Rhizosphere Aeration Improves Nitrogen Transformation in Soil,and Nitrogen Absorption and Accumulation in Rice Plants
XU Chunmei, CHEN Liping, CHEN Song, CHU Guang, WANG Danying, ZHANG Xiufu
(China National Rice Research Institute, Chinese Academy of Agricultural Sciences, Hangzhou 310006, China)
Abstract: Two rice cultivars (Xiushui 09 and Chunyou 84) were used to evaluate the effects of various soil oxygen (O2) conditions on soil nitrogen (N) transformation, absorption and accumulation in rice plants.The treatments were continuous flooding (CF), continuous flooding and aeration (CFA), and alternate wetting and drying (AWD). The results showed that the AWD and CFA treatments improved soil N transformation, rice growth, and N absorption and accumulation. Soil NO3- content, nitrification activity and ammonia-oxidising bacteria abundance, leaf area, nitrate reductase activity, and N absorption and accumulation in rice all increased in both cultivars. However, soil microbial biomass carbon and pH did not significantly change during the whole period of rice growth. Correlation analysis revealed a significant positive correlation between the nitrification activity and ammonia-oxidising bacteria abundance, and both of them significantly increased as the total N accumulation in rice increased. Our results indicated that improved soil O2 conditions led to changing soil N cycling and contributed to increases in N absorption and accumulation by rice in paddy fields.
Key words: ammonium-nitrogen content; nitrate-nitrogen content; nitrification activity; nitrate reductase activity; rhizosphere oxygen condition; ammonia-oxidising bacteria abundance; rice; nitrogen use efficiency
Nitrogen (N) is an essential nutrient for rice plants and is the most limiting nutrient for rice production (Dong et al, 2015; Ladha et al, 2016). In addition to the small amount of organic N compounds, such as amino acids that can be used directly, a large amount of organic N in the soil can be absorbed and used by plants only if it is converted into inorganic N through the action of soil microorganisms. Inorganic N is the main N form directly absorbed by rice, but it only accounts for approximately 1% of the total N in the soil, being produced mainly from mineralisation in the soil. Both crop yield and N turnover can be influenced by management strategies that manipulate the levels of soil N. Therefore, N supply in the soil is usually one of the main factors that limit crop yield and grain quality (Dotaniya and Meena, 2015; Wu et al, 2016).In order to obtain high yields, the use of mineral N fertilizers is continually increasing (Chen et al, 2008;Geng et al, 2016). China is now the world’s largest producer, consumer, and importer of chemical fertilizers. However, N utilization rates have tended to decrease, and excessive use of N fertilizers has given rise to severe ecological and environmental problems,such as soil degradation, groundwater pollution, and increasing greenhouse gas emission (Spiertz, 2010;Ollivier et al, 2011; Cammeron et al, 2013; Zhou et al,2014). Therefore, finding a new way to improve N utilization rate is an urgent problem to be solved.
Rice is a semi-aquatic plant that has adapted to survive submergence during heavy rainfall or floods.Rice plants often encounter low oxygen (O2) and anoxic stress due to the slow diffusion of gases in water (Kirk et al, 2014; Jackson and Ismail, 2015).The unique growth habit of rice indicates that N transformation and metabolism in paddy fields differ from other crop species. Soil N decomposition is slow and incomplete in low-O2environments (Swift et al,1979; Schlesinger, 1991) and often leads to the accumulation of organic materials. Due to the reduction of soil N mineralisation and nitrification,increased denitrification, and a reduction in soil N availability, the rhizosphere O2conditions play an important role in paddy field ecosystems (Ponnamperuma,1972; Reddy and Gretz, 1998; Tian et al, 2018).Therefore, regulating O2conditions in paddy soils to improve N utilization in rice represents an effective way to solve this problem.
Aerobic cultivation is an important agricultural practice, which plays a vital role in rice production.This practice aims to increase yields and reduce irrigation (Yoichiro and Midori, 2010). Different aeration strategies, such as alternate wetting and drying (AWD, also called aerobic irrigation) and chemical aeration (in which rice plants are fertilized with CaO2at the tillering and booting stages), can affect the growth, root physiology and N absorption of rice (Zhao et al, 2009). In addition, the soil redox potential, pH value, ion morphology and microbial activity are all affected by O2conditions in the rhizosphere. Furthermore, these parameters directly or indirectly affect N transformation in soil, and N absorption and utilization in rice (K?gel-Knabner et al,2010; Mishra and Salokhe, 2010; Li and Wang, 2013;Yan et al, 2019). Ammonium-nitrogen (NH4+-N) and nitrate-nitrogen (NO3--N) are the main forms of inorganic N in the soil. These two forms of N are absorbed and follow different pathways, and their contributions to N utilization in plants are different.However, the interaction of the two N forms affects the N absorption and metabolic processes in plants(Dash et al, 2015; Islam et al, 2016). Kronzucker et al(1999) reported that the presence of NO3-can enhance the plasma membrane transport, cytoplasmic accumulation, and metabolism of NH4+in cells, but these processes are significantly inhibited by NH4+itself. Some studies have shown that different N sources exert different regulatory effects on the transcription levels of N transporters and the key enzymes involved in various N metabolic processes(Dong et al, 2015; Dewi et al, 2018). Compared with NO3-or NH4+as the only source of N, the mixtures of ammonium and nitrate fertilizers increase the N net absorption and transport to underground tissue, which is more conducive to rice growth (Duan et al, 2006).Because N in the soil is mobile and dynamic, it can easily change from one form to another, such as from NH4+to NO3-(Cao et al, 2016). Soil O2conditions affect the form and distribution of N in the soil.Moreover, the response of rice to different N nutrients is different under various soil O2conditions. Therefore,studying the mutual transformation of different forms of N under various soil O2conditions is important to improve the N use efficiency of rice. To date, several studies concerning these two aspects have made great progress, but most of them treat soils and rice plants separately (Li et al, 2008; Ishii et al, 2011; Konishi et al,2015), with reports concerning both the two aspects being scarce. Therefore, the objectives of this study were to compare the differences in paddy soil parameters related to N transformation [NH4+, NO3-,soil nitrification activity, microbial biomass carbon(MBC), pH, respiration, and ammonia-oxidising bacteria (AOB) abundance] under different soil O2conditions, and to evaluate the effects of rhizosphere aeration on soil N transformation and its consequent effects on rice N absorption and accumulation. The results of this study can provide new insights in improving N utilization in rice and reducing the amount of N fertilizer input in rice-growing systems.
