Medical therapy for nephrolithiasis:State of the art
Igor Sorokin ,Mrgret S.Perle *
a Department of Urology,University of Massachusetts,Worcester,MA,USA
b Department of Urology,UT Southwestern Medical Center,Dallas,TX,USA
c Charles and Jane Pak Center for Mineral Metabolism and Bone Research,UT Southwestern Medical Center,Dallas,TX,USA
KEYWORDS Medical management;Kidney stones;Medical therapy;Pharmacology;Nephrolithiasis
Abstract The prevalence of nephrolithiasis is increasing worldwide.Understanding and implementing medical therapies for kidney stone prevention are critical to prevent recurrences and decrease the economic burden of this condition.Dietary and pharmacologic therapies require understanding on the part of the patient and the prescribing practitioner in order to promote compliance.Insights into occupational exposures and antibiotic use may help uncover individual risk factors.Follow-up is essential to assess response to treatment and to modify treatment plans to maximize therapeutic benefit.
1.Introduction
It is estimated that 50%of symptomatic kidney stone events could be prevented by addressing modifiable risk factors that can reduce the burden of stone disease on the general population [1,2].Unfortunately,medical management strategies are underused and inconsistent[3].The high prevalence and recurrent nature of kidney stones contribute to the large economic burden on society related to stone disease.Indeed,in 2000 the cost of stone disease to the United States.health care system was estimated at$5 billion[4].This is further compounded by the cost of missed work by working-age adults,who are disproportionately afflicted by stone disease compared with children or the elderly.Estimates from the Urologic Diseases in America project in 2012 suggest the cost is now up to$10 billion,making stone disease one of the most expensive urologic conditions[5].The cost of stone disease has been predicted to rise to more than$15 billion by 2030 as a consequence of population growth and the rising prevalence of obesity and diabetes,both risk factors for stone disease[6].Furthermore,stone disease significantly affects individual well-being,with lower quality of life(QoL)demonstrated even in those with asymptomatic stones[7].
Both the American Urologic Association(AUA)[8]and the European Association of Urology(EAU)[9]published recent guidelines on the prevention of recurrent nephrolithiasis,with specific recommendations regarding evaluation and dietary and pharmacologic therapy.The purpose of this state-of-the-art review is to examine strategies of medical management according to the latest published evidence and in accordance with recent guidelines.In addition,we will highlight recent efforts to improve compliance and QoL in patients with recurrent kidney stones.
2.Epidemiology
Nephrolithiasis is a highly prevalent disease worldwide with rates ranging from 7%-13%in North America,5%-9%in Europe,and 1%-5%in Asia[10].In the United States,there has been a linear increase in stone prevalence over the last 4 decades,with the prevalence in women rising disproportionately compared with men.While traditionally men have demonstrated a higher prevalence of stone disease than woman,the gender gap is narrowing[11,12].However,the difference in prevalence between men and women is not consistent worldwide,with male to female ratios varying from 2.7 to 4 among populations in Germany,Saudi Arabia,and China(Taiwan)[10].
The most common stone composition is calciumbased(70%-85%),followed by uric acid(5%-10%),struvite(1%-5%),and cystine(1%)[13],although one community found calcium stones present in 93.5%of kidney stone formers[14].In a retrospective review of 1516 patients at a single-institution from 1980 to 2015,the proportion of uric acid stones increased from 7%to 14%,with uric acid stone formers consistently older and with a higher body mass index(BMI)than calcium stone formers[15].While calcium stones remain common,analyzing stone composition remains beneficial to identify non-calcium stone formers,who tend to have higher rates of recurrence and require specific preventive strategies.
3.Screening evaluation
In addition to the common potential sequelae associated with kidney stones,such as pain,infection and obstruction,nephrolithiasis is also considered a risk factor for chronic kidney disease[16].As such,a thorough screening evaluation to identify patients with kidney stones who are at high risk of recurrence is prudent.The screening evaluation includes a detailed medical and dietary history,serum chemistries,urinalysis,stone analysis,and imaging studies.Those identified as high risk stone formers are then targeted for further metabolic testing with 24-hour urine collections to identify modifiable urinary risk factors that can be corrected with diet and medication.
A detailed medical history is aimed at identifying conditions associated with stone disease such as primary hyperparathyroidism,distal renal tubular acidosis(RTA),gout,metabolic syndrome,type 2 diabetes,obesity and malabsorptive gastrointestinal disorders.Metabolic syndrome is estimated to affect 25%of adults in the U.S.and is associated with a 30%increase in risk of nephrolithiasis[17].Gastrointestinal disorders associated with chronic diarrhea,such as intestinal resection,pancreatic insufficiency,celiac sprue,Crohn’s disease and ulcerative colitis cause predictable urinary abnormalities that lead to stone formation.A history of first-time versus recurrent stones also helps to stratify risk.In addition,some medications are associated with stone disease either by altering urinary analytes in an unfavorable way(topiramate,acetazolamide,zonisamide)or by direct precipitation of the drug or its metabolites in the urine(triamterene,ciprofloxacin).Finally,a detailed dietary history involving fluid intake,salt use,animal protein consumption and supplement use can reveal dietary risk factors for stone formation.
Serum chemistries should include calcium,creatinine,potassium,bicarbonate and uric acid,as abnormal values may suggest underlying medical conditions associated with stone disease,such as high serum calcium and primary hyperparathyroidism,or low serum bicarbonate and potassium and high chloride implicating distal RTA.Serum intact parathyroid hormone is considered an optional test that is indicated when primary hyperparathyroidism is suspected,such as when serum calcium is high or high normal,urinary calcium is exceptionally high,or with pure calcium phosphate stone composition.Urine should be evaluated by dipstick and microscopic analysis,as low or high pH can raise suspicion for uric acid(pH<5.5)or infection stones(pH>7),respectively,and the presence of bacteria and/or pyuria can implicate infection stones.
