A rare case of primary well-differentiated angiosarcoma of the right atrium
Wei-Zi SUN, Qiu-Ji WU, You WANG, Min CHEN, Jun ZHANG,Gong ZHANG,#, Yun-Feng ZHOU,#
1Department of Radiation and Medical Oncology, Zhongnan Hospital of Wuhan University, Wuhan, China
2Hubei Key Laboratory of Tumor Biological Behaviors, Zhongnan Hospital of Wuhan University, Wuhan, China
3Hubei Cancer Clinical Study Center, Zhongnan Hospital of Wuhan University, Wuhan, China
Keywords: Angiosarcoma; Cardiac tumor; Chemoradiotherapy; Literature review; Tumor resection
Primary cardiac angiosarcoma is rare in clinic. However,it is the most common malignant tumor in the heart, accounting for 15%-35% of all cardiac tumors.[1,2]It is a very aggressive disease characterized by high rates of local recurrence and systemic metastases, and it is a poor prognosis.[3]Echocardiography, computed tomography (CT) and magnetic resonance imaging (MRI) are important tools for diagnosing cardiac angiosarcoma and are valuable in guiding surgical resection and in monitoring treatment efficacy.
At present, the rarity of this disease and a lack of large-scale clinical studies render it difficult to standardize the treatment. Nevertheless, surgical treatment is preferred as the primary treatment.[4]Here, we present a case of a 38-year-old female patient with a well-differentiated angiosarcoma of the right atrium, with an intention to draw attentions of clinicians on this aggressive disease, and to provide some experience on its diagnosis and treatment.
A 38-year-old female patient presented with facial swelling for one week without obvious cause was admitted to the local hospital in October 2017 (Figure 1A).Chest-enhanced CT examination (Figure 1B) revealed a large mass about 8 cm × 6 cm × 5 cm occupying the right atrium and the superior and inferior vena cava with pericardial and bilateral pleural effusion. On November 8th2017,she underwent a surgical resection of the tumor. The tumor was found to be located at the top of the right atrium and the interatrial septum, and it is closely attached to the left atrium and the posterior wall of the aortic root. The tumor extended down until the inferior vena cava inlet and the tricuspid opening, partially blocking the superior and inferior vena cava inlet and the tricuspid valve outlet. The majority of the tumor was removed, while about 20% of the tumor at the top of the residual apex and interatrial septum was unresectable. Macroscopically, we found a gray-red solid cardiac tumor about 5 cm × 4 cm × 3 cm, incompletely capsulated. The tumor was soft and necrotic (Figure 1C). Histophathological study indicated a well-differentiated angiosarcoma (Figure 1D). Immunohistochemical staining showed Vimentin (+), CD31 (+), CK (-), CD34 (+), SMA(+), Desmin (-), MyoD1 (-), Myogolbin (-) (data not shown). The patient's facial swelling improved significantly after surgery (Figure 1E). Postoperatively, on December 13th2017 and January 3rd2018, two cycles of EI regimen(pyrubicin, ifosfamide, mesna sodium) were administered.After the first cycle of chemotherapy, the echocardiography showed a 3.4 cm × 2.1 cm slightly flow signal.
On January 16th2018, the patient came to our department for radiotherapy of the residual tumor. Myocardial enzymes and tumor markers showed no obvious abnormalities. Electrocardiogram showed sinus rhythm with T wave changes in some leads. Bone scintigraphy showed abnormal bone metabolism in the upper sternum. Cardiac MRI examination was not considered because of postoperative stapling device remain. Instead, a contrast-enhanced chest CT scan (Figure 1F) showed a 3.0 cm × 2.7 cm slightly enhanced mass in the right atrium, small amount of effusion in the right thoracic cavity and the pericardium, multiple slightly enhanced nodules in the liver. Subsequent liver ultrasonography indicated intrahepatic cystic lesions (cysts) and right hepatic hyperechoic nodules (large likelihood of hemangioma). An echocardiography showed a heterogeneous slight hyperechoic mass about 4.0 cm × 2.7 cm in the right atrium. The patient underwent an intensity-modulated radiotherapy (PTV-GTV= 60 Gy/30 fractions) to right atrial mass from January 30th2018 to March 18th2018. Grade I leukopenia and occasional self-relieving slight back pain occurred during the radio-therapy. No other obvious severe treatment-related toxicities were noticed.