MATERIALS AND METHODS
Site description and experimental design
Pot experiments (plastic buckets, 80 cm × 60 cm × 80 cm) were carried out at the China National Rice Research Institute (CNRRI), Hangzhou, Zhejiang Province, China (120.2° E, 30.3° N, altitude of 11 m above sea level), from June to November 2017 in a greenhouse. The indoor temperature of the greenhouse was maintained at (32 ± 3) °C during the day and at(22 ± 3) °C during the night. Natural illumination was maintained throughout the pot experiment. Each pot was filled with 20 kg paddy soil (0-15 cm depth), and blended with 25 g compound fertilizer (N : P : K as 15 : 12 : 15). The soils were analyzed for their primary soil properties before fertilization. Chemical analyses included the determination of soil pH (1 : 2.5 water extraction), soil organic matter, total N, available N,available phosphorous (P), available potassium (K),gravimetric soil moisture content and bulk density (Lu,2000) (Table 1).
The pots were arranged in a completely random design that consisted of three treatments with six replicates: continuous flooding (CF), continuous flooding and aeration (CFA), and alternate welting and drying (AWD). CF (also called anaerobic irrigation)treatment served as the control, and the rice plants were flooded with 3-5 cm of water during the entire growing period. The rice plants in the CFA treatment were grown in a similar manner to those in the control.Before rice planting, a gas vent with holes was embedded in the tested pot, and a ventilation pipe was then connected to a ventilation pump. Ventilation was performed (controlled by a timer) for 2 h at the first time. Afterward, each ventilation was performed for 10 min for every 2 h. Ventilation was conducted throughout the day. In the AWD (also called aerobic irrigation) treatment, the rice plants were allowed to dry naturally after an initial flooding and then were irrigated again in a cycle until harvest. Two rice cultivars, Xiushui 09 (conventional japonica rice) and Chunyou 84 (hybrid japonica rice), were planted as single-cropping rice. The seeds were directly sown into plastic buckets. After germination, the seedlings were thinned to 36 seedlings (18 seedlings of Xiushui 09 and Chunyou 84 each) per bucket.
Measurement of soil O2 diffusion rate
The diffusion rate of O2dissolved in the soil was measured using an O2diffusion meter equipped with an additional guard cathode (EK070 14.36, Holland).A cylindrical platinum electrode was inserted into the soil to measure the amount of electrical current required for the reduction of all the O2at the surface.Upon reaching a steady-state, the flow of O2in the pores filled with air, and the water film that passed through the electrode surface was measured.
Measurements of rice leaf area, aboveground dry matter accumulation, and N accumulation
The leaf area, aboveground dry matter accumulation,and N content of rice were determined at the tillering,heading and maturity stages. Six seedlings weresampled per treatment for each rice cultivar. All samples were separated into green leaf blades, stems(culm + sheaths), and panicles (at the heading and maturity stages). The dry matter of each part was determined after oven-drying at 105 °C for 30 min,followed by drying at 80 °C to a constant weight. Then,each part was weighed, ground into powder, and digested for total N determination via the Kjeldahl method (Bremner, 1960). N uptake was calculated by multiplying the N concentration by the aboveground dry matter of the plant (Li et al, 2008). Nitrogen use efficiency (NUE) was expressed as the whole-plant dry matter relative to N accumulation (Koutroubas and Ntanos, 2003).
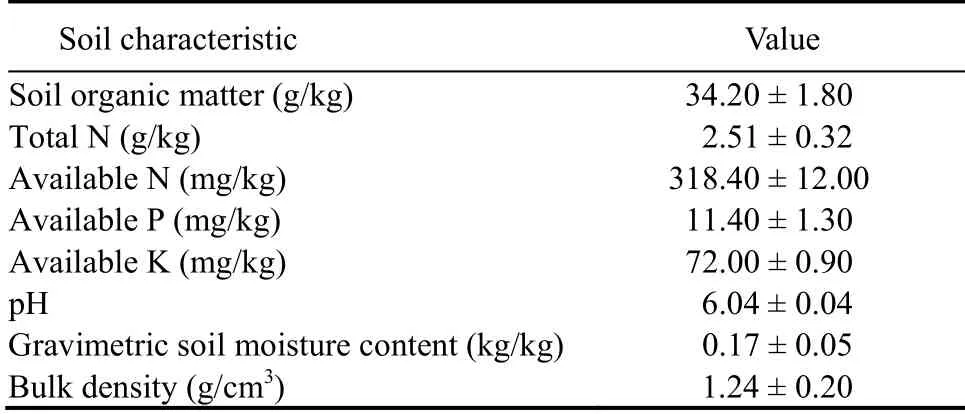
Table 1. Characteristics of tested pot soils.
