Optimized combinations of statins and azoles against Acanthamoeba trophozoites and cysts in vitro
Ines Sifaoui, Carmen M Martín-Navarro, Atteneri López-Arencibia, María Reyes-Batlle,Basilio Valladares, José E. Pi?ero?, Sutherland K Maciver, Jacob Lorenzo-Morales
1University Institute of Tropical Diseases and Public Health of the Canary Islands, University of La Laguna, Canary Islands, Spain
2Laboratoire Matériaux-Molécules et Applications, IPEST, LA Marsa, University of Carthage, Tunisia
3Centre for Integrative Physiology, Biomedical Sciences, Edinburgh Medical School, University of Edinburgh, Scotland, UK
Keywords:Keratitis Voriconazole Posaconazole Atorvastatin Fluvastatin Simvastatin
ABSTRACT Objective: To evaluate the combination of several statins (atorvastatin, fluvastatin and simvastatin) and azoles (voriconazole, posaconazole and itraconazole) against Acanthamoeba spp.Methods: The efficiency of the different drug combinations against the trophozoite stage of different Acanthamoeba strains were evaluated by Alamar Blue assay. Effect on the cyst stage was observed by inverted microscope. Cytotoxicity of combinations of azoles and statins was evaluated by measuring the release of lactate dehydrogenase from a murine macrophage cell line.Results: Combinations of any of the tested statins and voriconazole or posaconazole were more efficient in inhibiting Acanthamoeba compared to statins or azoles individually. The drug combinations at the combined inhibitory concentrations 50% showed lower toxicity compared to that of the compounds alone.Conclusions: The combinations of statins together with voriconazole and posaconazole are more efficient than these drugs alone, and these combinations have lower cytotoxicity in mammalian cell lines.
1. Introduction
Among several genera of free-living Amoebae, Acanthamoeba genus are responsible for different diseases such as Acanthamoeba keratitis, a sight-threating ulceration of the cornea, granulomatous amoebic encephalitis and various disseminated infections (mostly cutaneous)[1,2]. Current therapy against Acanthamoeba keratitis is based on topical applications of antimicrobials including various combinations of propamidine isethionate and neomycin or biguanides[1,2]. However, Acanthamoeba can form a double wall cyst stage, which is highly resistant to external agents including those mentioned above that significantly complicates therapy[3].
This means that there is an urgent to discover drugs and drug regimens that are active against both trophozoites and cysts of Acanthamoeba[1,2,4-6].
Statins are hypolipidemic agents widely used to lower the cholesterol levels and to prevent atherosclerotic cardiovascular disease[7]. Our previous studies have also demonstrated the in vitro efficacy of statins against Acanthamoeba trophozoites and cysts, indicating them as a novel effective therapeutic approach against these pathogens[5]. Another agent which has shown high activity against both trophozoites and cysts of Acanthamoeba is voriconazole[6,8]. Voriconazole belongs to the triazole family which has been shown to inhibit 14-α-demethylase resulting in the reduced production of ergsoterol in Acanthamoeba[9]. Ergosterol and 7-dehyrostigmasterol have been reported as the major sterols presented in Acanthamoeba[10,11]. The high efficacy of voriconazole against Acanthamoeba strains in vitro and in clinical cases has been reported in previous studies[6,8,12]. Other members of the family of triazoles that have been used as antifungals are posaconazole and itraconazole. Posaconazole has been previously reported to have a wide microbial activity spectrum and has recently been evaluated against Acanthamoeba cysts in vitro[9,13]. Although itraconazole has been included in successful treatment combinations against skin lesions caused by Acanthamoeba, it is reported to be less active compared to other azoles[3,14]. In many cases, combinations of therapeutic drugs are found to be more efficient than the individually applied drugs. In the case of Acanthamoeba, this phenomenon has been demonstrated recently using combination of biguanides[4].
Although the activity of statins and triazoles has been already described against Acanthamoeba strains, they have not been tested in combination. In the present study, combinations of these molecules were tested for their amoebicidal and cysticidal activity as well as for their cytotoxicity in a mammalian cell line. We report that these drugs are more efficient in combination, which suggests a better approach for the treatment of Acanthamoeba infections.
2. Materials and methods
2.1. Acanthamoeba strains
We have used 3 Acanthamoeba isolates in this study. One type strain Acanthamoeba castellanii Neff (ATCC 30010, genotype T4) and 2 clinical strains previously isolated by our group CLC-16 (genotype T3), and CLC-51. l (genotype T1)[15]. All strains were cultured axenically at room temperature in Peptone Yeast Extract Glucose Broth (PYG) medium supplemented with 40 μg/mL gentamicin(Sigma-Aldrich Chemistry Ltd., Madrid, Spain).