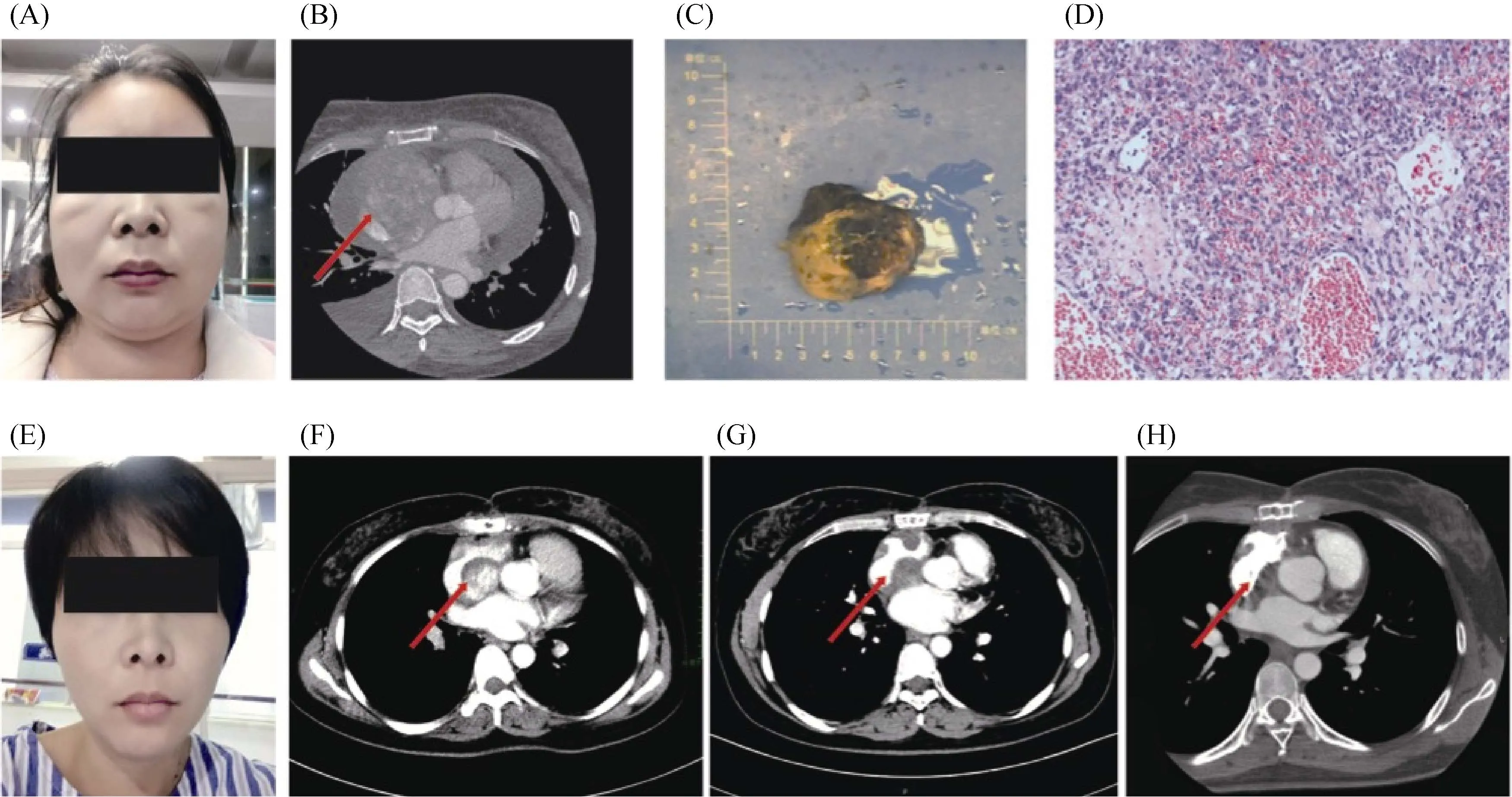
Figure 1. Clinical manifestation, pathological result, and imaging data of the patient during the treatment. (A): Facial sign before surgery; (B): CT image before surgery, the tumor measured about 8 cm × 6 cm × 5 cm, with heterogeneous enhancement ( red arrow); (C):gross specimen; (D): HE staining (×10); (E): facial sign after surgery; (F): sixty days after surgery (before radiotherapy), the residual mass measured about 3.0 cm × 2.7 cm, with heterogeneous enhancement (red arrow); (G): one month after radiotherapy, the tumor size reduced to 2.6 cm × 1.8 cm, with no obvious enhancement (red arrow); (H): four months after radiotherapy, almost no obvious mass was noticed (red arrow). CT: omputed tomography; HE: hematoxylin and eosin
One month after radiation therapy, the patient was reexamined on 17thApril 2018, and contrast-enhanced chest CT scan (Figure 1G) showed the residual tumor in the right atrium, which was about 2.6 cm × 1.8 cm without enhancement. Four months after radiotherapy, a contrast-enhanced chest CT scan (Figure 1H) showed no obvious sign of mass and abnormal enhancement in the right atrium on 23rdJuly 2018. The application of intensity-modulated radiation therapy allows precise delivery of acquired dose to the tumor while better protect organs at risk. In this case, we delivered 60 Gy to the remaining of the tumor (Figure 2).Organs at risk including bilateral lungs, the heart, the spinal cord and the esophagus received less than tolerated doses.With this technique and radiation doses, the remaining of the tumor continued regressed and the patients did not develop obvious severe treatment-induced toxicities.