Measurement of rice leaf chlorophyll content
Rice leaves at the tillering and heading stages were harvested, immersed in liquid N, and then lyophilised.Leaf chlorophyll content (mg/g) was determined via an 80% acetone extract of 0.1 g leaf tissue (Henriques,1989).
Measurement of nitrate reductase activity (NRA)in rice leaves
NRA in leaves was measured spectrophotometrically by determining the nitrite-producing capacity (Hageman and Reed, 1980) at the tillering and heading stages. A specific amount of tissue was homogenised in 5 mL phosphate buffer (pH 7.5) that contained 1 mmol/L EDTA, 10 mmol/L cysteine, and 25 mmol/L potassium phosphate, passed through 4 layers of cheesecloth; and then centrifuged at 25 000 × g for 20 min at 0-4 °C.After the samples were passed through glass wool, 0.5 mL phosphate buffer (pH 7.5), 0.4 mL nicotinamide adenine dinucleotide phosphate (NADPH) (2 mmol/L),and 0.7 mL distilled water were added to the supernatant(0.2 mL), which was subsequently incubated at 30 °C for 15 min. The reaction was terminated by the rapid addition of 1 mL sulphanilamide, followed by the addition of 1 mL naphthyl ethylenediamine reagent.The absorbance was recorded at 540 nm after 90 min,and the enzyme activity was calculated via the standard curve of sodium nitrate.
Soil samples
At the tillering, heading and maturity stages,rhizospheric soil samples (0-15 cm depth) were collected from each pot to analyze NH4+-N and NO3--N contents in soil, nitrification activity, soil MBC, respiration, and abundance of AOB.
Measurements of soil NH4+and NO3–contents NH4+and NO3-were extracted with 2 mol/L KCl by shaking at 250 r/min for 60 min at 25 °C. NH4+and NO3-contents were measured using the indophenol blue and phenol disulphonic acid methods via spectrophotometry, respectively.
Determination of soil nitrification activity, MBC and respiration
Soil nitrification activity was determined via the shaken slurry method, and the soil MBC was estimated using the chloroform fumigation extraction method (Lu, 2000). Soil respiration was measured using an automated soil respiration system (model LI-8100A fitted with an LI-8150 multiplexer, LI-COR,Nebraska, USA).
Quantification of soil AOB abundance
AOB abundance was quantified via the most probable number (MPN) method, which is based on scoring the presence or absence of bacteria with an extinction dilution in which replicate tubes of a special dilution are used. Griess-Ilosvay reagent was used to detect the NO2-produced by AOB. The culture medium contained(NH4)2SO4(0.5 g/L), NaCl (0.3 g/L), FeSO4·7H2O(0.03 g/L), K2HPO4(1 g/L), MgSO4·7H2O (0.3 g/L)and CaCO3(7.5 g/L), and was adjusted to pH 7.8. The test cultures were incubated at a constant temperature of 25 °C for 14 d (Li et al, 1996).
Statistical analysis
The data were analyzed using the SPSS/STAT statistical analysis package (Ver.11.0, Chicago, IL).The means and standard deviations were reported for each of the measurements. The different O2treatments(CF, AWD and CFA) for each cultivar were subjected to one-way analysis of variance followed by a least significant difference test at the 0.05 level. The Pearson’s correlation coefficient (r) was used to determine correlations among the total N accumulation of rice, the soil nitrification rate and AOB abundance.
RESULTS
Soil O2 diffusion rate
Soil O2diffusion rate differed among the treatments(Fig. 1). There was no significant difference (P < 0.05)among the treatments at the early stage of rice growth(0-20 d after transplanting). However, after 20 d,compared with the control (CF), the soil O2diffusion rates in the AWD and CFA treatments significantly increased. The soil O2diffusion rate decreased continually during the entire growth period in the CF treatment, whereas the rates fluctuated in the AWD and CFA treatments.
Rice leaf area and dry matter accumulation
The leaf areas per hill of the two cultivars in the CFA treatment at the tillering and heading stages increased significantly compared with those in the CF treatment(Table 2). The leaf area of Xiushui 09 increased by 22.50% and 6.71% at the tillering and heading stages,respectively, while those of Chunyou 84 were 5.41%higher at the tillering stage and 4.85% higher at the heading stage. At the tillering stage, no significant difference (P > 0.05) in total dry matter accumulation was recorded under different soil O2conditions.However, at the heading stage, the total dry matter accumulation of both cultivars significantly increased in the AWD treatment compared with the control, with Xiushui 09 increased by 22.60%, and Chunyou 84 increased by 18.90%. The biomass of both cultivars also increased in the CFA treatment compared to the control. At the maturity stage, Xiushui 09 exhibited the same trend as at the heading stage under different soil O2conditions. When compared to the control, dry matter accumulation increased by 33.56% and 16.23%in the AWD and CFA treatments, respectively.However, the trend of Chunyou 84 differed from that of Xiushui 09. The dry matter accumulation was the lowest in the AWD treatment, which was 2.54% lower than that in the control. The CFA treatment presented an increase of 2.49% compared to the control.
