On the regulation of PP2A and its role in controlling sister chromatid cohesion and microtubule-kinetochore attachment
Jin-Wei Yuan
1Tianjin Medical University,Tianjin,300070,China.
Abstract
Protein phosphatase 2A (PP2A) plays a critical multi-faceted role in the regulation of the cell cycle.PP2A is involved in such diverse processes by the formation of structurally distinct families of holoenzymes,which are regulated spatially and temporally by specific regulators.Mitosis requires the correct arrangement of sister chromatids so that replicated chromosomes can bind correctly to microtubules and segregate towards opposite poles.A large number of studies have shown that PP2A is mainly involved in a series of phosphorylation processes during the G2/M phase transition and the termination of M phase.Moreover,PP2A shows a more substantial contribution to sister chromatid cohesion and microtubule-kinetochore attachment.These processes are all crucial for proper cell survival and proliferation and are often deregulated in cancer and other diseases.
Keywords:PP2A,Cell cycle,Sister chromatid cohesion,Microtubule-kinetochore attachment
Background
Phosphorylation and dephosphorylation of proteins are involved in regulating the various biological processes in cell growth,including cell proliferation,development,apoptosis,and metabolism,et al.It is jointly regulated by protein kinases (PK) and protein phosphatases (PP) [1].Previous studies on reversible phosphorylation of proteins have focused on the regulation of PK.And recent studies solidify PP importance,it is now believed that PP,like PK,is closely regulated and plays an important role.There are four families of phosphatases with diverse active sites and mechanisms.And the four classes of phosphatases are: 1) protein serine/threonine phosphatases (PSPs),2) protein tyrosine phosphatases(PTPs),3)dual-specificity phosphatases(DSPs),and 4)histidine phosphatases[2,3].
The PSPs can form a large number of diverse oligomeric complexes and further divided into three families: 1) phosphoprotein phosphatases (PPPs),2)metal-dependent protein phosphatases (PPMs),3)Haloacid dehydrogenases (HAD) (Figure1) [2,3].The PPPs is the largest family of phosphatases,and protein phosphatase 2A (PP2A) is one of the most abundant members of the PPP family.PP2A is ubiquitously expressed and contributes to 0.3-1% of the total cellular protein in eukaryotic cells [4].PP2A is an important player in many cellular functions.It controls cell cycle,DNA replication,transcription and translation,cell metabolism,cell proliferation,apoptosis,cytoskeleton dynamics,signal transduction[5-8].In particular,many studies in recent years have demonstrated that PP2A also plays an important role in cancer [9-11].Therefore,scientists have recently invested more energy to sort out and analyze the upstream and downstream signaling pathways of PP2A,the structure of PP2A and how it functions as a phosphatase,and have made some extremely important progress.Given their vital importance for cell cycle progression,PP2A will be discussed in greater detail.
Structure the PP2A holoenzyme
PP2A is a heterotrimeric complex.Each PP2A holoenzyme is formed by a combination of three subunits: a scaffolding subunit (A),a catalytic subunit(C)and a regulatory subunit(B).The A and C subunits each exist with two possible isoforms α and β,with Aα and Cα accounting for the majority of each subunit in most cells[3-5].The four classes of the B subunit are:1) B (B55/PR55),2) B' (B56/PR61),3) B''(PR48/PR59/PR72/ PR130),4) B''' (PR93/PR110)(Figure2).Each class contains 2-5 isoforms(Table1).This variety of forms regulates PP2A activity,cellular localization and specificity towards different substrates.Studies have revealed that there is a close relationship between PP2A and the oncogene c-Myc.The PP2A-B56α selectively binds to the N-terminus of c-Myc,causing a significant decrease in c-Myc expression levels.Using short hairpin RNA (shRNA)to release the binding between PP2A-B56α and c-Myc can lead to over-expression of c-Myc,increased phosphorylation level of c-Myc Ser62 and enhanced function of c-Myc (PP2A-B56α associates with c-Myc and negatively regulates c-Myc accumulation).These results uncover a new protein involved in regulating c-Myc expression and reveal a critical interconnection between c-Myc,and a well-documented tumor suppressor,PP2A.
PP2A:catalytic subunit(PP2Ac)
PP2A catalytic subunit (PP2Ac) has the unique catalytic activity of PP2A.PP2Ac shares a high degree of sequence and 3D structural homology with the catalytic subunits of its closely related family members,and is the most conserved of known enzymes [2,12].PP2Ac exists in two isoforms Cα and Cβ.Their sequence was 97% consistent.The protein expression level of PP2Ac in the cell is regulated translationally to maintain a constant level[13,14].
