OsABA8ox2, an ABA catabolic gene, suppresses root elongation of rice seedlings and contributes to drought response
Yn Zhng*, Xioping Wng,Ynzhong Luo Ln Zhng Yun Yo, Lu Hn,Zhenhu Chen Lei Wng*, Yuin Li*
aBiotechnology Research Institute, Chinese Academy of Agricultural Sciences,Beijing 100081,China
bCollege of Life Science and Technology,Harbin Normal University,Harbin 150025,Heilongjiang, China
ABSTRACT In rice,OsABA8ox encodes abscisic acid(ABA) 8′-hydroxylase,which catalyzes the committed step of ABA catabolism. The contribution of ABA catabolism in root development remains unclear.We investigated the role of OsABA8ox2 in root growth and development and drought response. GUS staining results showed that OsABA8ox2 was expressed mainly in roots at seedling stage and was strongly expressed in the meristematic zone of the radicle.OsABA8ox2 expression in roots was markedly decreased after 0.5 h polyethylene glycol(PEG)treatment and increased after 0.5 h rehydration, implying that OsABA8ox2 is a drought-responsive gene.OsABA8ox2 knockout mediated by the CRISPR-Cas9 system increased drought-induced ABA and indole-3-acetic acid accumulation in roots,conferred increased ABA sensitivity,and promoted a more vertically oriented root system architecture (RSA) beneficial to drought tolerance.OsABA8ox2 overexpression suppressed root elongation and increased stomatal conductance and transpiration rate.Consequently,OsABA8ox2 knockout dramatically improved rice drought tolerance,whereas OsABA8ox2 overexpression seedlings were hypersensitive to drought stress,suggesting that OsABA8ox2 contributes to drought response in rice.Compared with wild type,functional leaves of OsABA8ox2 knockout seedlings showed higher ABA levels, whereas overexpression lines showed lower ABA levels,suggesting that OsABA8ox2,as an ABA catabolic gene,modulates ABA concentration through ABA catabolism.OsABA8ox2 and OsABA8ox3 were both localized in the endoplasmic reticulum.Together,these results indicate that OsABA8ox2 suppresses root elongation of rice seedlings,increases water transpiration,and contributes to drought response through ABA catabolism, and that OsABA8ox2 knockout dramatically improves rice drought tolerance. They highlight the key role of ABA catabolism mediated by OsABA8ox2 on root growth and development. OsABA8ox2, as a novel RSA gene, would be a potential genetic target for the improvement of rice drought tolerance.
1. Introduction
Abscisic acid(ABA)regulates many key processes during plant growth and development, including seed maturation and dormancy, germination, leaf morphology and cold-induced pollen sterility [1-6]. ABA also plays vital roles in plant responses to various environmental stresses including drought, salt, and cold [7-10]. In these key roles, endogenous bioactive ABA level is under fine-tuning through de novo biosynthesis,catabolism,conjugation and transportation[11].
A prominent subject in ABA research is how ABA regulates plant development and physiological status in response to drought stress, allowing the plant to survive in water-poor growth environments.In rice(Oryza sativa),reduced stomatal aperture and transpiration rate evoked by changes in ABA level or signal transduction enhanced water retention capacity, resulting in increased tolerance to drought stress [10,12].Root system architecture (RSA) is also crucial for plant survival. Drought-resistant plants could appropriately adapt their RSA to water-poor soil environments.The modulation of root system in plants under drought stress is mediated by ABA and the crosstalk with other phytohormones,i.e.indole-3-acetic acid (IAA) [13]. GmWNK1, which can be downregulated by ABA, is involved in the repression of lateral root formation[14].GmWNK1 can interacts with GmCYP707A1,an ABA 8′-hydroxylase in soybean. Under control condition, PEG and ABA treatments, primary root elongation rate and root hair density were significantly lower in the ABA biosynthetic mutant aba3-1 than in wild-type (WT) Arabidopsis, demonstrating the effect of ABA on root tip responses under moderate water stress [15]. ABA and the auxin signaling pathway interact to modulate root growth under drought stress [15,16]. ABA accumulation modulates auxin transport in the root tip, which increases proton secretion and thereby maintains or promotes primary root elongation and root hair development under moderate water stress[15].
ABA catabolism is important in contribution to the ABA concentration in plant. The hydroxylation of the 8′-methyl group of ABA is the committed step of ABA catabolism. It is catalyzed by ABA 8′-hydroxylase, which is encoded by the CYP707A gene in Arabidopsis[17,18]and the OsABA8ox gene in rice [19]. ABA catabolism plays important roles in plant growth and development and response to diverse endogenous and environmental signals [20-24]. The cyp707a3-1 mutant accumulated more stress-induced ABA than WT plants and showed increased drought tolerance [22].Wheat TaABA8’OH1 deletion lines accumulated higher ABA levels in spikes and caused higher levels of pollen sterility, indicating that ABA and ABA 8′-hydroxylase play an important role in controlling anther ABA homeostasis and reproductive-stage abiotic stress tolerance in cereals[23].bHLH122 bound directly to the G-box/E-box cis-elements in the CYP707A3 promoter and repressed its expression, resulting in substantially increased cellular ABA levels [24]. Transgenic plants overexpressing bHLH122 displayed higher resistance to drought, NaCl and osmotic stresses than WT plants [24]. The increase of endogenous ABA, which induced prompt stomata closure in Abz-F1 (an ABA 8′-hydroxylase inhibitor) treated leaves, may depend on inhibition of MdCYP707As [25]. However, the contribution of ABA catabolism to modulation of root growth and development under drought stress remains unclear.