Radiographic imaging provides a baseline assessment of stone burden and can reflect the level of metabolic activity.Multiple stones on initial imaging,even with a first stone event,imply recurrent stone disease.Nephrocalcinosis is typically associated with either anatomic anomalies(medullary sponge kidney)or medical conditions associated with recurrent stones,such as primary hyperparathyroidism or distal RTA.
Environmental exposures,the so-called exposome,may contribute to nephrolithiasis and comprise potential targets for modification[18].High risk occupations that involve excessive heat exposure or conditions that preclude adequate hydration because of lack of access to water or bathroom facilities predispose to stone formation.In a survey of healthcare professionals,physicians working in an operating room setting were found to have the highest prevalence of nephrolithiasis(17.4%)and reported lowerfluid intake and higher stress levels[19].A recent study of 100 steel workers noted a stone prevalence of 16%,with dehydration,defined as urine osmolality>700 mOsm,noted in 57%of workers by the end of their work shift[20].Welders and spray painters have also been reported to have a higher risk of stone disease as a result of exposure to nephrotoxic agents contained in paint,such as cadmium and oxalic acid[10].Overuse of antibiotics may lower colony counts of Oxalobacter formigenes,a stoneprotective bacterium that uses intestinal oxalate as its sole substrate[18].Living in urban environments may enhance stone risk because of exposure to warmer areas created by lack of vegetation and effects of urban architecture[18].
All patients who have formed stones can benefit from general dietary counseling.However,for those in whom the screening evaluation identifies factors associated with an increased risk of stone recurrence,national guidelines support further metabolic testing with one or two 24 h urine collections[8,9].While some have criticized 24 h urine testing for its complexity,inconvenience,cost,and limited ability to predict recurrence[21],spot urine collections,and particularly the spot calcium-to-creatinine ratio,have been shown to be inferior to 24 h urine samples,but are sometimes used in children or other individuals for whom 24 h urine testing is impractical or impossible[22].A 12 h nighttime urine collection has recently been proposed as an alternative to 24 h urine testing because it showed a strong correlation with 24 h collections but was less cumbersome for patients[23].Further studies are needed to validate this simplified urine collection.While imperfect,the 24 h urine still remains the best tool to assess specific modifiable urinary stone risk factors.
4.Dietary management
Diet modification is an inexpensive,safe,and proven method to improve lithogenic risk factors and should be considered alone or along with pharmacotherapy for idiopathic calcium oxalate stone formers.A variety of dietary factors can be targeted for modification to reduce stone formation,including increasing fluids,maintaining normal calcium intake,limiting foods rich in oxalate,consuming sodium and animal protein in moderation and enriching the diet in fruits and vegetables(Table 1).Specific dietary measures based metabolic testing and follow-up have been shown to be more successful than general dietary recommendations and no specific testing in preventing recurrent stones[24].One study recommended that practitioners prioritize dietary recommendations,as three or few recommendations are associated with higher patient recall[25].
4.1.Fluids
High fluid intake is recommended in all stone formers,regardless of stone composition,because it has been shown to be beneficial and cost effective[26].A long-term randomized controlled trial(RCT)among recurrent idiopathic calcium oxalate stone formers demonstrated that a fluid intake of at least 2 L/day reduced the risk of stone recurrence by about 56%[27].A recent meta-analysis evaluating the effect of high fluid intake on recurrent stone formation revealed a pooled risk ratio(RR)of 0.40(95%CI:0.20-0.79,p=0.008)among two RCTs comprising 269 patients and 0.49(95%CI:0.34-0.71,p=0.0002)among seven observational studies comprising 273 685 patients[28].A fluid intake under 2 L/day was also recently shown to be the strongest risk factor for incident stone formation among male health care professionals(40-75 years old)enrolled in the longitudinal Health Professionals Follow-up Study(HPFS)[1].The AUA Medical Management of Kidney Stones Guideline recommends a fluid intake that will yield a urine output of at least 2.5 L/day for stone formers,and even more(3 L/day)for higher risk groups such as patients with cystinuria[8].
The benefit of fluids other than water has been investigated less vigorously.Large epidemiologic studies of both men and women showed a reduced risk of incident stone formation with coffee,tea,beer,red wine and orangejuice,while sugar sweetened beverages were associated with an increased risk of stone formation[29].A consensus statement by a multidisciplinary group of stone experts recommended against the consumption of sugar-sweetened beverages and encouraged intake of orange juice with no added sugar as potential measures to protect against calcium nephrolithiasis[16].The effect of lemonade is controversial.Although a small,uncontrolled metabolic study[30]and two retrospective studies[31,32]showed a beneficial effect with regard to increasing urinary citrate and reducing recurrent stone formation,two controlled,metabolic studies showed that orange juice and potassium citrate,but not lemonade,promoted a citraturic response and increased urinary pH[33,34].