Primary cardiac neoplasms are extremely rare, with an incidence of approximately 0.0001%-0.030%.[5]Almost 75% of these tumors are benignant, the remaining 25% are malignant, and angiosarcomas are the most common histologic subtypes in adults.[6]Cardiac angiosarcoma is often overlooked as an initial diagnosis because of its rarity. Most patients with angiosarcomas are diagnosed with metastasis and they have a poor prognosis. Distant metastasis accounts for 47%-89% in these patients, the most common metastatic sites are lungs, followed by the brain, bone and colon.[7]Cardiac angiosarcomas usually originate in the right atrium,followed by the left atrium, right ventricle and left ventricle.[1]These tumors typically occur in the third to fifth decade of life, with a male to female ratio of 2–3:1.[1,8,9]Primary cardiac malignancies may have different clinical manifestations depending on the location and characteristics of their growth and the degree of dysfunction caused by local infiltration. Angiosarcoma is most commonly found in the right atrium, and it frequently interferes with neighboring structures, resulting in congestive heart failure and arrhythmia.[10,11]Pericardial effusion, cardiac tamponade and pulmonary or systemic thromboembolism are also common initial findings.[8,9]In addition, necrosis of the myocardial wall can lead to myocardial rupture.[12]
Echocardiography remains the initial test, and it has been the cornerstone to diagnose cardiac tumors. The sensitivities of transthoracic echocardiography (TTE) and trans-esophageal echocardiography (TEE) to detect these masses are 93% and 97%, respectively.[8]CT and MRI can not only show the tumor invasion of the myocardium, pericardium,large blood vessels and mediastinal lymph nodes, but also evaluate the feasibility of surgery and help determine the extent of surgical resection. Cardiac MRI is superior to CT in distinguishing heart chamber thrombosis and soft tissue.PET/CT is of great value in identifying the good and malignant masses, and it could suggest whether distant metastasis occurs. In this case, chest enhancement CT examination was used to monitor treatment efficacy instead of MRI due to the stapling device. There was significant uneven enhancement before and after surgery, suggesting that the blood supply is rich but not uniform, and that there may be necrosis in the local area. After radiotherapy, the tumor is significantly reduced, indicating high radiosensitivity in this case.