In rice, OsABA8ox1, OsABA8ox2, and OsABA8ox3 encode ABA 8′-hydroxylase [26]. OsABA8ox1 was induced by cold stress within 24 h and OsABA8ox2 and OsABA8ox3 were not[27]. OsABA8ox2 and OsABA8ox3 were ABA-inducible, but OsABA8ox1 was not [27]. The diversity of inductive expressions of OsABA8ox implies the diversity of their physiological functions.The rapid decrease in ABA levels in submerged rice shoots is controlled partly by ethylene-induced expression of OsABA8ox1 [26]. OsABA8ox1 was dramatically induced by rehydration, ultimately reducing ABA content in rice leaves[28]. Transgenic rice lines overexpressing OsABA8ox1 showed decreased levels of ABA and increased seedling vigor during cold stress [27]. Both OsABA8ox2 and OsABA8ox3 are induced early in seed germination and lead to a decrease in ABA level during seed germination [5]. Little is known about the role of rice ABA catabolic genes during drought stress except OsABA8ox3. OsABA8ox3 was the most highly expressed gene of the OsABA8ox family in rice leaves and was rapidly induced by rehydration after PEG-mimic dehydration [29]. Compared with WT seedlings, OsABA8ox3 RNAi lines showed significantly increased drought stress tolerance, whereas overexpression lines were hypersensitive to drought stress[29].
This study aimed to investigate the role of OsABA8ox2 in root growth and development and drought response.
2. Materials and methods
2.1. Plant materials
The rice japonica cultivar Kitaake was used as wild type (WT).The OsABA8ox2 knockout (KO) and overexpression (OE) lines were generated in the Kitaake background. Rice plants were grown in a plant growth chamber under a 14 h light/10 h dark photoperiod at 28/25°C.
2.2. Vector construction and rice transformation
To construct a vector for OsABA8ox2 (LOC_Os08g36860)knockout, a 19-bp sequence (5'-GGAGGAGAGATGTTGGACA-3′) at nucleotide positions 92-110 of the 1533-bp full-length coding sequence (CDS) of OsABA8ox2 was selected for the generation of single-guide RNA(sgRNA)using E-CRISP(http://www.e-crisp.org/E-CRISP/designcrispr.html). The primer pair OsABA8ox2-CRIS (Table S1) was phosphorylated using T4 polynucleotide kinase (3′ phosphatase plus; NEB, Ipswich,MA, USA), annealed and inserted into the BsaI site of the linearized sgRNA scaffold vector pHUN4c12[30].
To construct a vector for OsABA8ox2 overexpression, the entire OsABA8ox2 CDS was amplified by reverse transcription polymerase chain reaction (RT-PCR) using the primer pair OsABA8ox2-OE (Table S1). The fragment was introduced into the modified pCAMBIA1303 vector by homologous recombination using a GBclonart Seamless Cloning Kit (GBI, Suzhou,China). The modified vector contains an Ubi-1 promoter to drive the transcription of the full-length CDS of OsABA8ox2.
For OsABA8ox2 promoter analysis, a fragment containing 1619 bp upstream of the ATG start codon and the first 25 bp of the CDS was amplified with the primer pair OsABA8ox2-Pro(Table S1) and inserted in front of the 5′ end of GUS in pCAMBIA1303 by homologous recombination using GBclonart Seamless Cloning Kit. For OsABA8ox1 promoter analysis, a fragment containing 894 bp upstream of the ATG start codon and the first 79 bp of the CDS was amplified with the primer pair OsABA8ox1-Pro (Table S1). For OsABA8ox3 promoter analysis, a fragment containing 1417 bp upstream of the ATG start codon and the first 199 bp of the CDS was amplified with the primer pair OsABA8ox3-Pro(Table S1).
The above constructs were verified by sequencing and then introduced into the WT plant by Agrobacterium-mediated transformation as described previously [31] with minor modifications. Hygromycin B (50 mg L?1) was used to select transformants.
To identify OsABA8ox2 KO lines generated by the CRISPRCas9 system,PCR amplification and sequencing of a genomic fragment containing the target sequence were performed using the primer pair OsABA8ox2-target(Table S1).To identify the OsABA8ox2 OE lines, PCR amplification and sequencing were performed using the primer pair OsABA8ox2-C (Table S1).
2.3. GUS staining
Histochemical staining of GUS activity was performed using a standard method. Plant tissue was incubated in X-Gluc staining solution (Coolaber, Beijing, China) at 37 °C. After incubation, tissue was decolorized in 70% ethanol. For microscopic observation, roots were immersed in fixing solution [50% ethanol, 5% glacial acetic acid, 3.7% formaldehyde] after GUS staining and then dehydrated in an ethanol gradient series. The samples were embedded in paraplast(BBI, Shanghai, China) after vitrification. Microtome sections(10 μm) were mounted on poly-L-lysine coated slides (Sigma,St. Louis, MO, USA) and dewaxed with xylene. They were mounted using neutral balsam and observed and photographed under a Zeiss Axio Imager M1 microscope(Carl Zeiss AG, Oberkochen, Germany).
2.4. RT-PCR and quantitative RT-PCR (qPCR)
Total RNA was extracted using TRIzol reagent (Invitrogen,Carlsbad, CA, USA). RNA quality was assessed by agarose gel electrophoresis prior to RQ1 RNase-Free DNase digestion.First-strand cDNA was synthesized from 5 μg total RNA using the SuperScript III First-Strand Synthesis System(Invitrogen). RT-PCR and qPCR experiments were performed with gene-specific primers (Table S1). The rice Actin1 or ubiquitin was used as the reference gene and internal control.qPCR was performed in the reaction system of SYBR Green Realtime PCR Master Mix (TOYOBO, Osaka, Japan) on the ABI 7500 Real-Time System. Values of relative mRNA levels in qPCR were calculated from the means of three biological replicates.Each cDNA template was assayed in triplicate.