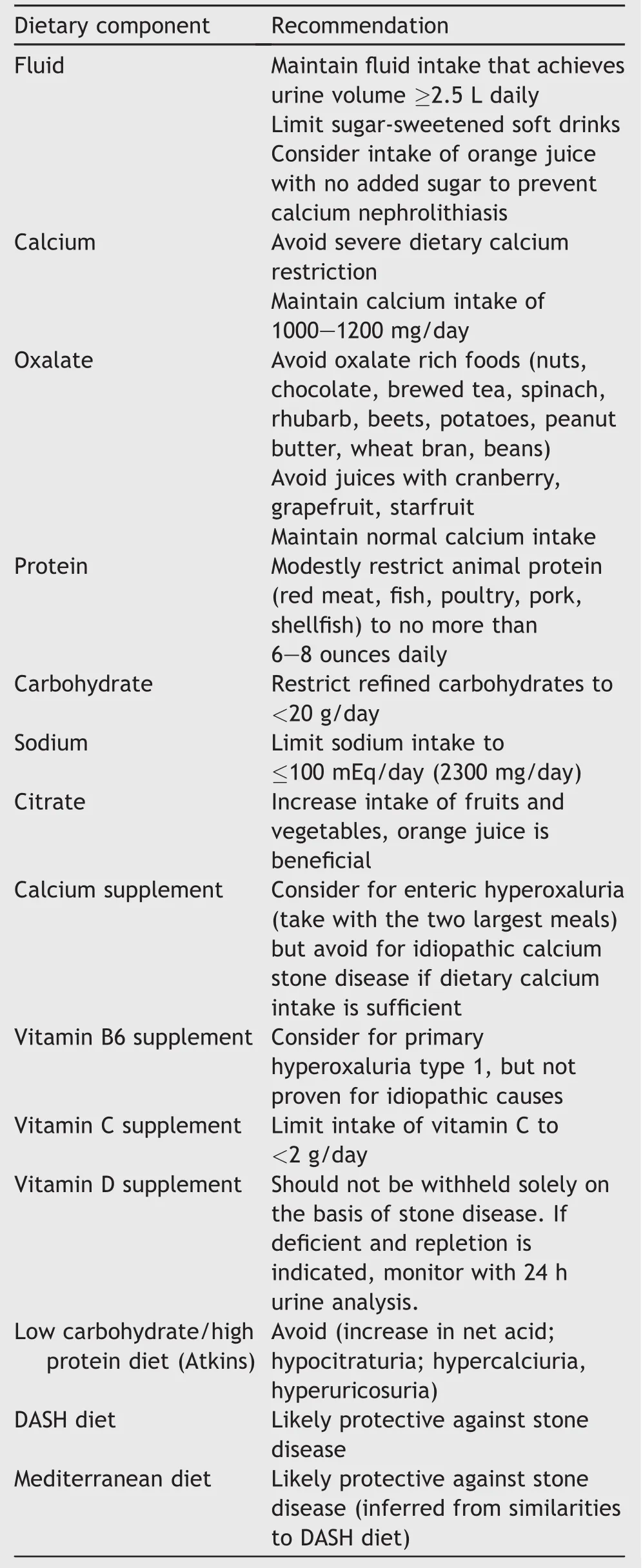
Table 1 Dietary recommendations to prevent kidney stone recurrence.
Increasing fluid intake has not proven to be a simple recommendation with which to comply[25].A questionnaire designed to estimate the success of achieving a highfluid intake among 302 stone formers noted perceived higher success rates in patients who preferred water and liked the “taste” of plain water compared to other beverages[35].The most common barrier to increasing fluid intake was noted to be “not feeling thirsty” (44.2%),followed by frequent urination(32.8%)and difficulty drinking while at school or work(20.7%).Using a container to carry water was noted to be a key strategy for success in maintaining a high fluid intake compared to simply making an effort to remember to drink.Compliance may be further improved with technologies such as “smart” water bottles that can accurately quantify 24 h fluid intake[36].
4.2.Calcium
Hypercalciuria is the most common risk factor among calcium oxalate stone formers[37].Hypercalciuria has been variously defined as 24 h urinary calcium>300 mg/day in men,>250 mg/day in women or>200 mg/day in either sex while on a diet restricted in calcium,sodium,and animal protein[38,39].Hypercalciuria may be a manifestation of systemic diseases such as primary hyperparathyroidism or sarcoidosis,but is considered idiopathic if no underlying cause can be identified.While idiopathic hypercalciuria has been shown to have a genetic predisposition in some cases,it can also be influenced by environmental factors such as diet[40].Although kidney stone formers have a higher risk of bone fracture than those in the general population[41],it is not clear that hypercalciuria is a cause.In a retrospective study of 250 men and 182 post-menopausal women on or off estrogen therapy,no significant relationship was found between urine calcium levels and bone mineral density.Hypercalciuria may not cause low bone mineral density and increased fracture rate among stone formers[42].
A normal calcium intake(1 000-1 200 mg/day elemental calcium or approximately three servings of dairy daily)is recommended for patients with idiopathic hypercalciuria[8,9].Both dairy and non-dairy dietary sources of calcium have been shown to have a protective effect against incident stone formation[43].On the other hand,severe calcium restriction should be avoided as it may accelerate bone loss and lead to hyperoxaluria due to the interaction between calcium and oxalate in the intestinal lumen by which calcium binds to oxalate and forms a calcium oxalate complex.In the setting of low calcium intake,excess uncomplexed oxalate is absorbed,ultimately leading to increased urinary oxalate excretion[37,44,45].Calcium in the form of food is preferred over calcium supplements as supplementation has been shown in epidemiologic studies to be associated with an increased risk of incident stone formation[46-48].If calcium supplements are indicated,they should be taken with meals,allowing the ingested calcium to complex with oxalate,thereby reducing intestinal oxalate absorption and counteracting the effect of increased urinary calcium.
Vitamin D deficiency has been shown to be more prevalent in the stone-forming population[49].However,repletion of vitamin D with supplements in this population has been controversial.The Women’s Health Initiative study demonstrated a 17%increased risk of stone formation among 36 282 postmenopausal women randomized to calcium carbonate plus vitamin D(1 000 mg/day and 400 IU/day,respectively)versus placebo[50].However,a recent small RCT comparing 6 weeks of low(1 000 IU/day)versus high(50 000 IU weekly)dose vitamin D supplementation among 21 vitamin D deficient stone formers found no significant change in urinary calcium after treatment in either group,although only the higher dose group showed a significant increase in serum vitamin D levels[51].If vitamin D repletion is indicated,urine calcium should be monitored with 24 h urine studies[52,53].
