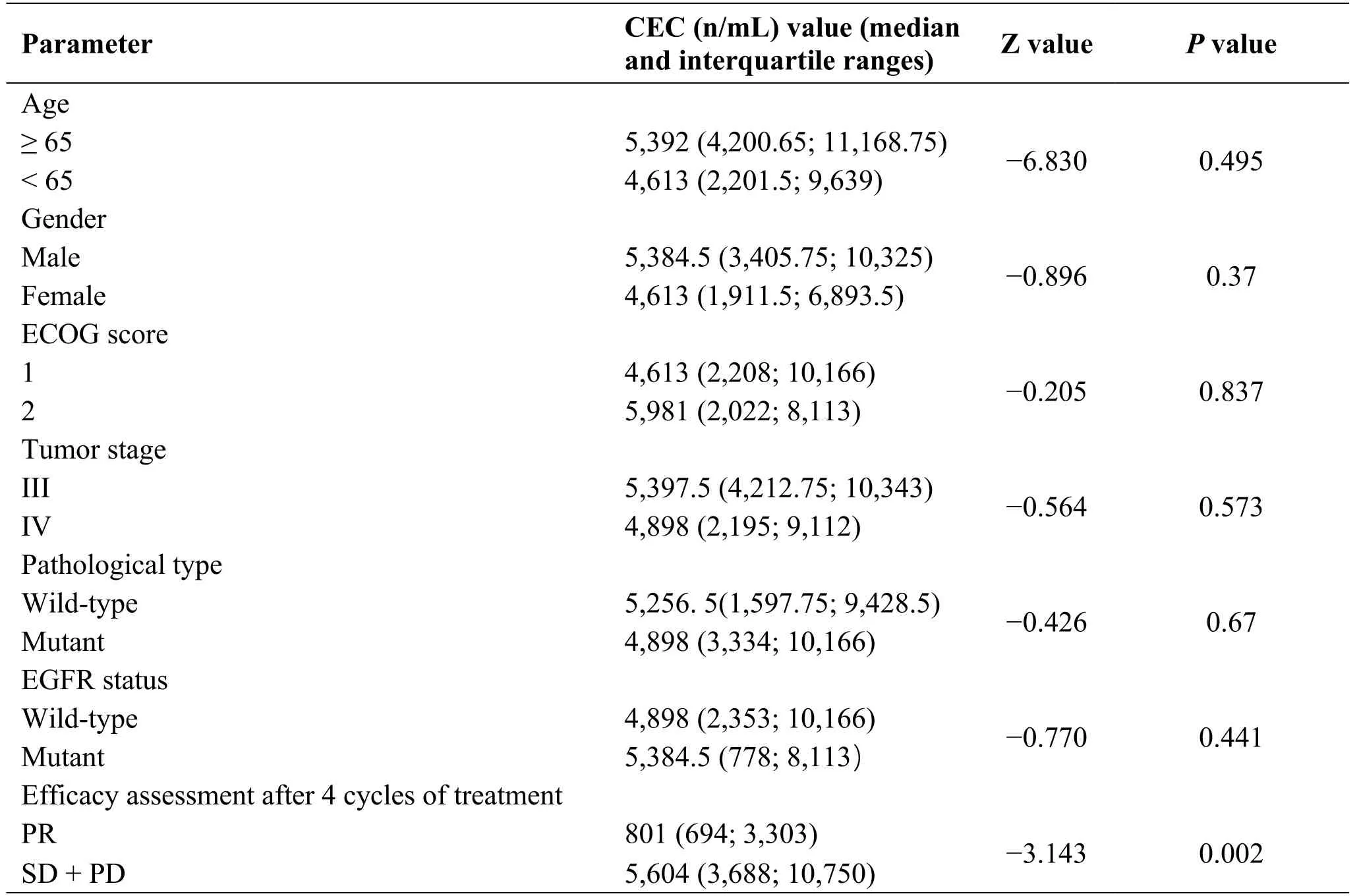
Table3 Differences in CECs for different clinical characteristics
Conclusion
Our study suggests that the level of pretreatment circulating endothelial cells significantly correlated with the efficiency of first-line therapy for non-smallcell lung cancer.Compared with low level of circulating endothelial cells,high level of circulating endothelial cells lead to poor efficacy.Therefore,circulating endothelial cell is indeed an effective indicator for predicting the efficacy of first-line therapy for advanced non-small-cell lung cancer.
- Cancer Advances的其它文章
- Anti-cancer drugs targeting using nanocarrier niosomes-a review
- Analysis of cancer incidence and mortality in Heilongjiang cancer registries,2016
- Clinical Research progress of traditional Chinese medicine in treating esophageal cancer
- Thick tongue coating:diagnostic markers for metastatic colorectal cancer?