2.5. Plant growth and drought stress treatment
To mimic physiological dehydration,roots of 3-week-old WT,OsABA8ox2 OE2 and KO1 seedlings were treated with 15%polyethylene glycol(PEG)6000 for 4 h,and then transferred to 1/2 Hoagland nutrient solution for rehydration. Roots were harvested at defined time points to analyze the expression profiles of OsABA8ox genes and measure ABA and IAA contents. Generally, 15% PEG-6000 treatment can produce a drastic dehydration effect and is appropriate for transient treatment, owing to its substantial physiological impacts.Therefore, 5-day 2% PEG-6000 treatment was performed to characterize root morphology of young seedlings under mild dehydration. And 7-day-old seedlings were grown on germination bags containing 2% PEG-6000 solution or sterile distilled water.Soil drought tolerance assays were performed at the five-leaf stage of WT, OsABA8ox2 OE and KO plants by withholding irrigation.
2.6. Measurement of ABA and IAA contents
Three-week-old roots after 4 h of PEG-mimic dehydration treatment and five-leaf-stage functional leaves of WT,OsABA8ox2 KO1 and OE2 plants were used to measure ABA and IAA contents,which were determined using a previously described method [32] with minor modifications. Briefly, this method employs an automatic liquid handling system for solid phase extraction and ultra-performance liquid chromatography (UPLC) coupled with a tandem quadrupole mass spectrometer (qMS/MS) equipped with an electrospray interface (ESI; UPLC-ESI-qMS/MS). ABA and IAA contents were assayed in three biological repeats.
2.7. Measurement of photosynthetic indices
Functional leaves of five-leaf-stage seedlings were used to measure stomatal conductance,transpiration rate,net assimilation rate and intercellular CO2concentration using a portable photosynthesis system(Li-6800,LI-COR Inc.,Lincoln,NE,USA)at a CO2concentration of 400 μmol mol?1,a flow rate of 500 μmol s?1to the sample cell, and a quantum sensor of 1200 μmol m?2s?1. Steady-state levels of reference CO2and H2O were observed before taking the measurements.
2.8. Measurement of malondialdehyde (MDA) content
MDA content was measured using an MDA Content Assay Kit(Solarbio, Beijing, China) according to the manufacturer's protocols. The condensation reaction of MDA with thiobarbituric acid produces brownish red 3,5,5-trimethyloxazolidine-2,4-dione (tridione), which has a maximum absorption at 532 nm. Leaf blades used to measure MDA content were collected from one-month-old seedlings under control conditions or after withholding irrigation for 10 days.MDA content was assayed in three biological repeats.
2.9. Subcellular localization
To investigate the subcellular localization of OsABA8ox2 and OsABA8ox3, CaMV35S:OsABA8ox2-GFP and CaMV35S:OsABA8ox3-GFP were constructed. The CDS of OsABA8ox2 and OsABA8ox3 were amplified by RT-PCR with primer pairs OsABA8ox2-SL and OsABA8ox3-SL, respectively (Table S1). The amplified product was cloned into the vector pRTL2 [33] by homologous recombination using a GBclonart Seamless Cloning Kit. Protoplast preparation and transfection were performed as described previously [34] with some minor modifications. Rice protoplasts were co-transformed with the following plasmids: (1) CaMV35S:OsABA8ox2-GFP and ERrk; (2) CaMV35S:OsABA8ox3-GFP and ER-rk; (3) CaMV35S:GFP and ER-rk. ER-rk was used as an ER marker [35]. The protoplasts were incubated in WI solution [0.6 mol L?1mannitol, 4 mmol L?1KCl, and 4 mmol L?1MES (pH 5.7)]. The GFP and ER-rk mCherry signals were observed under a Zeiss LSM 700 Meta confocal microscope (Carl Zeiss AG, Oberkochen,Germany).
2.10. Statistical analysis
Significant differences between means were identified by Student's t-test(P <0.05, P <0.01) using“T.TEST”in Microsoft Excel (Microsoft, Redmond, WA, USA) or Duncan's multiple range test using SPSS V17(SPSS Inc.,Chicago,IL,USA).
3. Results
3.1. Rice OsABA8ox2 is a drought-responsive gene in roots
Transgenic rice plants separately harboring the PROOsABA8ox1:GUS, PROOsABA8ox2:GUS, and PROOsABA8ox3:GUS constructs were examined by GUS staining. The results indicated that OsABA8ox3 was expressed mainly in leaves of seedlings (Fig.1-A),and OsABA8ox2 mainly in roots(Fig.1-B).OsABA8ox1 was hardly detected at seedling stage(Fig.1-A,B).OsABA8ox2 was expressed mainly in the meristematic zone of the radicle(Fig.1-C-E), and was also observed in the stele and lateral root primordia (LRP) but not in the root cap (Fig. 1-E, F). Examination of the cross section of the GUS-stained roots revealed that OsABA8ox2 expression was strong in the primary meristem(Fig.1-H),and was also detected in epidermis cell,exodermis,and residual cortical parenchyma cells (Fig. 1-G). In seedlingstage roots,higher levels of OsABA8ox2-GUS expression were observed in young adventitious roots and lateral roots than in old roots.