4.3.Oxalate
Hyperoxaluria occurs in approximately 10%-15%of calcium stone formers.Increased urinary oxalate can occur as a consequence of excessive dietary intake,endogenous oxalate overproduction or intestinal oxalate overabsorption.In normal individuals,approximately 10%of ingested oxalate is absorbed while the rest is eliminated through the stool[54,55].For reasons not clearly understood,calcium oxalate stone formers absorb a slightly higher proportion of oxalate from the intestine than do normal subjects[54].Oxalate absorption depends not only on the amount of dietary oxalate,but also on dietary calcium intake.Higher calcium diets lead to reduced oxalate absorption,while calcium-restricted diets are associated with enhanced oxalate absorption and subsequently increased urinary oxalate excretion[55,56].As such,a normal calcium diet in association with oxalate restriction is recommended in hyperoxaluric patients.High oxalate foods such as spinach,rhubarb,beets,nuts,chocolate,potatoes,bran,legumes and tea should be avoided[57].Some juices have been found to have a high oxalate content,including cranberry [58],grapefruit[59]and carambola juice(starfruit)[60].Spinach is frequently added to homemade fruit and vegetable juices,raising the oxalate content[61].Since the leaves are not cooked or processed to enhance removal or reduction of soluble oxalate,this practice may pose a threat to calcium oxalate stone formers.The addition of small amounts of calcium ions,especially in the form of calcium chloride,has been recommended by some to convert the oxalate into an insoluble form that is less likely to be absorbed in the digestive tract[61].However,any added calcium should be considered when calculating total dietary calcium intake.
Interestingly,no studies have directly shown a correlation between urinary oxalate and recurrent idiopathic calcium oxalate stone formation,and therefore recommendation of dietary oxalate restriction is empiric.Indeed,Noori and colleagues[62]randomized 57 patients with hyperoxaluria and recurrent calcium oxalate stones to a low oxalate diet or to the Dietary Approaches to Stop Hypertension(DASH)diet,which is a diet high in fruits,vegetables,nuts and legumes(high oxalate content)and low in sodium and red and processed meats.Although urinary oxalate increased in the group assigned to the DASH diet and decreased in those adhering to a low oxalate(4.8 mg/day vs.-4.2 mg/day,respectively,p=0.08),urinary saturation of calcium oxalate declined more on the DASH diet(-2.14)than on the low oxalate diet(-0.90,p=0.08),suggesting that other dietary measures had greater impact on reducing urinary stone risk than a low oxalate diet.
Enzymatic defects in the oxalate biosynthetic pathway lead to markedly high levels of urinary oxalate leading to aggressive calcium oxalate stone formation and oxalosis.Among the three forms of primary hyperoxaluria(I-III),renal failure is typically seen only with primary hyperoxaluria type 1.Strict dietary oxalate restriction is recommended in all forms of primary hyperoxaluria[63].
Patients with malabsorptive disorders from intestinal resection,roux-en-Y gastric bypass surgery,Crohn’s disease,celiac sprue,pancreatitis or use of fat-malabsorbing medications such as orlistat[64],are at risk of enteric hyperoxaluria because luminal calcium binds to poorly absorbed fatty acids,leading to higher levels of uncomplexed oxalate that is subsequently absorbed and excreted in the urine.In these patients,strict dietary oxalate restriction,along with a low fat diet and use of calcium supplements with meals to bind luminal oxalate is an effective strategy for stone prevention.
Finally,O.formigenes is a Gram-negative anaerobic bacterium that resides in the intestine and uses oxalate as its sole source for energy and growth[65].Animal models have shown that absence of O.formigenes colonization can result in reduced degradation of oxalate in the intestinal lumen as well as reduced enteric oxalate secretion[66].Other animal models demonstrated that colonization with O.formigenes in addition to a low fat diet decreased urinary oxalate excretion[67].A case-control study in human subjects noted that despite a strong inverse correlation between colonization and risk of recurrent stone formation,no significant difference in median urinary oxalate levels was detected between patients who were or were not colonized with O.formigenes[68].Further work is needed to clarify the therapeutic role of this organism or its enzymes in preventing calcium oxalate stone formation.
4.4.Citrate
Hypocitraturia has been reported in 15%-63%of patients with kidney stones and is often seen in conjunction with other metabolic disorders[69].Citrate is an important inhibitor of calcium stone formation because it directly inhibits nucleation,agglomeration and growth of calcium oxalate and/or calcium phosphate crystals and by complexing with calcium to reduce urinary saturation of calcium salts[70].Renal citrate excretion is modulated primarily by acid-base status;acidosis increases citrate reabsorption and alkalosis enhances citrate production and excretion in the renal proximal tubule.
Fruits and vegetables increase urinary citrate because of their high alkali content,but not all fruits and juices have the same citraturic effect.Orange juice has shown the most consistent benefit because it has a high content of potassium citrate that confers an alkali load [33,71,72].Lemonade,which is high in citric acid,does not affect urine pH and has less citraturic effect.While fruit juices offer a more palatable and less costly therapy than potassium citrate medication,fruit juices can be high in calories and oxalate content and this may temper their use[59,73].Fruits with a high malic acid(precursor to citrate)content,such as pears,may theoretically increase urinary citrate but few studies have examined this[74].Unfortunately,no citrus fruits or juices have been tested in a randomized trial to assess their benefit in reducing stone recurrence rates.
The DASH diet,as an overall healthy diet,has been suggested to reduce the rate of stone formation[75].The high alkali content,among other factors,may contribute to improvement in urinary stone risk factors[76].Among three large cohorts of both men and women[Nurses’Health Study I(NHS I),Nurses’Health Study II(NHS II),and HPFS],those subjects adhering most closely to the DASH diet had the lowest risk of incident stone formation on multivariate analysis[1].That is to say,when patients were given a score relating to how closely their diet resembled the DASH diet,those in the lowest quintile of DASH scores had the highest rate of incident stone formation in HPFS(odds ratio(OR)=1.53,95%CI:1.31-1.78),NHS I(OR=1.47,95%CI:1.25-1.73),and NHS II(OR=1.37,95%CI:1.21-1.55).
4.5.Sodium
Lowering salt intake is essential for stone prevention.Sodium increases stone risk by increasing urinary calcium excretion and decreasing urinary citrate[77,78].Sodiuminduced calcium excretion is further compounded by animal protein-induced hypercalciuria[79].Along with ample fruits and vegetables,reduction of sodium intake is a primary component of the DASH diet and likely contributes to its lower stone risk[75].