2.2. Chemicals
Posaconazole and voriconazole were purchased from Sigma-Aldrich Chemistry Ltd. (Madrid, Spain) and itraconazole was purchased from the Cayman Chemical Company (Vitro, Madrid,Spain). Simvastatin, atorvastatin, and fluvastatin were purchased from the Cayman Chemical Company (Ann Arbor, MI, US).
2.3. Activity assays
The anti-Acanthamoeba activities of the drugs were determined by the AlamarBlue? assay, as previously described[15,16]. Briefly, 50 μL of Acanthamoeba trophozoite cells (104cells/mL) were seeded in 96-well microtiter plates. Amoebae were allowed to adhere for 15 min and 50 μL of different dilutions of the drugs. Finally,10 μL of the Alamar Blue Assay Reagent (Bioresource, Europe,Nivelles, Belgium) to each well. Plates were then incubated for 120 h at 28 ℃ with slight agitation and were measured using an EnSpire? Multimode Plate Reader (Perkin Elmer, Madrid, Spain).A test wavelength of 570 nm and a reference wavelength of 630 nm were used. Inhibitory concentration 50% (IC50) and inhibitory concentration 90% (IC90) were calculated by non-linear regression analysis with 95% confidence limits. The IC50s of each drug alone were calculated and drug combinations were then tested at various concentration to determine the lowest IC50s of each drug combination.
2.4. Evaluation of the cysticidal activity of posaconazole
The effects of posaconazole against cysts were evaluated against the 3 strains of Acanthamoeba. Mature cysts were prepared as previously described[17]. Briefly, trophozoites were transferred from PYG medium to Neff's encystment medium where they were cultured for a week under slight agitation to obtain mature cysts.After that, the cells were harvested and washed twice with PYG medium. A concentration of 104cysts/mL was transferred to plates containing fresh PYG medium and incubated with posaconazole at the previously calculated IC50s and IC90s. During a week, the number of trophozoites, cysts, and non-viable were counted in a Neubauer counting chamber each 24 h, as previously described by Martín-Navarro et al[5,6]. A negative control including only mature cysts in Neff's encystment medium was included to the experiments.After 7 d of incubation, the supernatant was replaced with fresh PYG medium and the cultures were observed for a second week to confirm the cysticidal activity.
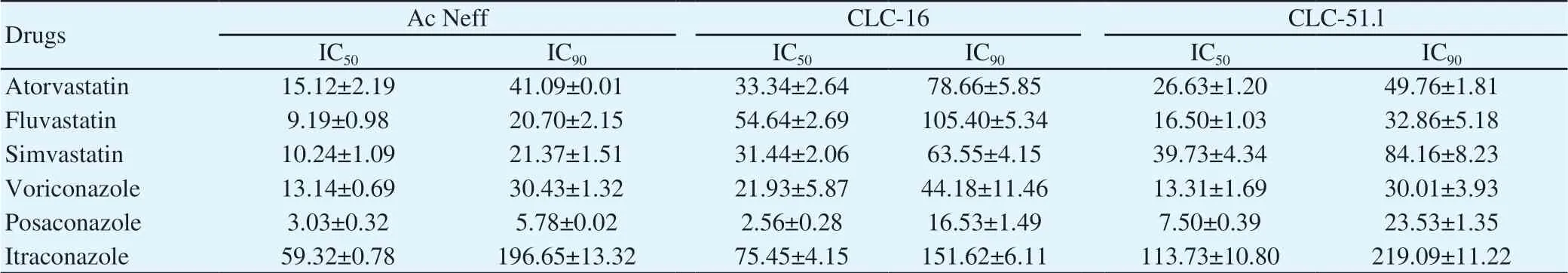
Table 1. IC50 and IC90 values of posaconazole against different strains of Acanthamoeba measured by Alamar blue assay after 96 h (μM).
2.5. Cytotoxicity evaluation
The murine macrophages cell line (ATCC TIB-67) was used to measure the cytotoxicity of the drugs individually and in combinations. This assay was evaluated by the measure of the release of lactate dehydrogenase by cytotoxicity detection kit (Roche Applied Science, Barcelona, Spain) according to the manufacturer's instructions. Cytotoxicity less than 10% was considered as being non-cytotoxic, levels between 10%-25%, low toxicity, levels between 25%-40%, medium toxicity and higher than 40%, highly cytotoxic.The results were compared by one-way ANOVA using Sigma Plot 12.0 software (Systat Software Inc., London, UK)[2,18].