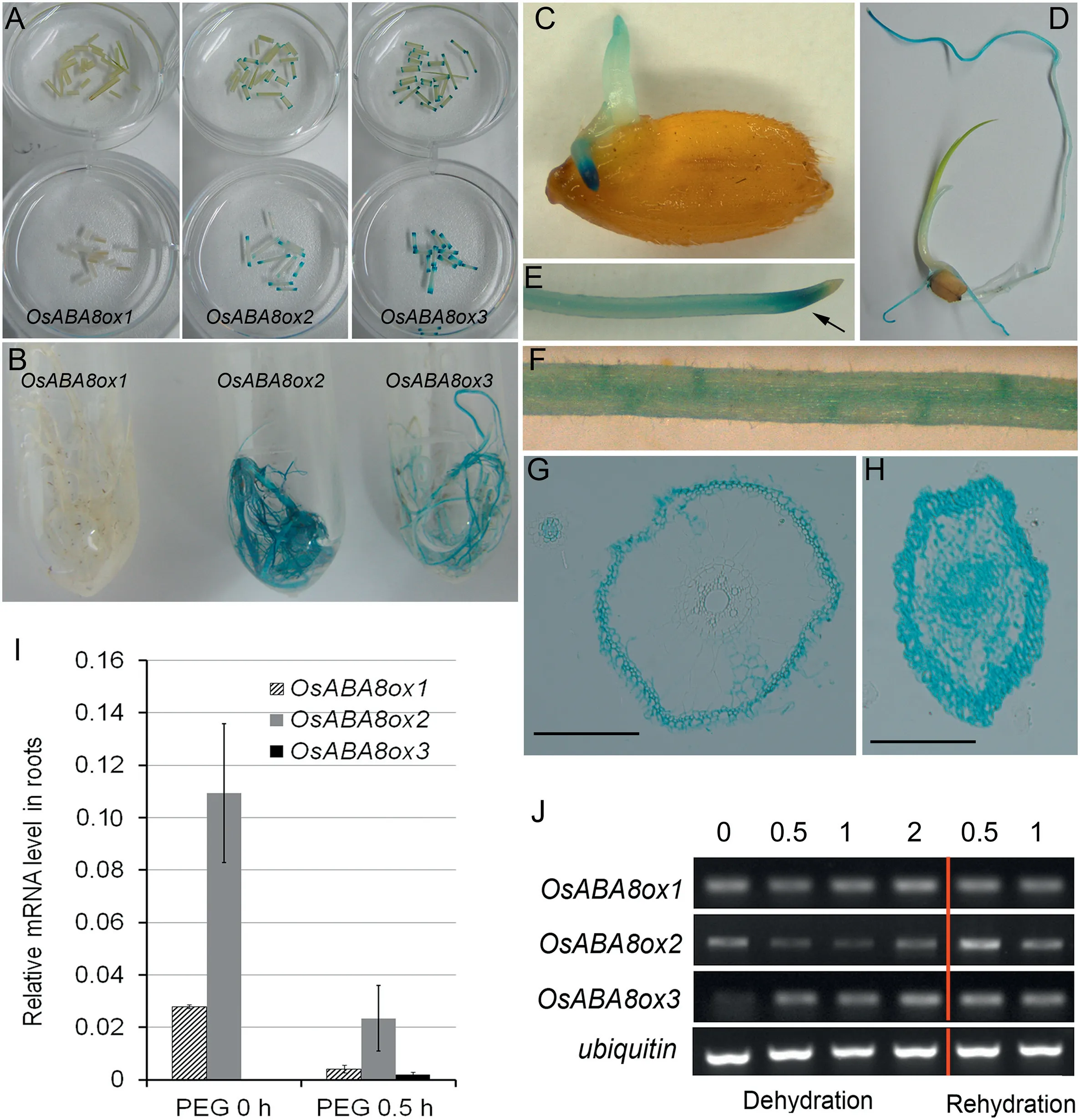
Fig.1-Expression analysis of OsABA8ox genes.GUS staining in PROOsABA8ox:GUS(A,B)and PROOsABA8ox2:GUS transgenic plants(C-H).(A)Leaf blade(top),leaf sheath(bottom).(B)roots.(C)Seed after 4-day germination.(D)Seven-day-old seedling.(E)Arrow indicates the meristematic zone of the radicle.(F)Lateral root primordial(LRP).(G,H)Cross sections of the roots of 3-week-old seedlings. Bars 100 μm.(H)Meristematic zone of root.(I, J)Changed expressions of OsABA8ox genes in 3-week-old rice roots after 15%PEG-6000 treatment.The rice ubiquitin gene was used as internal control and reference gene.Values are mean and standard deviation(SD)of three independent experiments(I).
To test whether OsABA8ox expressions in roots are drought-responsive, expressions in roots under 15% PEG-6000 treatment were measured by qPCR and RT-PCR (Fig. 1-I,J). In seedling-stage roots, OsABA8ox2 showed the highest expression level among the three OsABA8ox genes (Fig. 1-I).OsABA8ox2 expression in roots was markedly decreased after 0.5 h PEG treatment and increased after 0.5 h rehydration(Fig.1-J), implying that OsABA8ox2 expression is drought responsive. Slight upregulation of OsABA8ox3 after 0.5 h PEG treatment was observed.
3.2. OsABA8ox2 knockout plants show increased rice drought tolerance
To investigate the role of OsABA8ox2 gene in drought stress,targeted mutagenesis of OsABA8ox2 using the CRISPR-Cas9 system was performed. The mutated target sequences of two homozygous KO lines are shown in Fig.S1-A.KO1 showed a G insertion between nucleotide positions 107 and 108 of the 1533-bp full-length CDS of OsABA8ox2. KO2 showed deletions at nucleotide positions 105 and 106. To investigate ABA catabolism-enhanced phenotype in rice, a vector construct containing Ubi-1 promoter-driven full-length CDS of OsABA8ox2 was transformed into WT rice to obtain OsABA8ox2 OE lines. Positive lines were identified by amplification of the exogenous CDS fragment of OsABA8ox2(Fig.S1-B).The transcripts of OsABA8ox2 in WT,OE and KO lines were quantified by qPCR,and the expression levels in OE lines were higher than that in WT(Fig.S1-C).
After 4 day of unwatering, two OsABA8ox2 OE lines (OE1 and OE2)exhibited curly leaves,whereas the leaves of WT and KO lines (KO1 and KO2) were still explanate (Fig. 2-B). After 5 day of unwatering, WT plants also exhibited curly leaves and OE lines showed severe wilting phenotype,whereas more than half of the leaves of KO lines were still explanate(Fig.2-C). The plants were rewatered after 7 days of soil drought treatment. Vigor of all the KO plants was recovered after 2 days of rewatering (Fig.2-D).After 2 days of rewatering,the survival rates of OE1 and OE2 were both much lower, while that of KO seedlings was much higher than that of WT(Fig.2-E). The survival rates after rehydration indicate that OsABA8ox2 KO lines showed increased drought stress tolerance, whereas OE seedlings were hypersensitive to soil drought stress in comparison with WT.