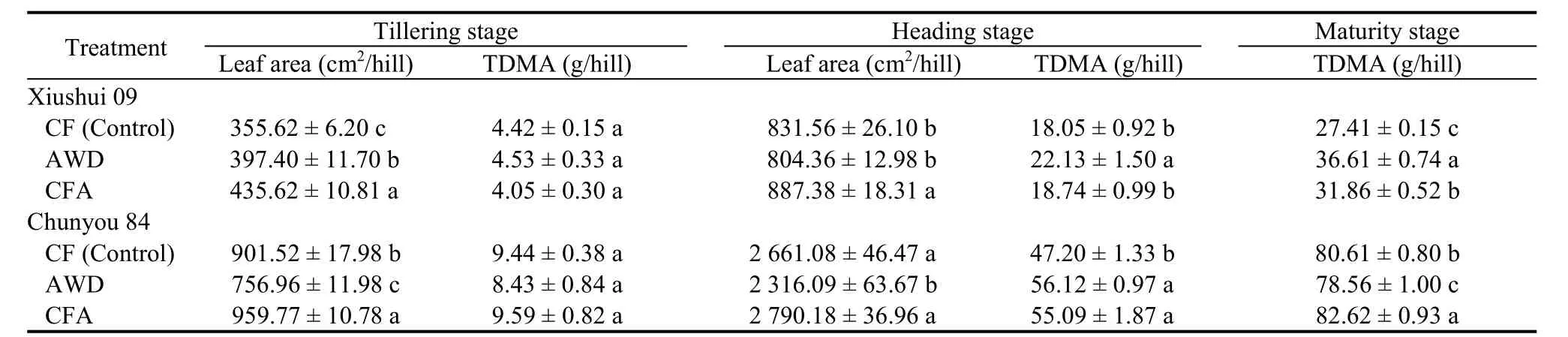
Table 2. Effects of different soil oxygen conditions on rice leaf area and biomass accumulation at the different rice growth stages.
Chlorophyll content
Chlorophyll content increased in the AWD and CFA treatments (Fig. 2). At the tillering stage (Fig. 2-A),the chlorophyll contents of Xiushui 09 in the AWD and CFA treatments increased by 10.98% and 25.10%,respectively, compared with that in the CF treatment,while those of Chunyou 84 increased by 8.17% and 25.00%, respectively. At the heading stage (Fig. 2-B),the AWD and CFA treatments also resulted in the increase of chlorophyll content. The rates of increase for Xiushui 09 in the AWD and CFA treatments were 11.70% and 11.59%, respectively, while those of Chunyou 84 were 8.70% and 7.61%, respectively.
Leaf NRA
At the tillering stage (Fig. 3-A), the leaf NRAs of the two cultivars significantly increased in the AWD treatment. The NRAs followed the order of AWD >CFA > CF for both cultivars. Compared with the CF treatment, the leaf NRA of Xiushui 09 in the AWD treatment increased by 91.12%, while that of Chunyou 84 increased by 61.81%. At the heading stage (Fig.3-B), the NRA of Chunyou 84 significantly increased in the AWD treatment, with 59.63% higher than that of the control.
Total N concentration, N accumulation and NUE in rice
N concentration
Compared with the CF treatment, the N concentrations in the stems and leaves of Xiushui 09 at the tillering stage increased by 23.03% and 20.61% in the CFA treatment, respectively (Table 3). Similarly, the increases of N concentration in the stems and leaves of Chunyou 84 were 54.81% and 18.87%, respectively,in the AWD treatment. At the heading stage, the N concentrations in the stems, leaves, and panicles of Xiushui 09 increased by 7.69%, 78.63%, and 7.32% in the CFA treatment, respectively. The N concentrations in the stems and panicles of Chunyou 84 was greater in the AWD treatment than in the CF treatment(increased by 35.85% and 7.21%, respectively).However, in the leaves, the N concentration was significantly higher in the CFA treatment than in the CF treatment (increased by 24.68%). At the maturity stage, the N concentration in the stems of Chunyou 84 was the greatest in the CFA treatment, being 31.82%higher than that in the CF treatment, whereas the greatest absorption of N in the leaves and panicles of Chunyou 84 occurred in the AWD treatment.
N accumulation
At the tillering stage, the highest accumulation of N in the stems and leaves of Xiushui 09 occurred in the AWD treatment (Table 4), which was 13.61% and 21.61% higher than those in the CF treatment,respectively. The N accumulation in the stems of Chunyou 84 was higher (33.79%) in the AWD treatment, and that in the leaves was higher (32.66%)in the CFA treatment than those in the CF treatment.At the heading stage, the N accumulation in the stems and leaves of Xiushui 09 significantly increased in theCFA treatment, which was 14.71% and 88.59% higher than those in the CF treatment, respectively. The highest N accumulation in the stems and panicles of Chunyou 84 occurred in the AWD treatment, while the maximum accumulation of N in the leaves occurred in the CFA treatment. At the maturity stage, the main differences in N accumulation between the two cultivars were manifested in the panicles. The greatest N accumulation of both rice cultivars occurred in the AWD treatment. Compared with the CF treatment, the N accumulation in the panicles in Xiushui 09 and Chunyou 84 increased by 70.48% and 17.56% in the AWD treatment and by 50.49% and 16.26% in the CFA treatment, respectively.