2.6. Statistical analysis
All experiments were performed 3 times each in duplicate. Data were analysed using ANOVA, multiple post hoc analysis, Tukey's test and a paired two-tailed t-test and P<0.05 were considered significant. Statistical analysis was done with the Sigma Plot 12.0 software program (Systat Software Inc., London, UK).
3. Results
All of the 3 Acanthamoeba strains tested were found to be sensitive to the statins (Table 1), but the activity of itraconazole was low and this drug was not used in further experiments. All combinations of statins and voriconazole and posaconazole were active against the strains of Acanthamoeba. However, the different drugs combined,produced lower IC50s compared to that of the molecules alone(Table 2 and Table 3).
The cysticidal activity of posaconazole was also evaluated and was found to reduce the viability of all 3 strains. The IC50and IC90of posaconazole for cysts was calculated (Figure 1). No cysts was able to revert into trophozoites after 168 h.
Simvastatin IC50(S50), posaconazole IC50(P50), voriconazole IC50(V50) and the following combinations of molecules: posaconazole and atorvastatin (P+A), posaconazole and fluvastatin (P+F),posaconazole and simvastatin (P+S), and voriconazole with atorvastatin, fluvastatin and simvastatin (V+A, V+F and V+S) did not induce cytotoxicity against macrophages. Whereas in the case of atorvastatin IC50and IC90(A50 and A90), fluvastatin IC50and IC90(F50 and F90), posaconazole IC90(P90) and voriconazole IC90(V90), low cytotoxicity values against macrophages were observed.Furthermore, moderate toxicity was shown in the case of cells incubated with simvastatin IC90(S90). Amphotericin B IC90(Amp B 90) and chlorhexidine IC90(Chx90) induced high levels of toxicity in the tested cell line (Figure 2). The statistical analysis revealed significant differences (P<0.001) in the cytotoxicity produced by the reference drugs (IC90of Chx and AnfB) and all treatments, excepting in the comparison between Chx90 and S90 (Figure 2).

Table 2. IC50 values of atorvastatin, fluvastatin, simvastatin alone or in combination with voriconazole at a concentration corresponding to the IC50 for each of the three strains of Acanthamoeba after 96 h (μM).

Table 3. IC50 values of atorvastatin, fluvastatin, and simvastatin alone or in combination with posaconazole at a concentration corresponding to the IC50 for each of the three strains of Acanthamoeba after 96 h (mM).
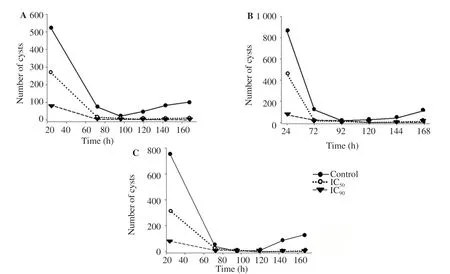
Figure 1. Number of cysts when they were incubated with posaconazole with previously calculated IC50 and IC90 in PYG medium of Ac Neff (A), CLC-16 (B) and CLC-51.l (C). Cysts were not viable after incubation with this drug, since amoebae were not able to excyst. Moreover, the number of cysts decreased with time and became non-viable cells.
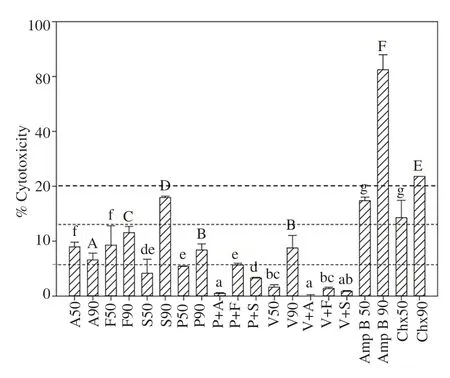
Figure 2. Cytotoxicity of atorvastatin (A), fluvastatin (F), simvastatin (S),posaconazole (P), voriconazole (V), amphotericin B (Amp B) and chlorhexidine (Chx) at IC50 and IC90 as well as the combination between azoles and statins produced in murine macrophages. S50, P50, V50 and the combinations of P+A, P+F, P+S, V+A, V+F and V+S were not cytotoxic against macrophages compared with the references drugs used; AmpB 90 and Chx90 showed high levels of cytotoxicity against macrophages. The cytotoxicity values showed significant differences with the cytotoxicity produced by chlorhexidine and amphotericin B. a-g: Different small letters represent statistically different results within the IC50 toxicity towards macrophages with P<0.05; A-F: Different capital letters represent statistically different results within the IC90 toxicity towards macrophages with P<0.05.