3.3. OsABA8ox2 KO plants show increased ABA and IAA accumulation in roots under drought stress
ABA contents in roots of WT and OsABA8ox2 KO1 seedlings were both increased after 4 h of PEG-mimic dehydration treatment (Fig. 3). KO1 plants showed a 1.3-fold increase in ABA level after dehydration treatment. KO1 plants showed higher ABA levels than WT after dehydration, possibly owing to impaired ABA catabolism. In contrast, the ABA content in OsABA8ox2 OE2 seedlings after dehydration treatment was almost unchanged. These results suggest that OsABA8ox2 knockout promotes ABA accumulation in roots, leading to response and adaptation to drought stress, and that OsABA8ox2 is a key gene regulating ABA accumulation in roots under drought stress.Impaired ABA catabolism in roots caused enhanced ABA accumulation when rice roots experienced water deficit, which was in agreement with the markedly decreased expression of OsABA8ox2 after 0.5 h PEG treatment.
The ABA and auxin signaling pathways interact to modulate root growth under drought stress[15,16].The IAA content in the roots of OsABA8ox2 KO1 seedlings was dramatically elevated after 4 h of PEG-mimic dehydration treatment,whereas that of WT was hardly changed(Fig.3).
3.4. OsABA8ox2 overexpression suppresses root elongation of rice seedlings, while KO seedlings develop more vertically oriented RSA beneficial to drought tolerance
Five-day 2% PEG-6000 treatment was performed to characterize root morphology of young seedlings under mild dehydration condition. Length of primary root and length and number of lateral roots of 12-day-old OsABA8ox2 OE2 seedlings were all much lower than those of WT seedlings whether under control or 2% PEG-6000 treatment (Fig. 4-A,Table 1). Density of lateral roots of OE2 was much higher than that of WT and KO1 under control conditions (Table 1).These results suggest that OsABA8ox2 promote lateral root germination and suppresses the elongation of primary and lateral roots of young seedlings. Number of lateral roots of KO1 was increased after 5-day PEG treatment (Table 1).Shoots of KO1 were shorter than those of WT under control conditions(Table 1).Shoot elongation of WT was suppressed under PEG treatment, whereas that of OE2 was not (Fig. 4-A,Table 1).OsABA8ox2 overexpression suppressed root elongation and promoted shoot elongation, resulting in a length ratio of root to shoot of KO1 more than twice that of OE2(Fig.4-A, Table 1).
Root morphology and parameters under control and soil drying were characterized. Soil drought stress was imposed on one-month-old seedlings by withholding irrigation for 2 weeks.Total root length,diameter of adventitious roots,and total root weight of OsABA8ox2 OE2 seedlings were all much lower than those of WT seedlings whether under control or drought treatment (Fig. 4-B, Table 2). Lateral root growth of WT and KO1 seedlings were markedly increased under soil drying (Fig. 4-B). After 2 weeks of unwatering, the total number of adventitious roots of KO1 was less than that under control condition,and the number of adventitious roots whose lengths ≥70%of total root length was greater than that of WT(Table 2),resulting in a more vertically oriented RSA of KO1.
3.5. OsABA8ox2 overexpression increases stomatal conductance and transpiration rate
Compared with WT, functional leaves of five-leaf-stage OsABA8ox2 KO1 seedlings showed higher ABA levels,whereas OE2 leaves showed lower ABA levels(Fig.5-A),suggesting that OsABA8ox2, as an ABA catabolic gene, modulates ABA concentration through ABA catabolism. In contrast, IAA content in OE2 leaves was higher than that of WT (Fig. 5-A).Stomatal conductance, transpiration rate, and net assimilation rate of OE2 leaves in daylight were much higher than those of WT (Fig. 5-B). Increased stomatal conductance and transpiration rate led to excessive transpirational water loss(Fig.5-C),probably leading to water stress in OE lines even under normal soil conditions. We further analyzed MDA content in leaves, as it is often used as a biomarker to evaluate cell membrane injury and cell and tissue oxidative damage [36]. The MDA content was significantly increased after 10-day drought-stress treatment in OE2 leaves,whereas it was not in WT and KO1 leaves(Fig.5-D).This result suggests that OsABA8ox2 OE2 seedlings suffer more severe injury than WT and KO1 seedlings under the same drought-stress treatment.
3.6. OsABA8ox2 knockout retards post-germination growth and increases ABA sensitivity
Hypersensitivity to ABA in plants is closely associated with increased drought tolerance [10,22,29,37]. To examine the ABA sensitivity of WT, OsABA8ox2 OE, and KO plants, seed germination and post-germination growth were treated with ABA (1 μmol L?1and 5 μmol L?1) and the root lengths after 6-day imbibition were measured (Fig. 6). Radicle growth of OsABA8ox2 KO1 was markedly retarded in comparison with that of WT under water treatment. Radicle growth of KO1 was strongly suppressed under 1 μmol L?1ABA treatment. After 5 μmol L?1ABA treatment, radicle growth of KO1 and WT were severely suppressed, while radicle growth of OE2 was moderately suppressed. Thus, radicle growth of OsABA8ox2 KO1 showed hypersensitivity to exogenous ABA, wherease OsABA8ox2 OE2 showed lower sensitivity than WT. Both OsABA8ox2 and OsABA8ox3 are significantly induced early in seed germination and are responsible for the decrease of ABA level during seed germination [5]. The result (Fig. 6) further substantiated that OsABA8ox2 contributes to seed germination and post-germination growth.