The AUA guideline recommends limiting sodium intake to no more than 2 300 mg/day.Healthy middle-aged women from the NHS I cohort who had a daily sodium intake in the highest quintile(median sodium intake 4 958 mg/day)demonstrated a higher risk of incident stone disease[46].However,the same correlation did not hold true in NHS II,the cohort of younger nurses,or in the cohort of men in HPFS[47,48].In two RCTs evaluating the effect of a low sodium diet on hypercalciuric stone formers,the groups randomized to a low sodium diet demonstrated lower mean urinary calcium excretion[80]or lower rate of stone recurrence[81]than the control groups.
4.6.Protein
Uric acid is the metabolic end product of purine metabolism and animal protein is a rich source of purines.Urinary uric acid has been shown to reduce the effectiveness of naturally occurring macromolecular inhibitors of calcium oxalate crystallization[82].In addition,protein derived from animal sources increases stone risk by increasing urinary calcium and oxalate and reducing pH and citrate.Animal protein provides an acid load through the high content of sulfur-containing amino acids,which leads to a state of mild chronic metabolic acidosis,low urine pH and hypocitraturia[83,84].Since high protein diets are often additionally devoid of sufficient fruits and vegetables,hypocitraturia may also ensue from the lack of alkali[85].Interestingly,a study comparing idiopathic calcium stone formers with a control group found a higher mean renal acid load in the stone formers,even though animal protein intake was similar between the two groups[86].The authors attributed these findings to a lower intake of fruits and vegetables among stone formers.Furthermore,a small metabolic study simulating the three phases of the Atkins diet(a low carbohydrate,high protein diet)demonstrated increases in urinary uric acid and calcium and decreases in pH and citrate during both the stringent induction phase and the less stringent maintenance phases of the diet compared to baseline[87].While the acid load conferred by animal protein has been presumed to promote hypercalciuria by increasing bone resorption[88],Maalouf and colleagues[89]found that administration of potassium citrate failed to prevent protein-induced hypercalciuria,suggesting that the hypercalciuria may be attributable to a renal etiology.
The recommended dietary allowance of protein is 0.8 g/kg/day[90],and animal protein restriction should include all forms of meat,including beef,poultry,and fish.A 3-phase randomized,crossover metabolic study in 25 normal subjects comparing three different animal protein sources revealed higher levels of urinary uric acid during the fish phase compared to the beef or poultry phase,although urinary saturation of calcium oxalate did not simply reflect urinary uric acid levels[91].Additionally,the question often arises among stone patients whether ingestion of whey protein,a dairy-derived protein supplement popular among athletes because it is thought to increase muscle mass and improve exercise performance,is also a risk factor for stone formation.A recent 2-phase metabolic study in which whey protein or albumin was given to 18 healthy volunteers on a controlled diet for 3 days showed no significant change from baseline in urinary stone risk factors with either supplement[92].
5.Pharmacologic management
When dietary measures fail or are inappropriate in patients with particular metabolic backgrounds,pharmacotherapy may be indicated to reduce stone recurrence(Table 2).A recent systematic review and meta-analysis of 21 RCTs comprising 2168 participants determined that drug therapy was more effective than dietary therapy in preventing stone recurrence among recurrent idiopathic calcium stone formers[93].Of note,the benefit of therapy was apparent only among those with two or more previous stone episodes.Although multiple RCTs have shown a benefit of pharmacotherapy in preventing stone recurrence,compliance with prescribed drug regimens is often suboptimal.Using medical claims data,Dauw and colleagues[94]found that only 30.2% of kidney stone patients who were prescribed medications for stone prevention adhered to their drug regimen.Factors that were independently associated with lower likelihood of adherence included combination drug therapy,female gender,less comprehensive health insurance and residence in the South or Northeast of United States.Older patients and salaried employees had a higher probability of adherence.Interestingly,adherence rates differed according to the prescribed drug,with only 13%of patients complying with citrate therapy compared to 42.5%for thiazides and 40%for allopurinol.
5.1.Calcium stones
5.1.1.Thiazides
Thiazide diuretics are recommended by both the AUA and EAU guidelines for patients with recurrent calcium-based stones and hypercalciuria[8,9].Thiazides enhance calcium reabsorption directly in the distal renal tubule and indirectly in the proximal renal tubule.A meta-analysis of six RCTs found that thiazides were associated with a 47%relative risk reduction in stone recurrence rates compared with placebo or no treatment[95].The benefit of thiazides may additionally extend to normocalciuric patients since the proportion of patients with documented hypercalciuria in these RCTs was variable,and indeed in some trials the metabolic background of patients was unknown.
Thiazide doses and regimens tested in RCTs include hydrochlorothiazide 25 mg twice daily,chlorthalidone 25 mg daily or indapamide 2.5 mg daily.Interestingly,one study noted that only 35%of patients treated with thiazides were prescribed a dose shown in RCTs to be beneficial for stone prevention[96].The most common side effect of thiazides is hypokalemia,which may lead to hypocitraturia due to intracellular acidosis.As such,potassium supplementation is recommended to prevent hypokalemia,to avoid increased cardiovascular risk and to minimize glucose intolerance.The choice of potassium citrate versus potassium chloride to prevent thiazide-induced hypokalemia should be based on initial or follow-up urine pH and citrate;if either is low,potassium citrate may be the preferred potassium supplement.
Because hypercalciuria has been shown to be a risk factor for osteoporosis,a multidisciplinary panel of stone experts authored a consensus paper in which they recommended that stone formers at increased risk of bone loss undergo measurement of bone mineral density by dual emission X-ray absorptiometry(DXA)[16].The fracture risk associated with stone disease varies by skeletal site and demonstrates a higher risk of incident wrist fracture,but not hip fracture,in women and men[97].The use of thiazides in hypercalciuric men was shown to reduce urinary calcium and improve bone mineral density in a short-term study[98].Likewise,Pak and colleagues[99]observed significant increases in bone mineral density at the lumbar spine,femoral neck and radial shaft in 28 recurrent idiopathic hypercalciuric stone formers treated with thiazides and potassium citrate for a mean of 3.7 years.