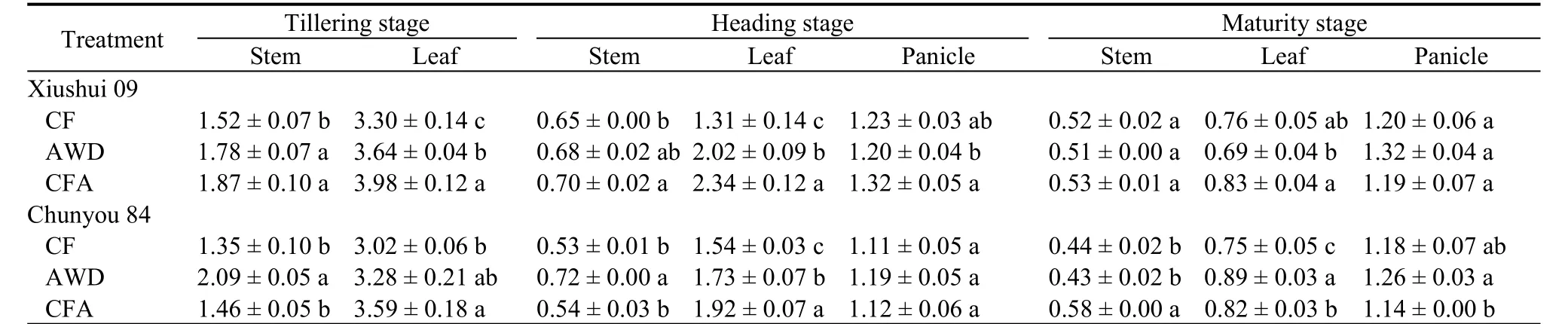
Table 3. Nitrogen concentrations under different soil oxygen conditions at different sampling stages. mg/g
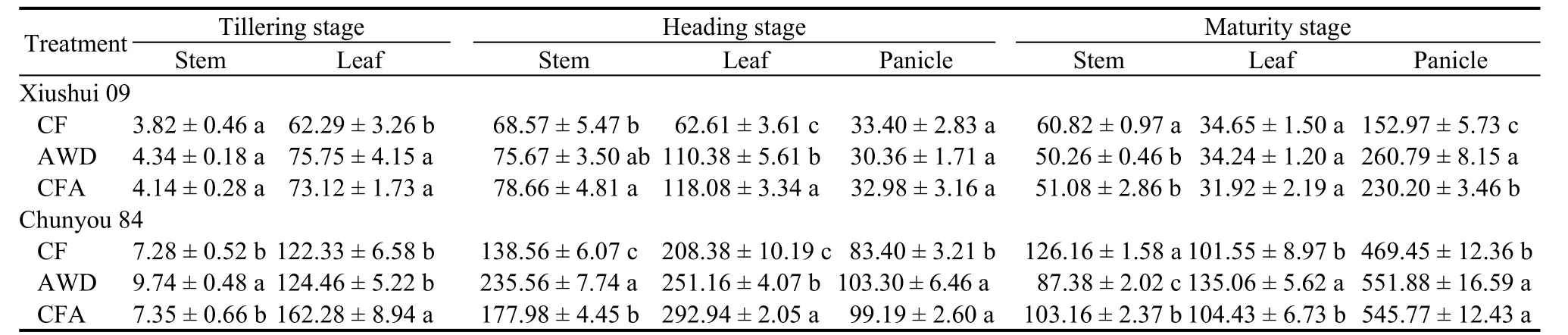
Table 4. Nitrogen accumulation under different soil oxygen conditions at different rice growth stages.mg/hill
NUE
At the tillering stage, the highest NUE of the two rice cultivars occurred in the CF treatment (Table 5), and there were significant differences (P < 0.05) among the treatments. At the heading stage, NUE of Xiushui 09 increased by 7.06% in the AWD treatment compared to the CF treatment. Moreover, NUE of Chunyou 84 in the AWD and CFA treatments waslower (13.29% and 7.36%, respectively) than in the CF treatment. At the maturity stage, the lowest NUE of Xiushui 09 was observed in the AWD treatment. NUE of Chunyou 84 in the CFA treatment was higher(5.38%) than that in the CF treatment.
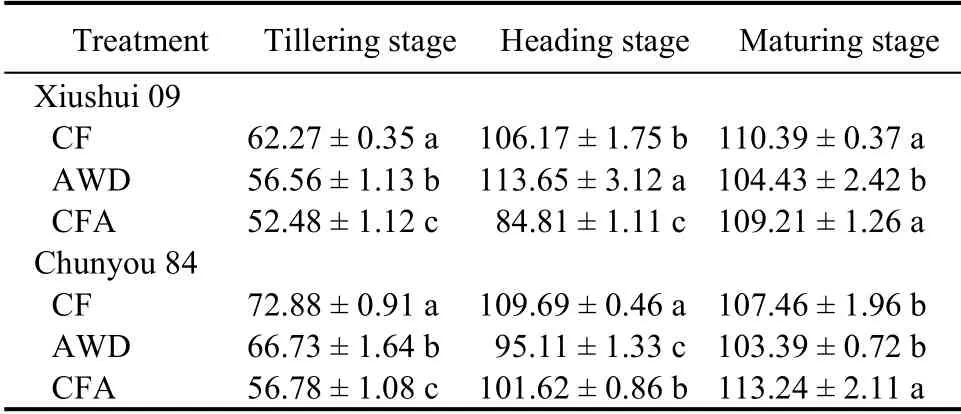
Table 5. Nitrogen use efficiency under different soil oxygen conditions at different sampling stages.