PP2A:Scaffold subunit(PP2A-A/PR65)
PP2A scaffolding subunit (PR65) is a scaffold combined with PP2Ac and B subunit,and is closely combined with PP2Ac.Similar to PP2Ac,the PR65 is encoded by two isoforms,Aα and Aβ.Both are ubiquitously expressed and highly homologous to one another [15].PR56 orchestrates the formation of the active holoenzyme through its conformational flexibility.PR56 escorting the PP2Ac shows a horseshoe shape structure.This flexible bending proposed to facilitate B and C subunit binding[16-19].
PP2A:Regulatory subunit(PP2A-B/B'/B''/B''')
The association of PP2A regulatory B subunit to the core enzyme provides substrate specificity as well as spatially and temporally controlled functions.There are four families of the B subunits,which include the B (B55/PR55),B' (B56/PR61),B''(PR48/PR59/PR72/PR130) and B''' (PR93/PR110),each with multiple isoforms encoded for by different genes.They are characterized by diversity and low sequence homology,but they have high specificity and different localization,which allows for broad and regulated substrate specificity involved in diverse cellular functions[20,21].
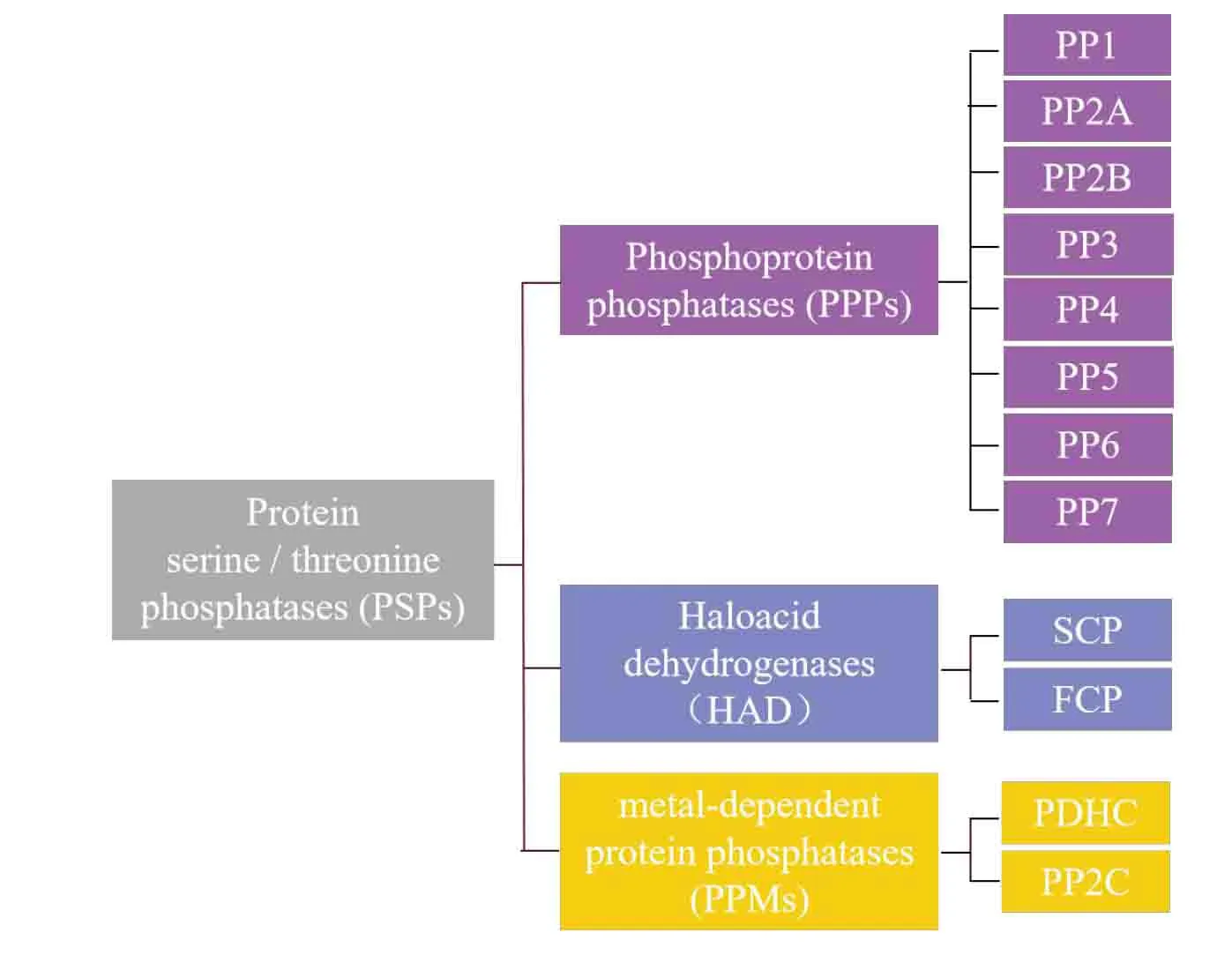
Figure1 The classification of protein serine/threonine phosphatases (PSPs).Protein serine/threonine phosphatases(PSPs)are classified based on the biochemical mechanisms.They are divided into three families,the phosphoprotein phosphatases (PPPs),the aspartate based phosphatases and the metal-dependent protein phosphatases(PPMs).