5.1.2.Potassium citrate
Potassium citrate is used to correct hypocitraturia,which occurs alone or in combination with other abnormalities in about 10%-60%of calcium stone formers[100].Barcelo andcoworkers[101]showed a benefit of potassium citrate therapy among 57 recurrent,hypocitraturic calcium stone formers randomized to receive either 30-60 mEq of potassium citrate daily or placebo in a 3-year trial assessing stone remission rates.Patients in the potassium citrate group had a significantly higher remission rate compared to the placebo group(72%vs.20%,respectively,RR=0.25,95%CI:0.09-0.70).Potassium citrate has also been shown to improve stone remission rates after shock wave lithotripsy.A recent meta-analysis evaluated the efficacy of potassium citrate in reducing stone recurrence rates 12 months after shock wave lithotripsy in four trials comprising 374 participants[102].A significantly lower stone recurrence rate was seen 12 months after shock wave lithotripsy in the potassium citrate group compared to the control group(RR=0.21,95%CI:0.13-0.31).Finally,patients with hypocitraturia due to distal RTA have also been shown to benefit from potassium citrate therapy,as the alkali load corrects the metabolic acidosis,increases urinary citrate excretion and reduces stone recurrence rates[103].
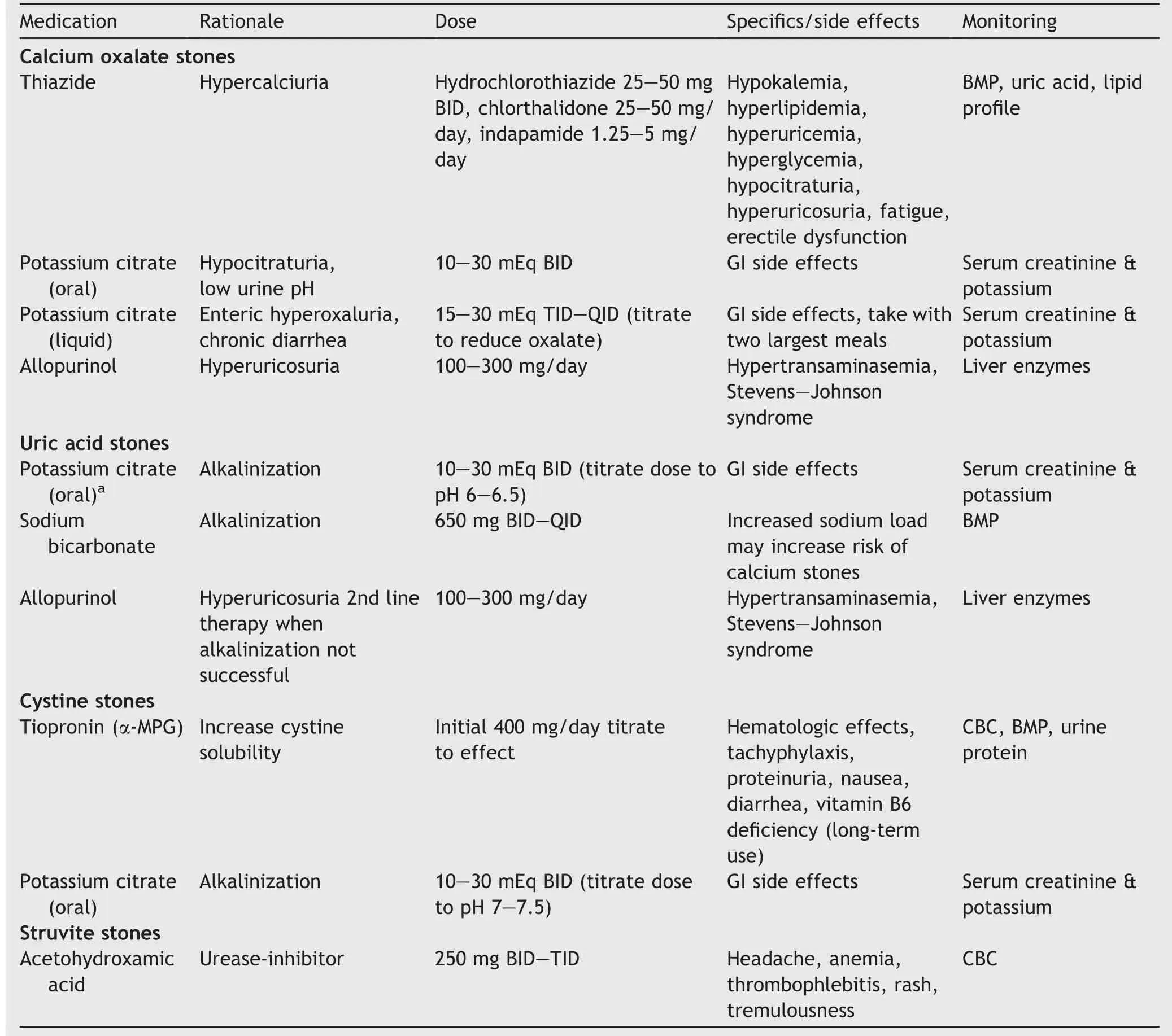
Table 2 Pharmacologic treatments by stone type.
Potassium citrate may also reduce urinary calcium excretion as an additional benefit of treatment.Song and associates[104]observed a mean decrease in urine calcium of 30%among 22 hypocitraturic calcium oxalate stone formers receiving 30-60 mEq of potassium citrate daily for at least 3 months.They speculated that the reduction in urinary calcium may be accounted for by a decrease in bone turnover due to systemic alkalinization,by binding of calcium by citrate in the intestine and reducing calcium absorption or as a result of a direct effect on the distal renal tubule affecting calcium reabsorption.