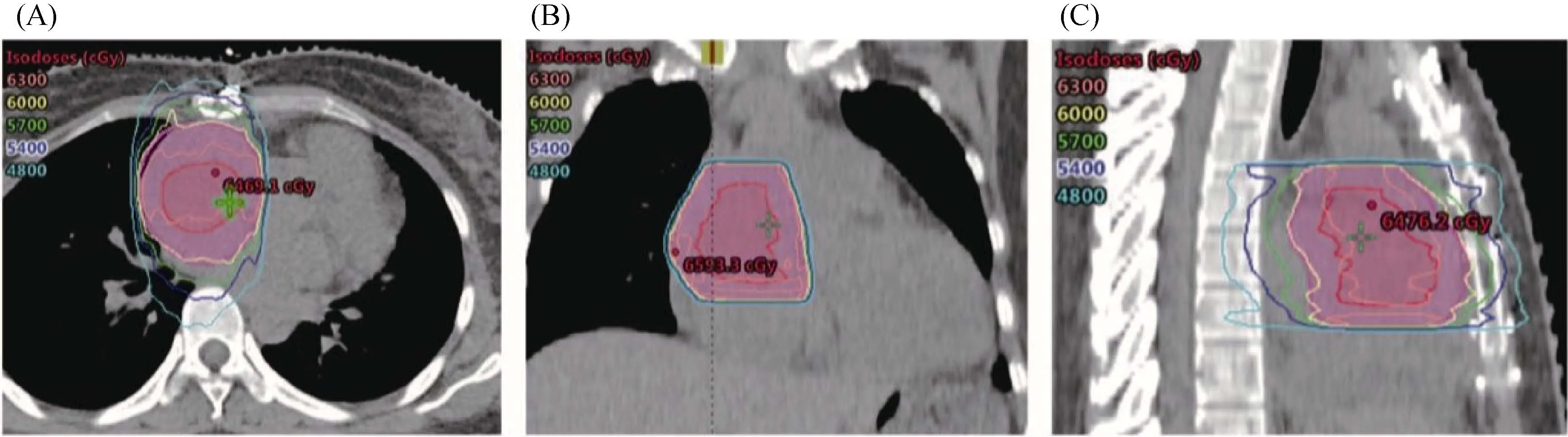
Figure 2. Target volume and dose distribution of the residual tumor in intensity-modulated radiation therapy. The dark red line delineated the GTV and the pink line delineated the PTV of the GTV. A total dose of 60 Gy in 30 fractions was prescribed to the PTV-GTV.(A): Representative dose distribution on transverse plane; (B): representative dose distribution on coronal plane; (C): representative dose distribution on sagittal plane. GTV: gross target volume; PTV: planning target volume.
There is currently no standard treatment for primary cardiac neoplasms. Most of published studies are restricted to case reports and small case series. To date, several retrospective studies have documented the outcomes of patients with cardiac angiosarcoma (Table 1). Among these limited cases, multidisciplinary approaches with adjuvant chemotherapy and radiotherapy after radical resection were shown to improve overall survival.[5,12-17]Therefore, surgical resection remains the first-line treatment and for locally advanced cases, integrated treatment with chemotherapy and radiotherapy may lead to shrinkage of the mass, allowing for radical surgery. Nevertheless, therapeutic regimens and radiation doses varied from case to case due to the lack of standard treatment modalities. At present, chemotherapeutic drugs used to treat cardiac angiosarcoma include Adriamy-cin, ifosfamide, cyclophosphamide, vincristine, or dacarbazine.[8,18]The doxorubicin-based chemotherapy regimen is the standard protocol for the treatment of local recurrence and distant metastasis of angiosarcoma. Furthermore, several phase II trials using sorafenib, bevacizumab and imatinib have shown efficacy of these anti-angiogenic drugs in the treatment of advanced or metastatic angiosarcoma.[11,18,19]For radiotherapy, postoperative adjuvant radiotherapy of 45–50 Gy should be considered for completely resected cardiac sarcomas.[7]However, postoperative adjuvant chemoradiotherapy following non-radical resection for massive tumors has not been reported.