Soil respiration and microbial biomass
The soil respiration clearly increased in the AWD treatment during the whole rice growth period, while it decreased at maturity in the CFA treatment compared with the CF treatment (Fig. 4-A). At the tillering stage, soil respiration increased by 51.12% in the AWD compared with the CF treatment, while no significant difference (P > 0.05) was recorded between the AWD and CFA treatments. At the heading stage, the soil respiration in the AWD and CFA treatments was higher than that in the CF treatment.At the maturity stage, it was also higher in the AWD treatment than in the CF treatment.
As shown in Fig. 4-B, the soil MBC increased as the rice growth progressed and peaked at the maturity stage. Increase of the soil MBC was different under different soil O2conditions. The rate of increase was the greatest in the CFA treatment, which was 127.0%higher at the maturity stage than at the tillering stage.At the tillering stage, the soil MBC in the AWD treatment was slightly higher than those in the CF and CFA treatments, while there was no significant difference between the CF and CFA treatments. At the heading and maturity stages, the values were 15.11%and 6.39% greater in the CFA treatment than in the CF treatment, respectively.
NH4+ concentrations, NO3– concentrations and pH values of the soils
The concentration of NO3-associated with different soil condition treatments did not significantly differ at the tillering stage, but a significant difference (P < 0.05)was observed between the treatments at the heading and maturity stages (Fig. 5-A). The concentration of NO3-decreased in the CF treatment as the rice plants grew, but increased in the AWD and CFA treatments at the heading stage. Nearly no NO3-was detected in the CF treatment at the maturity stage. During the rice growth stage, the average NO3-concentrations in the CF, AWD and CFA treatments were 0.71, 1.59 and 1.49 mg/kg, respectively.
During the rice growth stage, the NH4+concentration significantly increased in the CF treatment and fluctuated in the AWD and CFA treatments (Fig. 5-B).Compared with the CF treatment, the average values of NH4+concentration in the AWD and CFA treatments were 57.76% and 64.42% lower, respectively.
As shown in Fig. 5-C, at the tillering stage, the soil pH in the AWD and CFA treatments was significantly higher than that in the CF treatments, but no significant differences (P < 0.05) were observed between the AWD and CFA treatments. At the heading and maturity stages, the soil pH was significantly higher in the CF treatment than in the AWD and CFA treatments, but no significant differences (P < 0.05)were observed between the AWD and CFA treatments.
Nitrification activity and AOB abundance in soil
The nitrification activities increased in the AWD and CFA treatments and decreased in the CF treatment during rice growth stage (Fig. 6-A). At the tillering stage, the nitrification activities were 2.96 and 1.99 times higher in the CFA and AWD treatments than that in the CF treatment, respectively. At the heading and maturity stages, the nitrification activities in the CFA and AWD treatments were also significantly higher than that in the CF treatment. This difference might be due to a greater abundance of AOB at different rice growth stages. The average abundance of AOB was the highest in the AWD treatment, relatively low in the CFA treatment, and the lowest in the CF treatment(Fig. 6-B). At the heading stage, the abundances of AOB in the AWD and CFA treatments were 3.39 and 2.73 times greater than that in the CF treatment,respectively. Both soil nitrification activity and AOB abundance were significantly and positively correlated with rice total N accumulation, and the correlation coefficient peaked at the heading stage. Soil respiration was also positively correlated with rice total N accumulation, and the correlation was extremely significant at the heading stage (Table 6).
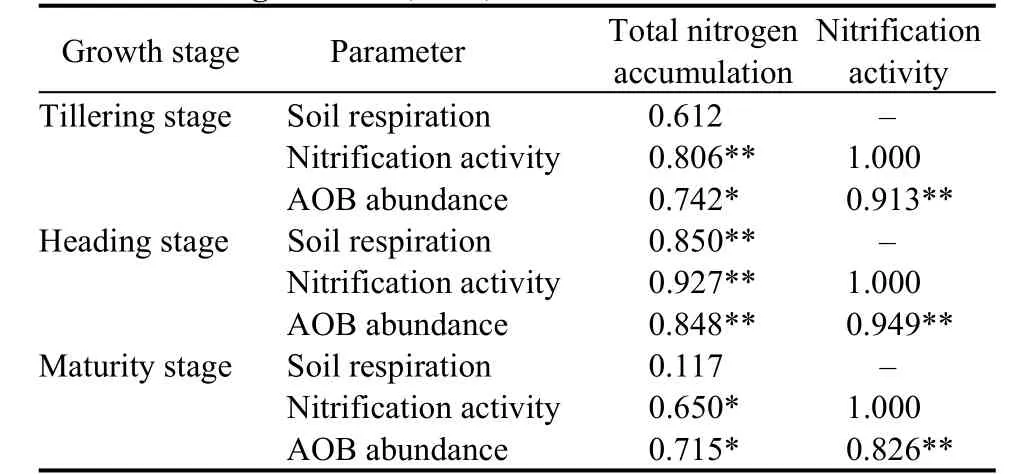
Table 6. Correlation coefficients for both treatments between total nitrogen accumulation, nitrification activity, and ammoniaoxidizing bacteria (AOB) abundance.