The optimal dose and formulation of potassium citrate remains to be determined[70].However,potassium citrate is usually administered as a sustained-release wax matrix tablet in doses ranging from 10-30 mEq twice daily.However,for patients with chronic diarrhea,use of a liquid formulation is advised because the rapid intestinal transit in these patients precludes sufficient absorption of sustained-release potassium citrate[105].
The most common side effects of potassium citrate are gastrointestinal upset,abdominal pain,and diarrhea.Taking the medication with meals may prevent these symptoms in some patients.Periodic monitoring with serum electrolytes and creatinine is recommended.For patients at risk of hyperkalemia or those who are unable to tolerate potassium citrate,sodium alkali such as sodium bicarbonate or a combination of sodium citrate and citric acid may be considered,although the sodium load conferred by these medications may induce hypercalciuria.
5.1.3.Allopurinol
Hyperuricosuria is seen in approximately 20%of patients with calcium stones[106].Although dietary restriction of animal protein is recommended as first line therapy in patients with hyperuricosuria,allopurinol,a xanthine oxidase inhibitor that lowers serum and urinary uric acid,is indicated in patients who are unable to normalize urinary uric acid with diet alone or in those with a genetic predisposition to hyperuricosuria.Allopurinol may additionally have antioxidant effects that contribute to the prevention of calcium stone formation,independent of xanthine oxidase inhibition[107].Among four RCTs evaluating the effect of allopurinol in recurrent calcium oxalate stone formers,the only trial to show a significant benefit in reducing recurrence rates is one in which only hyperuricosuric,normocalciuric patients were enrolled[108].
Allopurinol is generally well tolerated but may,in rare cases,be associated with Stevens-Johnson syndrome.Any patient reporting a rash with allopurinol should be instructed to discontinue the medication immediately.Hypertransaminasemia is another side effect that is generally reversible with discontinuation of the medication.Liver enzymes should be monitored soon after starting allopurinol and periodically thereafter[109].
5.1.4.Pyridoxine
Nondietary urinary oxalate is derived from the conversion of glyoxylate to oxalate by the enzyme lactate dehydrogenase[110,111].Pyridoxine,a component of vitamin B6,is a necessary cofactor for alanine:glyoxyalate aminotransferase(AGT)which is involved in the alternate metabolic conversion of glyoxylate to glycine.Patients with primary hyperoxaluria type I have a deficiency in AGT by which they are unable to divert glyoxylate metabolism to the alternate pathway.While the only curative treatment for this disease is liver transplantation,pyridoxine can be used to decrease urinary oxalate.A prospective,uncontrolled open label trial in which 12 patients with primary hyperoxaluria type I were given pyridoxine incrementally up to a dosage of 20 mg/kg per day showed a 25.5%mean relative reduction in urinary oxalate,with benefit seen in 50%of patients[112].
Pyridoxine has been proposed as a preventative treatment in patients with idiopathic hyperoxaluria as well.One retrospective study evaluating the effect of pyridoxine on urinary oxalate in 95 idiopathic hyperoxaluric stone formers found that 75%of patients treated with pyridoxine and dietary counseling versus only 52%of patients treated with dietary counseling alone showed a reduction in urinary oxalate[113].On the other hand,three large cohort studies(HPFS,NHS I,NHS II)found no association between vitamin B6 intake and risk of incident kidney stones[114].The AUA Medical Management of Kidney Stones Guideline found insufficient evidence to recommend pyridoxine for the management of patients with idiopathic hyperoxaluria[8].However if it is used,pyridoxine should be started at a low dose and titrated in a stepwise fashion to no more than 200 mg/day[115].Doses excessing 500 mg/day should be avoided because of the risk of developing severe sensory neuropathy.
5.2.Non-calcium based stones
5.2.1.Uric acid stones
Uric acid stones account for approximately 10%of all kidney stones in the United States[116].Low urine pH is the primary risk factor for idiopathic uric acid nephrolithiasis,although low urine volume and hyperuricosuria are additional contributors.Gout is a well-established risk factor for pure uric acid stones due to a defect in renal production of ammonia leading to acidic urine[117].Additionally,uric acid stones have recently been observed to comprise a greater proportion of stones among individuals with metabolic syndrome,type 2 diabetes,or obesity due to a defect in ammoniagenesis as well as increased net acid excretion,both of which lead to acidic urine[118].One study noted that metabolic syndrome conferred a 93%increased OR of forming uric acid stones,particularly in those with higher fasting serum glucose and hypertriglyceridemia[119].
The prevention of uric acid stone formation involves the administration of alkali to increase urine pH.A target pH of 6-6.5 can effectively prevent and dissolve uric acid stones[120].Potassium citrate,titrated to achieve the target pH,is first-line therapy for uric acid stone formers,with sodium alkali(sodium bicarbonate)reserved for those unable to tolerate potassium citrate or for whom renal dysfunction or hyperkalemia precludes its use.Of note,even large amounts of uric acid are soluble if the urine pH is sufficiently high;in contrast,even small amounts of uric acid will precipitate out in acidic urine.As such,allopurinol should not constitute first line therapy for idiopathic uric acid stone formers and is reserved only for patients who continue to form uric acid stones despite adequate urinary alkalinization[8].In addition to pharmacologic treatment to prevent uric acid stones,lifestyle changes such as weight loss and exercise should be recommended by physicians for patients with metabolic syndrome.