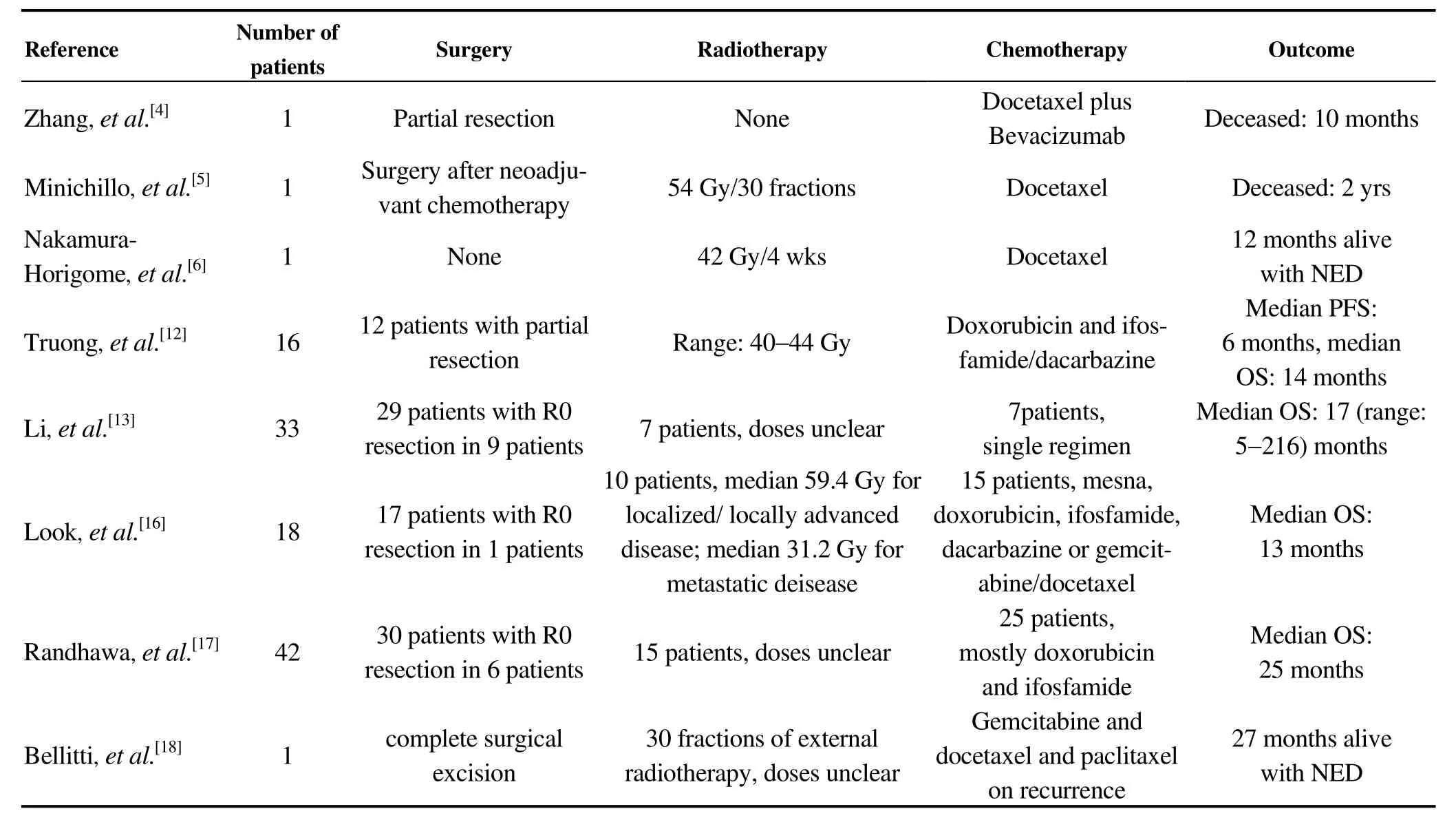
Table 1. Treatment and outcomes in cardiac angiosarcoma.
In this case, we reported a 38-year-old woman with a well-differentiated angiosarcoma who firstly underwent a non-radical surgical resection followed by two cycles of chemotherapy regimen with pirarubicin, ifosfamide and mesna. Intensity-modulated radiation therapy (60 Gy/30 fractions) was subsequently delivered targeting the residual lesions. The treatment was well-tolerated. Although there was no significant regression of the residual tumor after two cycles of chemotherapy, a quasi-disappearance of the residual tumor was noticed four months later after radiotherapy.To date, the patient recovered well with no sign of tumor recurrence and no obvious severe treatment-related adverse effects. Therefore, subtotal resection followed by sequential chemoradiotherapy might be an effective strategy for radically unresectable large primary cardiac angiosarcoma that warrants further validation in prospective studies.
 Journal of Geriatric Cardiology2019年2期
Journal of Geriatric Cardiology2019年2期
- Journal of Geriatric Cardiology的其它文章
- Removal of refractory thrombus by 5F child catheter in patients with subacute myocardial infarction
- Impact of main vessel calcification on procedural and clinical outcomes of bifurcation lesion undergoing provisional single-stenting intervention:a multicenter, prospective, observational study
- Bleeding risk assessment in elderly patients with acute coronary syndrome
- Frailty and acute coronary syndrome: does gender matter?
- Frailty in patients admitted to hospital for acute coronary syndrome: when,how and why?
- Clinical and prognostic implications of delirium in elderly patients with non–ST-segment elevation acute coronary syndromes