Figure2 The composition of protein phosphatase 2A(PP2A).PP2A is a heterotrimeric complex consisting of a scaffolding subunit (PP2A-A),a regulatory subunit (PP2A-B) and a catalytic subunit (PP2A-C).The A and C subunits each exist with two possible isoforms α and β.B subunit consists of four classes: B (B55/PR55),B'(B56/PR61),B'' (PR48/PR59/PR72/PR130)and B''' (PR93/PR110).Each class contains 2-5 isoforms.This variety of forms regulates PP2A’s activity and cellular localization and specifificity towards different substrates.

Table1 Various isoforms of different subunits of PP2A
Activation of the PP2A holoenzyme
PP2A catalytic efficiency,substrate specificity and intracellular localization are regulated by many factors,such as post-translation modification,inhibiting proteins,auto-regulation,subunit diversity and substrate protein interaction [23].Methylation and phosphorylation are two major post-translation modifications that have been shown to modulate PP2A activity [24,25].Phosphorylation by the receptor-associated tyrosine kinases or auto-phosphorylated-activated protein kinases decreases the PP2A activity via inhibiting the interaction PP2Ac with the B subunit [26,27].PP2A methylation is also essential for cellular function,it is reversibly controlled by PP2A-specific methyltransferase,which known as leucine carboxyl methyltransferase (LCMT-1),and by PP2A-specific methylesterase 1 (PME-1).LCMT-1 leads to methylation at position Lys309 of PP2Ac and activates PP2A activity.PME-1 can lead to the demethylation of Lys309 and inhibit aggregation and activity of the PP2A subunit (Figure3).Besides,PP2Ac methylation also fluctuates during the cell cycle,indicating that regulation of PP2Ac methylation and holoenzyme assembly is required for cell cycle regulation [28].In fact,PP2Ac expression is tightly regulated in the cell at the translational level but not at the transcription level[23].
Functions of PP2A: PP2A as a master regulator of the cell cycle
PP2A plays a key multi-faceted role in the regulation of the cell cycle.It is known to dephosphorylate more than 300 substrates involved in the cell cycle,regulating almost all diverse and complex signaling pathways and checkpoints of the cell cycle (Figure4).Mitosis requires the correct arrangement of sister chromatids so that replicated chromosomes can bind correctly to microtubules and segregate towards opposite poles.This process relies on highly-dynamic and tightly regulated phosphorylation of numerous cell cycle proteins.A large number of studies have shown that PP2A is mainly involved in a series of phosphorylation processes during the G2/M phase transition and the termination of M phase.Phosphorylation of kinetochore proteins is also controlled by PP2A,which shows a more substantial contribution to sister chromatid cohesion and microtubule-kinetochore(MT-KT)attachment.
Among various B subunits,the PP2A-B56 (B′)family members (α,β,γ,δ,and ε) regulates several major mitotic processes,including sister chromatid cohesion,MT-KT attachment and the spindle assembly checkpoint (SAC) and localize to kinetochores during mitotic.Given their vital importance for mitotic,PP2A-B56 (B′) family will be discussed in greater detail.
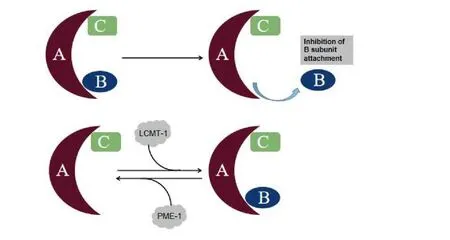
Figure3 Post transitional modification of PP2A.Methylation and phosphorylation are two major post-translation modififications that have been shown to modulate protein phosphatase 2A(PP2A)activity.