DISCUSSION
Effects of oxygen in the rhizosphere on growth and nitrogen absorption in rice
The results of this study indicated that increasing soil O2conditions is beneficial to rice growth and the absorption and utilization of soil N. Among cereal crops, rice is unique because it can form a well-developed aerenchyma system that allows the roots and rhizosphere to be oxidised, thus alleviating most of the stress experienced from hypoxia (Limami et al, 2014). However, paddy soils are generally flooded for certain time periods during the ricegrowing season. In addition, microbial respiration in the soil and plant roots will quickly deplete the O2content in the soil, and the flooded plant roots will quickly change from aerobic to anoxic. Therefore, the O2content in the rhizosphere is a key factor limiting the growth of rice roots (Hodge et al, 2009). A sustained hypoxic environment can cause the accumulation of reducing toxic substances in paddy fields. Different aeration modes in paddy fields can enhance the root physiological functions and improve N absorption and utilization (Zhu et al, 2012; Li and Wang, 2013). Rejesus et al (2011) reported that intermittent irrigation is more conducive to form leaf area index and increase yield than flooding irrigation.Belder et al (2004) reported that CF inhibits the growth of rice, significantly decreasing the accumulation of dry matter and the final yield by 46.46%. However,these findings are believed to be caused by soil moisture conditions. In fact, the effects of AWD and CF treatments on rice growth are the result of differences in soil O2conditions. Plants under flooding conditions present the similar symptoms as those presented when water is hypoxic. Similar results were found in this study. The leaf area of the two cultivars increased in the CFA treatment at the tillering and heading stages (Table 2). Since pot experiments differ from field experiments, a period of time is necessary for the flood to dry. The dry matter accumulation of the two cultivars subjected to the AWD treatment did not present an advantage throughout the whole growth period, and the effect of the AWD treatment on the leaf area of the two cultivars was not definitive. However, the two treatments still promoted dry matter accumulation at the heading stage. The N concentration and accumulation in plants tended to be greater in the AWD and CFA treatments than those in the CF treatment (Tables 3 and 4) due to their greater chlorophyll content (Fig. 2) and NRA(Fig. 3). An increase in chlorophyll content is conducive to photosynthesis and rice growth, and the relationship between leaf N and chlorophyll content is strongly linear (Schlemmer et al, 2013). Nitrate reductase (NR) is a key enzyme involved in NO3-assimilation in crops, and its activity strongly depends on the NO3-concentration (Kaiser and Huber, 2001).A relatively high NRA means that rice plants are growing well and can absorb much more N from the paddy soils (Galib, 2015). Since the NUE was related to the rice biomass accumulation and the N uptake and accumulation, and the effects of different O2conditions on these two indicators were not completely consistent, the NUE of rice in the different O2conditions were not obvious (Table 5).
Effects of oxygen in the rhizosphere on soil nitrogen transformation
In flooded paddy fields, where the soil is hypoxic to anaerobic, the main form of plant-available N is NH4+(Zhao et al, 2008), which is the preferred N species taken up by rice (Cai, 2002; Chen et al, 2008; Sun et al,2015). However, as rice roots can aerate the surrounding soil (rhizosphere) by excreting O2, the rhizosphere always occurs as a mixture of NH4+and NO3-(Cao et al, 2008). NH4+and NO3-contents in paddy soils were affected by O2conditions in the rhizosphere, and their ratios in the rhizosphere were dynamic. Increasing the amount of O2in the rhizosphere significantly affects the presence and proportions of NH4+and NO3-in the soil (Fierer and Schimel, 2002; Duan et al, 2004). The present results confirmed this phenomenon. Improved O2conditions in the rhizosphere (AWD and CFA treatments)significantly increased the soil NO3-content, while the hypoxic (CF) treatment increased the soil NH4+content. In particular, the soil NO3-content was very small (Fig. 5-A) in the CF treatment at the maturity stage. This may be related to the strengthening of denitrification caused by hypoxic environments(Zhang et al, 2016). Although NH4+is usually considered as the preferred N source of rice, the role of NO3-in rice growth cannot be ignored. Under some conditions,the beneficial effects of NO3-for rice are very clear(Zhang et al, 2004; Duan et al, 2006). The simultaneous provision of NH4+and NO3-promotes plant growth, increases dry matter accumulation and yields, and improves the N absorption and utilization,both in hydroponic systems and soils (Qian et al, 2004;Ruan et al, 2007; Wang et al, 2016). Furthermore, soil culture and field experiments have shown increased rice yields in response to NO3-applications (Qian et al,2004). We also observed this phenomenon in the current study. At the maturity stage, the NO3-content in the CF treatment was very low, and the NH4+content in the CFA and AWD treatments also decreased, although the content was still greater than 2 mg/kg (Fig. 5-A and -B). These findings show that,during the rice growth stage, the roots in the CFA and AWD treatments were actually exposed to a mixed N supply. This supply would have provided additional N sources and helped rice make full and effective use of the organic N in the soil. Therefore, the N absorption and accumulation in the AWD and CFA treatments were higher than those in the CF treatment (Tables 3 and 4). Moreover, the improvement in the N assimilation ability resulted in higher leaf area and dry matter accumulation (Table 2). It is well known that an increased amount of dissolved O2in the rhizosphere will cause an increase in NO3-content in the soil, which also provides a large amount of substrate for denitrification, while whether it causes N loss in rice fields is controversial. Tan et al (2013) reported that,compared with the CF treatment, the AWD treatment can significantly reduce the infiltration of N fertilizers,however, the AWD treatment increases the loss of N fertilizers due to leaching and reduces the N utilization rate. Guo et al (2009) reported that low O2conditions in sludge are more conductive to nitrification and denitrification, and that the amount of N lost in sludge is relatively high. Some researchers believe that the main factor that determines the loss of N caused by soil nitrification and denitrification is soil pH (Hao et al,2003; Yang et al, 2016). The results in this study showed that the soil pH in the CF treatment was higher than those in the AWD and CFA treatments(Fig. 5-C), which may be the reason for the small amount of N uptake accumulated in the CF treatment(Tables 3 and 4). Owing to the specificity of the rice paddy ecosystem and rice N uptake, it is still necessary to carry out relevant research to address if increasing the amount of O2in the soil causes more N loss in the future.