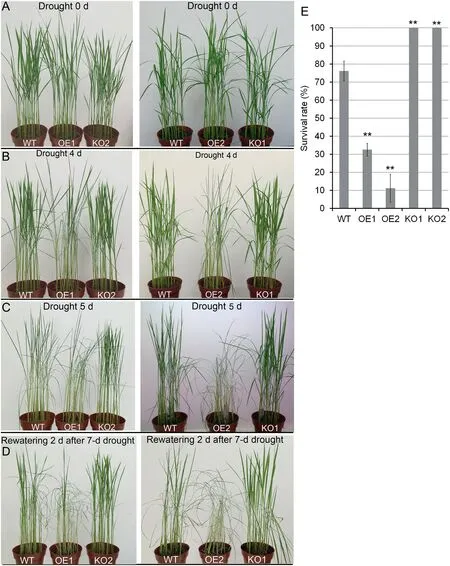
Fig.2- Drought stress tolerance assays.OE1 and OE2,two OsABA8ox2 OE lines.KO1 and KO2,two OsABA8ox2 KO lines.(A-D)Performance of WT,OE,and KO lines after soil drought stress and rewatering.(B)OE1 and OE2 showed curly leaves.(C)KO1 and KO2 showed higher drought tolerance.(D)Distinct performances of WT,OE,and KO lines after rewatering.(E)Survival rate of rice seedlings after drought stress and rewatering.Values are mean±SD(n =36).Asterisks indicate significant differences between the WT and KO(or OE)seedlings(Student's t-test,** P <0.01).
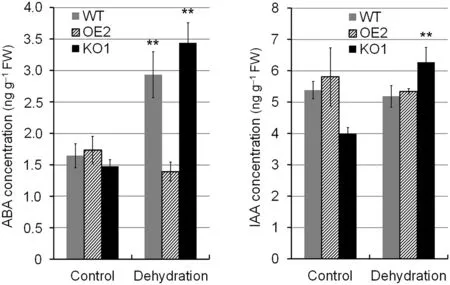
Fig.3-ABA and IAA contents in the roots of WT,OsABA8ox2 OE2 and KO1 transgenic seedlings.Roots of 3-week-old seedlings were treated with 15%PEG6000 for 4 h.Samples(approximately 300 mg)were quickly frozen with liquid nitrogen.Values are mean±SD of three independent experiments. Asterisks indicate significant differences between control and PEG treatment(Student's t-test,**P <0.01).
3.7. OsABA8ox2 and OsABA8ox3 are both localized in endoplasmic reticulum (ER)
OsABA8ox2 and OsABA8ox3 were predicted to be located in the ER by Plant-mPLoc (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/). To verify this prediction, OsABA8ox2-GFP and OsABA8ox3-GFP fusion protein driven by the CaMV 35S promoter were transiently expressed in rice mesophyll protoplasts respectively. The merged confocal image showed the co-localization of OsABA8ox2-GFP and ER marker, indicating the ER localization of OsABA8ox2, as well as OsABA8ox3 (Fig. S2). It has been reported that OsABA8ox1 protein is localized in the ER [26]. The ER localization of OsABA8ox1, OsABA8ox2 and OsABA8ox3 implies that ABA catabolism via 8′-hydroxylation occurs in the ER.
4. Discussion
4.1. Expression specificity of OsABA8ox2

Fig.4- Root morphology of WT,OsABA8ox2 KO1 and OE2 transgenic seedlings.(A)Twelve-day-old seedlings grown on germination bags after 5-day 2%PEG-6000 treatment.Seedlings grown on germination bags containing sterile distilled water were used as control.White frame indicates the altered length ratio of root to shoot of KO1 and OE2.(B) Root of 6-week-old seedlings under soil drought stress imposed by withholding irrigation for 2 weeks.Lateral root growth of WT and KO1 was clearly increased under drought stress(top panel).Total root length of OE2 was much lower than those of WT and KO1.
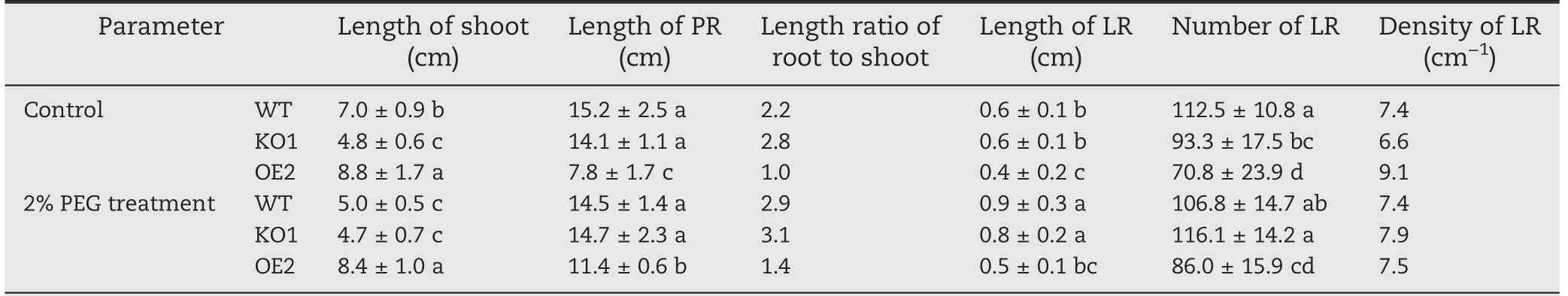
Table 1-Root and shoot parameters of the 12-day-old WT,OsABA8ox2 KO1 and OE2 transgenic seedlings.
The OsABA8ox family consists of three members, OsABA8ox1,OsABA8ox2 and OsABA8ox3, whose protein sequence similarity was shown in Fig. S3. The amino acid sequences of OsABA8ox2 and OsABA8ox3 display very high identity, but they showed distinct expression patterns. In a previous study [29], seedling-stage OsABA8ox expression at transcription level was consistent with the GUS staining results shown in the present study. At seedling stage, OsABA8ox2 was expressed mainly in roots (Fig. 1-B, I) whereas OsABA8ox3 was expressed mainly in leaves (Fig. 1-A), implying distinct roles of OsABA8ox2 and OsABA8ox3 in rice seedling growth and development.