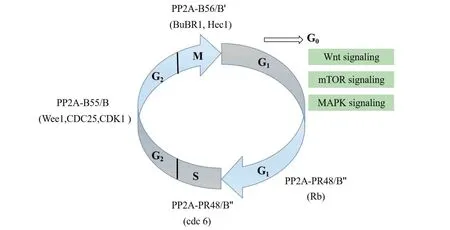
Figure4 Holoenzyme of PP2A involved in the regulation of cell cycle.Protein phosphatase 2A (PP2A) plays a critical multi-faceted role in the regulation of the cell cycle.It is known to dephosphorylate over 300 substrates involved in the cell cycle,regulating almost all major pathways and cell cycle checkpoints.PP2A is involved in such diverse processes by the formation of structurally distinct families of holoenzymes,which are regulated spatially and temporally by specific regulators.
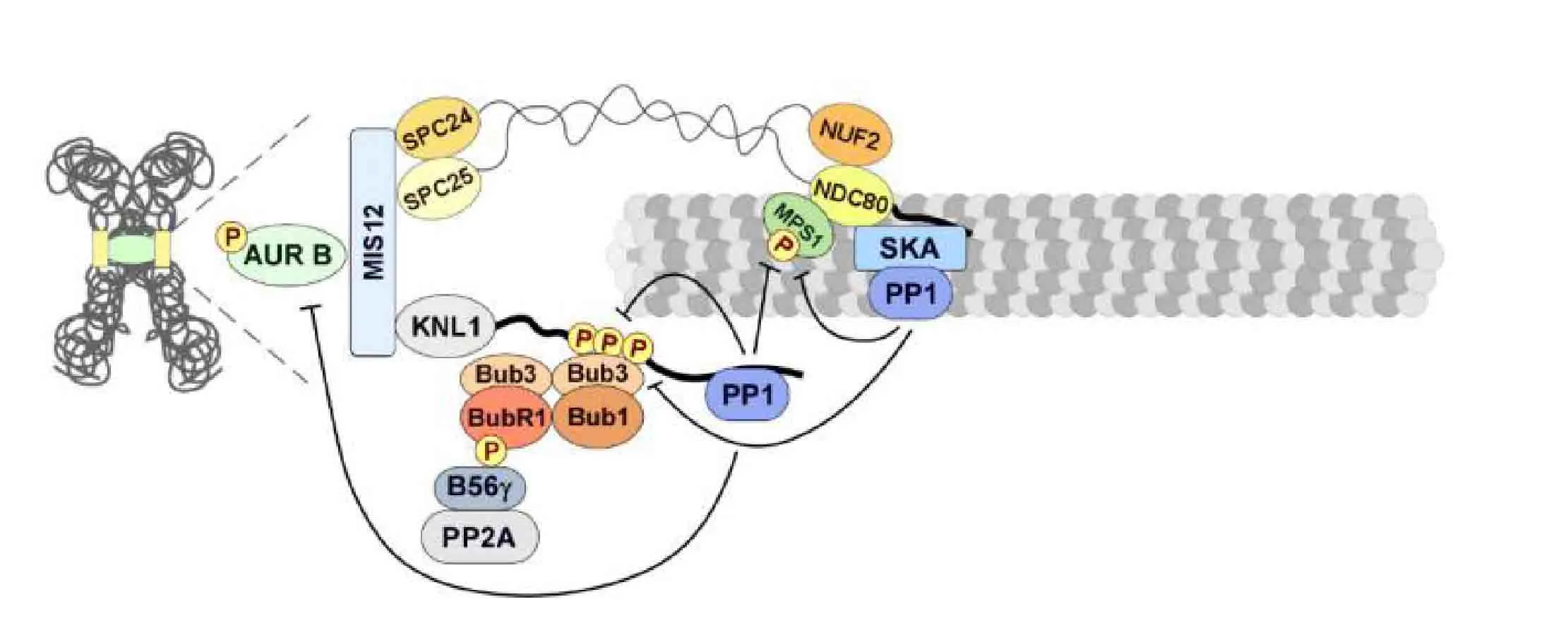
Figure5 Kinetochore PP2A-B56 promote the stability of microtubule attachments and efficient Spindle Assembly Checkpoint silencing in animal cells
Sister chromatid cohesion: PP2A-B56 binds them together
During mitosis,sister chromatids are held together by cohesin,from their creation during DNA replication until their disjunction at anaphase.Cohesin ensures that sister kinetochores attach to microtubules emanating from opposite spindle poles [29,30].Cohesion must,persist at centromeres until the kinetochores of all chromosomes are correctly attached to microtubules.Centromeres are protected against WAP1-dependent cohesin removal by the activity of SGO1-associated PP2A-B56 [31,32-35].Recruitment of PP2A-B56 to centromeres is mainly mediated by SGO1 [36-38].Structural analysis of an N-terminal fragment of SGO1 bound to PP2A-B56 reveals a bipartite interaction between SGO1 and the regulatory and catalytic subunits of the PP2A-B56 holoenzyme [38].This interaction likely places PP2A-B56 to counteract Aurora B,cyclin-dependent kinases(CDK1),and cyclin-dependent kinases(PLK1)destabilizing phosphorylations and thereby prevent the dissociation of centromeric cohesin complexes.