Effects of oxygen in the rhizosphere on soil microorganisms and AOB abundance
Soil microorganisms are vital to the cycle of life on Earth. They play an important role in the soil,supporting ecosystems (Hiinninghaus et al, 2017) as well as fertility (Brady, 2002; Bhat, 2013). N transformation in paddy soil mainly includes biological N fixation, ammoniation, nitrification and denitrification, all of which require microbial involvement (Canfield et al, 2010). Microorganisms that respond quickly to environmental changes mediate transformations in the N cycle (Haefele et al,2008; Sooksa-nguan et al, 2009). Nitrification, the conversion of ammonia (NH3) to nitrite (NO2-) and then to NO3-, is a dominant process in the N cycle (Li et al, 2008). These steps require two different microbes, AOB and nitrite-oxidising bacteria (NOB)(Warrington, 1878). Ammonia oxidation is considered a rate-limiting step for nitrification (De-Boer, 1990).Rice cultivation results in increased microbial biomass,respiration, and bacterial and fungal abundance in paddy soils, which are collectively called the rhizosphere effect (Zhang et al, 2016), and affect soil microorganisms. Our results show that the AWD treatment significantly increased the soil MBC at the tillering stage, while the CFA treatment significantly increased the soil MBC at the heading and maturity stages. Moreover, the soil respiration increased in the AWD and CFA treatments at the tillering and maturity stages (Fig. 4-A). A plausible explanation for this pattern may be because we measured the total MBC in the soil and did not distinguish between aerobic microorganisms and anaerobic microorganisms.Aerobic microorganism biomass in the soil may have increased in the AWD and CFA treatments, whereas anaerobic microorganism biomass may have increased in the CF treatment. Soil MBC and respiration indicate soil microbial activity (Yan et al, 2008). The microbial activity has the potential to increase soil N mineralisation in the rhizosphere, thereby enhancing N uptake by the plants (Hamilton and Ftank, 2001).Our results also showed that soil respiration positively correlated with N accumulation (Table 6). Thus,improving soil O2will be useful for the N absorption by rice. Soil aerobic conditions might also be an important factor determining AOB abundance (Chu et al, 2009). Our results verified this conclusion. The results showed that the CF treatment reduced the abundance of AOB. On the contrary, the AWD and CFA treatments, with potentially higher O2, may be more appropriate for AOB, which, in turn, directly affect soil nitrification (Fig. 6-A and -B), the most important process in the N cycle (Yang et al, 2016).O2availability can stimulate the soil nitrification activity in rice rhizospheres (Li et al, 2013). The correlation analysis between soil nitrification activity and N accumulation also revealed that the nitrification activity was positively correlated with N accumulation at different rice growth stages (Table 6). These findings indicate that the increase of the N utilization in rice can be achieved by increasing the O2concentration in the rhizosphere and soil nitrification.Soil respiration, nitrification, and MBC are closely related to soil O2concentration. The effects of rhizospheric O2concentration on the soil environment require a certain time, and the effects of different O2concentrations on these indicators at the early rice growth stage (tillering stage) were not significant.Contrarily, these effects were more definitive during the middle and late rice growth stages. Therefore, we believe that regulating the O2content in the rhizosphere in order to improve the N utilization rate of rice may be an effective way to reduce the applications of N fertilizers.
ACKNOWLEDGEMENTS
This research was supported by the National Key Research and Development Program of China (Grant No. 2016YFD300507), the National Natural Science Foundation of China (Grant No. 31401343), and the National Rice Industry Technology System of China(Grant No. CARS-01-04A).
- Rice Science的其它文章
- INSTRUCTION FOR CONTRIBUTORS
- Expression Profiles and Protein Complexes of Starch Biosynthetic Enzymes from White-Core and Waxy Mutants Induced from High Amylose Indica Rice
- Rice Heavy Metal P-type ATPase OsHMA6 Is Likely a Copper Efflux Protein
- OsSRK1, an Atypical S-Receptor-Like Kinase Positively Regulates Leaf Width and Salt Tolerance in Rice
- Genetic Relationship and Structure Analysis of Root Growth Angle for Improvement of Drought Avoidance in Early and Mid-Early Maturing Rice Genotypes
- Marker-Assisted Introgression of Quantitative Resistance Gene pi21 Confers Broad Spectrum Resistance to Rice Blast