4.2. OsABA8ox2 contributes to drought-induced ABA and IAA accumulation in roots
The dehydration-induced accumulation of endogenous ABA contents in OsABA8ox2 KO1 roots was greater than that of WT (Fig. 3), perhaps owing to the impairment of ABA catabolism caused by OsABA8ox2 knockout. ABA catabolism mediated by OsABA8ox2 was involved in drought-induced ABA accumulation in roots. OsABA8ox3 RNAi lines showed increased drought resistance and higher accumulation of the endogenous ABA contents than WT [29]. Thus, both OsABA8ox2 and OsABA8ox3 contribute to drought-induced ABA accumulation.
Liang et al. found that when3H-ABA was loaded into roots, the half-life of fed3H-ABA was prolonged during soil drying compared with that in normal condition, indicating a slowed ABA catabolism in roots caused by soil drying [38]. In this study, OsABA8ox2 expression in roots was markedly decreased after 0.5 h PEG treatment (Fig. 1-I, J). A previous gene chip analysis showed that OsABA8ox2 expression in flag leaves under drought stress was also downregulated [37]. Thus, impaired ABA catabolism caused by downregulated OsABA8ox2 expression is found in both root and leaf of rice seedlings under drought stress. OsABA8ox2 expression was increased again after 2 h PEG treatment, perhaps owing to the induction by accumulated ABA. Therefore the decreased OsABA8ox2 expression in roots under PEG treatment was a temporary event.
Increasing evidence points to the important role of auxin in mediating root architecture changes during drought stress [39-41]. The increased IAA content in OsABA8ox2 KO roots under drought stress might be required for the maintenance or promotion of root growth during drought stress, and crosstalk between OsABA8ox2 and auxin signaling may be involved in the adaptation of RSA.
4.3. OsABA8ox2 shows sophisticated modulation on root growth and root architecture
Lateral root germination of 7-day-old OsABA8ox2 OE seedlings preceded that of KO seedlings (Fig. S4), but elongation of primary and lateral roots of 12-day-old OE lines fell far behind that of KO lines (Fig. 4-A, Table 1). The ability of root elongation to reach deeper soil layers for adequate water and nutrients is crucial for the survival and growth of rice plants. We infer that suppressed root elongation of rice seedlings caused by OsABA8ox2 overexpression (Fig. 4-A, B; Table 1, Table 2) was one of the main reasons for the much lower drought tolerance capacity of OE lines. A narrower and more vertically oriented RSA is typically more drought tolerant [42], and OsABA8ox2 knockout could lead to this architecture and corresponding adaptation to water deficit.
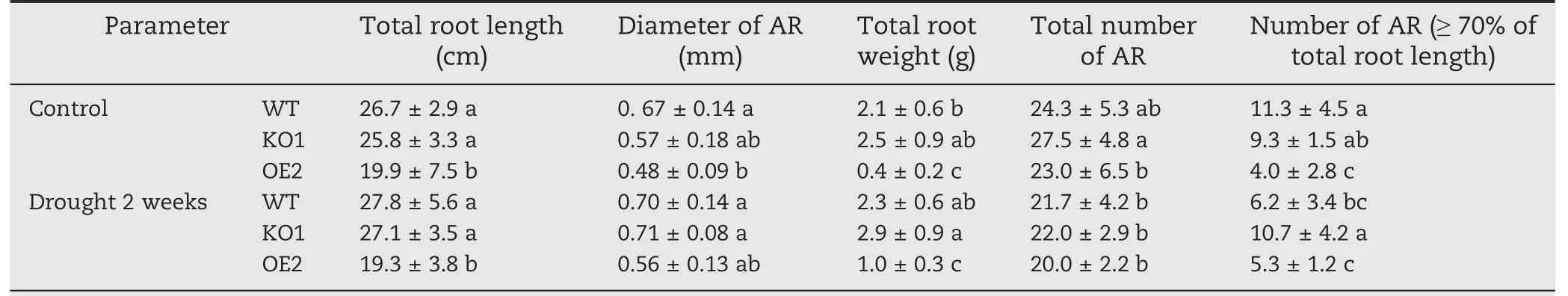
Table 2-Root parameters of the WT,OsABA8ox2 KO1 and OE2 transgenic seedlings under soil drought stress.
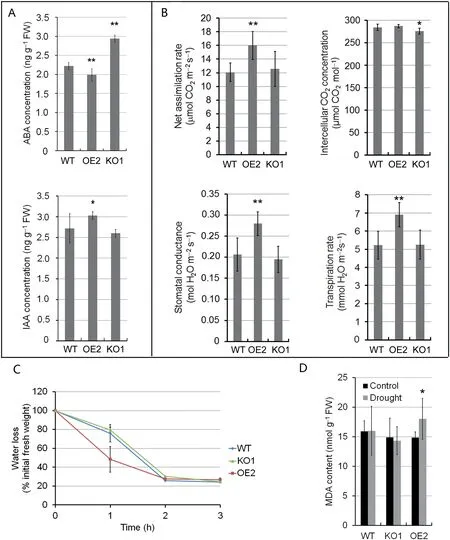
Fig.5-Physiological changes in leaves of WT,OsABA8ox2 KO1 and OE2 seedlings at five-leaf stage.(A)ABA and IAA contents in leaves.Values are mean±SD of three independent experiments.(B) Photosynthetic indices.Values are mean± SD(n= 6).Asterisk in(A)and(B)indicates significant difference between WT and OE2(or KO1)(Student's t-test,*P <0.05,**P <0.01).(C)Water loss from detached leaves.Detached leaves were placed on the laboratory bench at 25°C.Water loss was expressed as the percentage of initial fresh weight.Values are mean±SD(n=12).(D)MDA contents in the leaves of seedlings under control condition or soil drought stress imposed by withholding irrigation for 10 days.Values are mean± SD of three independent experiments.Asterisk indicates significant difference between control and drought treatment(Student's t-test,*P <0.05).