MT-KT attachment:PP2A-B56 keep them stable
To ensure faithful genome partitioning,kinetochores must become attached to microtubules [39].However,the initial contacts occurring between kinetochores and the microtubules are asynchronous and stochastic,which implies that amphitelic attachments are often preceded by immature or erroneous microtubule interactions.Consequently,accurate chromosome segregation requires the destabilization of the inadequate MT-KT attachment and the formation of robust amphitelic end-on attachments,events that are orchestrated by the coordinated activities of molecular motors,kinases,and phosphatases [40-42].In that respect,it is important to consider that the early contacts that kinetochores establish with microtubules are predominantly lateral and that PP2A-B56 at outer-kinetochores was shown to play a critical role in antagonizing Aurora B dependent phosphorylations.
The localization of the PP2A-B56 on unattached kinetochores,and their levels are reduced (α,ε) or undetectable (β,γ,δ) on attached kinetochores.When PP2A-B56 is exhausted,the levels of KNL1 and Dsn1 phosphorylation by Aurora B and BubR1 phosphorylation by Plk1 increase,and MT-KT attachment is compromised (Figure5).This reduced MT-KT attachment is rescued by inhibiting Aurora B,indicating that PP2A-B56 antagonizes Aurora B to support stable MT-KT attachment.To sum up,studies to date suggest a division of labor between mitotic phosphatases in which PP2A-B56 promotes dephosphorylation of kinetochore substrates at unattached kinetochores to promote MT-KT attachment[43].
Conclusion
In the past decades,we have witnessed the emergence of protein phosphatases as critical regulators of mitosis.In recent years,the list of identified substrates and regulators has increased considerably,as has our understanding of phosphatase roles in mitotic progression.Protein phosphatase plays a critical role in the life activities of cells.PP2A is a vital member of the protein phosphatase family.The successive cracking of the crystal structure of PP2A core enzymes and holoenzymes had a significant impact on the in-depth understanding of the interaction between PP2A's structure and subunits,and the mechanism of its interaction with binding proteins.With the continuous emergence of a series of new research results on the correlation between PP2A and tumors,PP2A has also shown a very crucial role in tumorigenesis and cell migration.It focuses on the composition and structure of PP2A,the particular modification of the catalytic subunit,the interaction between the subunits,and the biological function of PP2A as a new tumor suppressor.
Understanding how these phosphorylations increase the binding between BubR1 and PP2A-B56 and how CDK1 and PLK1 monitor MT-KT attachment will be an essential topic of the cell cycle for the future research.Also,PP2A represents a target in cancer and a better understanding of its roles in cell cycle will lead to the development of promising therapeutics for multiple malignancies,as cancer cells are essentially the continuous division and proliferation of cells caused by the uncontrolled cell cycle.A better understanding of which PP2A regulatory subunits will further enrich the regulatory mechanism of mitosis and provide new clues for the study of cell cycle regulation and tumorigenesis and development.
- Cancer Advances的其它文章
- The practical value of serum TK1 concentration expression in clinical research of malignant tumors
- Dehydrocostus lactone exerts the antitumor effect in non-small cell lung cancer H1299 cells
- Clinical efficacy of Qingxin Fupi Jieyu formula in the treatment of 28 cases with digestive tract cancer-related cognitive impairment
- Tumor-associated macrophages,exosomes and tumor metastasis: a mini-review