4. Discussion
Drugs are often combined for their synergize activities to increase their therapeutic potency. There are many instances where this applies (e.g. HIV therapy) and this has also been found with Acanthamoeba, where polyhexamethylene biguanide combined effectively with chlorhexidine[4]. This has also been found with various other drug combinations[19]. It is also an advantage if the cytotoxicity produced by this combination will be the same or even lower than that produced by the individual drugs themselves. Such a decrease in the toxicity has been reported by the combination of PHMB and chlorhexidine on epithelial cells[4].
Statins and voriconazole were used in this study because of their proven amoebicidal and cysticidal activity and their relatively low cytotoxicity. The activity of atorvastatin, fluvastatin, simvastatin and voriconazole against Acanthamoeba was previously evaluated by our group[5,6,8,16]. These in vitro results can be compared with in vivo experiment where voriconazole has been successfully used to treat amoebic infections, even against resistant Acanthamoeba strains[12].Initially, those drugs were chosen because their molecular target,ergosterol was known to be a valid one. Statins inhibit 3-hydroxy-3-methylglutaryl-coenzyme A reductase, an enzyme that catalyse the conversion of HMG-CoA to mevalonate, which is a precursor of cholesterol in vertebrates and ergosterol in fungi and some protozoa such as Acanthamoeba[5,20]. Voriconazole is a triazole antifungal agent that causes demethylation of ergosterol. As both drugs act to inhibit different parts of the same ergosterol pathway, we suspected that these 2 drugs may be combined successfully.
Itraconazole has been included in some successful treatment regimens used against skin lesion in a lung transplant patient with disseminated acanthamoebiasis and against Acanthamoeba keratitis[14,21]. However, resistance to azoles has been reported.Acanthamoeba from skin nodules of a fatal cutaneous infection in an HIV patient, was resistant to a combination of drugs with itraconazole in vitro[21]. We confirmed that only a weakly amoebicidal effect of itraconazole, so it was discarded for further experiments. We could find only one study in which posaconazole has been used against Acanthamoeba, showed a cysticidal activity in clinical and culture collection isolates[9]. This previous study reported minimal cysticidal concentrations [(43.75-52.50) μM] that are higher than the IC50found in the present study [(2.56-7.50) μM].We have reported that statins and voriconazole lead to the death of Acanthamoeba by the activation of programmed cell death[8].We have also reported that caffeine and maslinic acid also activate programmed cell death but we do not yet know the molecular targets of either drug. It remains to be seen if either caffeine or maslinic acid treatment will allow even lower concentrations of statins,posaconaole or voriconazole to be effective in human therapy[18].
The combination of statins together with voriconazole and posaconazole is more efficient than these drugs by themselves,and these combinations have lower cytotoxicity in mammalian cell lines. We anticipate that treatments based on combinations of statins and azoles will be more effective and better tolerated than present treatment regimens against Acanthamoeba infections of humans.
Conflict of interest statement
The authors declare no competing financial interests.
Foundation project
This work was supported by PI18/01380 from Instituto de Salud Carlos III and FEDER, Spain and CTQ2014-55888-C03-01/R (MINECO). MRB: RICET [RD16/0027/0001 project, from Programa Redes Temáticas de Investigación Cooperativa, FIS(Ministerio Espa?ol de Salud, Madrid, Spain). IS and ALA were supported by the Agustín de Betancourt Programme.
 Asian Pacific Journal of Tropical Medicine2019年6期
Asian Pacific Journal of Tropical Medicine2019年6期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Community-acquired pneumonia with Acinetobacter radioresistens bacteremia in an immunocompetent host: A case report
- Anticancer activity of Mahonia leschenaultii methanolic root extract and berberine on Dalton’s ascitic lymphoma in mice
- Symptoms of dengue at the acute and post-infection stage in the Western Province, Sri Lanka: A cross-sectional study
- Epidemiology and immunodiagnostics of Strongyloides stercoralis infections among migrant workers in Malaysia
- Laboratory diagnosis of schistosomiasis mansoni: Current status and future trends