4.4. Excessive transpirational water loss could partially account for the severe wilting phenotype of OsABA8ox2 OE seedlings under drought stress
ABA-induced stomatal closure reduces transpiration and prevents further water loss from leaves [43,44]. Constitutive expression of CYP707A3 could reduce endogenous ABA and increase transpiration [22]. In the present study, excessive transpirational water loss caused by increased stomatal conductance and transpiration rate could be the other main reason for the severe wilting phenotype of OsABA8ox2 OE seedlings under drought stress. We presented evidence that appropriate regulation of OsABA8ox2 expression influences the water retention capacity of rice seedlings.
4.5. Conclusion and perspective
Compared with WT, functional leaves of OsABA8ox2-KO rice seedlings mediated by CRISPR-Cas9 system showed higher ABA levels, whereas OE lines showed lower ABA levels,suggesting that OsABA8ox2, as an ABA catabolic gene,modulates ABA concentration through ABA catabolism.OsABA8ox2 knockout conferred increased ABA sensitivity and retarded post-germination growth. OsABA8ox2 overexpression suppressed root elongation and increased transpirational water loss through enhanced ABA catabolism,resulting in decreased drought tolerance (Fig. 7). OsABA8ox2 overexpression suppressed root elongation and promoted shoot elongation,resulting in decreased length ratio of root to shoot(Fig. 7). Compared with WT, OsABA8ox2 knockout rice dramatically improved drought tolerance and promoted a more vertically oriented RSA beneficial to drought tolerance.These results indicate that OsABA8ox2 suppresses root elongation of rice seedlings and contributes to drought response through ABA catabolism.
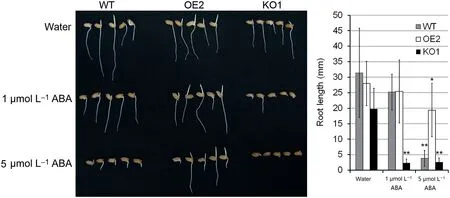
Fig.6- ABA sensitivity of WT,OsABA8ox2 OE2 and KO1 seeds during post-germination growth.(A)Seeds were treated with water and ABA(1 μmol L?1 and 5 μmol L?1)and imbibed at 28°C for 6 days.(B) Root lengths of WT,OE2,and KO1 after 6-day imbibition.Values are mean± SD(n= 10).Asterisk indicates significant difference between control and ABA treatment(Student's t-test,*P <0.05,**P <0.01).
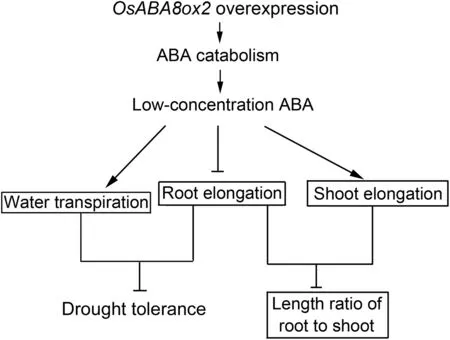
Fig.7- Schematic diagram of the role of OsABA8ox2 in rice growth and development and drought tolerance.Arrows indicate promotion or increase(positive effects).Blocked arrows indicate suppression or decrease(negative effects).OsABA8ox2 overexpression suppresses root elongation and increases transpirational water loss through enhanced ABA catabolism,resulting in decreased drought tolerance.OsABA8ox2 overexpression suppresses root elongation and promotes shoot elongation,resulting in decreased length ratio of root to shoot.
Recently, it has been reported that GmSIN1, a soybean salinity-induced NAC transcription factor, promotes root elongation and salt tolerance by boosting cellular ABA and ROS contents [45]. Appropriate ABA level contributes to root elongation. Here we highlighted the key role of ABA catabolism mediated by OsABA8ox2 on root growth and development. The elongation of the aboveground part of OsABA8ox2 OE seedlings was increased (Fig. 2-A, Fig. 4-A), whereas the root growth of 12-day-old and subsequent OE seedlings was severely impaired (Fig. 4). The consequences of OsABA8ox2 overexpression in rice plants before drought stress include longer leaves and impaired root growth, whereas OsABA8ox2 knockout leads to shorter leaf blades. The partitioning of carbohydrate produced by leaf blade (“source”) can modulate root (“sink”) elongation in Arabidopsis thaliana [46]. Further study of the role of OsABA8ox2 in leaf growth and development would be helpful for essential understanding of root elongation modulated by OsABA8ox2. OsABA8ox2, as a novel RSA gene, would be a potential genetic target for the improvement of rice drought tolerance. Since OsABA8ox2 knockout rice maintains better root growth for drought tolerance than WT, OsABA8ox2 knockout rice could be superior in water-deficient areas or areas where rainfall varies greatly. But biomass and grain yield also must be orchestrated. The clarification of the role of OsABA8ox2 in leaf growth and development would support the targeted use of OsABA8ox2 in future.
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2019.08.006.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31501244), Chinese Academy of Agricultural Sciences Elite Youth Program Grant to Yubin Li and the Fundamental Research Funds for Central Non-profit Scientific Institution (1610392019001).
- The Crop Journal的其它文章
- Brief Guide for Authors
- Crop genome editing: A way to breeding by design
- Less and shrunken pollen 1 (LSP1) encodes a member of the ABC transporter family required for pollen wall development in rice (Oryza sativa L.)
- Mutagenesis reveals that the rice OsMPT3 gene is an important osmotic regulatory factor
- Targeted mutagenesis of amino acid transporter genes for rice quality improvement using the CRISPR/Cas9 system
- Precise base editing of non-allelic acetolactate synthase genes confers sulfonylurea herbicide resistance in maize