Dissolution therapy for uric acid stones is also achievable with alkalitherapy using a targeturine pHof6-6.5.However,the successofdissolution therapy relieson accurate diagnosis of pure uric acid stones.One group of investigators determined that computed tomography(CT)Hounsfield units≤500,pH≤5.5 and stone size>4 mm on non-enhanced CT had an 86%sensitivity,98%specificity,and 90%positive predictive value fordiagnosing uric acid stones[121].In an in vivo study of30 patients,dualenergy CTwasshown to have a 100%positive predictive value for detecting uric acid stones[122].Patients with 100%pure uric acid stones tend to be older(60 vs.55 years,p=0.05),heavier(34.3 vs.29 kg/m2,p<0.0001),have higher serum uric acids(6.53 vs.5.89 mg/dL,p<0.0006)and have lower pH(5.62 vs.5.89,p=0.03)than those with 10%-20%uric acid stone composition[123].Combining these demographics with stone size and Hounsfield units on CT scan may contribute to better diagnostic accuracy in identifying patientswith pure uric acid stones who may be successfully treated with dissolution therapy.Complete dissolution may occur in as little as 6 weeksto 6 monthsormore[124,125],likely due to the varying percentages of uric acid stone composition[123].
5.2.2.Cystine stones
Cystinuria is a rare inherited disorder in the renal and intestinal transport of the dibasic amino acids,caused by mutations in the SLC3A1 and/or SLC7A9 genes[126].The worldwide prevalence of cystinuria is estimated at 1:7000,although it is regionally variable[127].The hallmark of cystinuria is aggressive and recurrent cystine stone formation stemming from defective cystine reabsorption in the proximal tubule,leading to accumulation of poorly soluble cystine in the urine.
The therapeutic goal in treating patients with cystinuria is to reduce cystine concentration or raise cystine solubility above the solubility limit of 250 mg/L.Reducing cystine concentration can be accomplished with increased fluid intake,typically enough fluid intake to produce a urine volume of at least 3 L/day,or by reducing cystine excretion.Restriction of sodium and animal protein have been shown in randomized trials to decrease urinary cystine excretion[128-130].Like all stone formers,cystinuric patients are advised to limit their sodium intake to less than 2300 mg/day(100 mEq/day)[8].
Cystine solubility is influenced by urine pH and the presence of urinary macromolecules.Because cystine solubility increases significantly only above pH 7.5 and most cystinuric patients have high urine pH at baseline,urinary alkalinization has a limited therapeutic role,but constitutes second line therapy after diet and fluids,with the goal of treatment to achieve a urine pH of 7.0-7.5.At pH>7,the risk of calcium phosphate stone formation becomes more pronounced,and repeat stone analysis,if one is available during treatment,should be pursued[131,132].Potassium alkali(potassium citrate)is preferred over sodium alkali for urinary alkalinization because sodium enhances urinary cystine excretion.
For more severe cystinuria, cystine-binding thiol drugs(CBTD)constitute third line therapy[133,134].Agents most commonly used include α-mercaptopropionyl glycine(tiopronin)and D-penicillamine.Thiol compounds contain sulfhydryl groups that undergo a disulfide exchange reaction with cystine to produce two molecules of cysteine bound to the CBTD,a complex that is 50 times more soluble than cystine.The effect of the drugs is dose-dependent.
Tiopronin,as a second generation CBTD,is considered the first line drug,with dosing starting at 200 mg two or three times daily and titrated to achieve a urine cystine concentration of less than 250 mg/L.Adverse effects include fever,gastrointestinal upset,asthenia,rash,joint aches,loss of taste,thrombocytopenia,aplastic anemia,proteinuria,and changes in mental status[135].
D-penicillamine has a more extensive side effect profi le than tiopronin and therefore is used much less commonly[136].Dosing typically starts at 250 mg two or three times daily and is titrated to effect.One study reported adverse effects in 65%of patients taking tiopronin compared with 84%of those taking D-penicillamine[137].Adverse reactions necessitating cessation of treatment were also less common with tiopronin(31%vs.69%,respectively).Long-term therapy with D-penicillamine may lead to pyridoxine(vitamin B6)deficiency,which may require oral supplementation(50 mg/day).
Captopril is a third generation angiotensin-convertingenzyme inhibitor that also contains a free sulfhydryl group and has been shown in vitro to increase cystine solubility[138].However,the recommended doses of captopril in vivo are not thought to be sufficient to induce a therapeutic effect on cystine stone formation and the drug has not been tested in rigorous clinical trials.
Recently,a widely available nutritional supplement,αlipoic acid,has been investigated as a treatment for cystinuria[139].Using the SLC3A1-/-mouse model which grows urinary bladder stones at a rate of 1 mm3/day,treatment with α-lipoic acid was found to not only attenuate growth of existing cystine stones but also prevent initiation of new stone formation.The protective effect is thought to be derived from increased urinary cystine solubility due to excretion of downstream α-lipoic acid metabolites into the urine.Clinical trials are necessary to prove its efficacy in humans.
Although traditionally the goal of treatment has been to reduce urinary cystine concentration below the solubility level[133],most assays are unable to distinguish free cystine from soluble thiol drug-cysteine complexes.A proprietary test,cystine capacity(Litholink Corporation,Chicago,IL,USA),has been shown to reliably estimate stone forming propensity even in the presence of CBTDs and offers an alternative means of monitoring therapeutic ef ficacy[140,141].
5.2.3.Struvite stones
Struvite stones form as a consequence of repeated infection with urease-producing organisms,leading to alkaline urine and precipitation of magnesium ammonium phosphate crystals.Because these stones incorporate bacteria inside them,aggressive surgical stone removal is essential to eradicate the bacteria and prevent recurrent infections and stone.Culture-specific antibiotic therapy is recommended both pre-operatively and for some time post-operatively to sterilize the urine and prevent re-infection.A recent multiinstitutional study on patterns of infection and colonization of struvite stones revealed that the bacteriology has shifted away from traditional urea-splitting organisms such as Proteus to Enterococcus and Escherichia coli[142].The high prevalence of infection with Enterococcus(18%)suggests that 1st and 2nd generation cephalopshorins may be ineffective and antibiotic coverage should include agents appropriate for Enterococcus.Nonetheless,there is a lack of evidence on the role of antibiotics in preventing stone growth/recurrence and the optimal duration of therapy.
Acetohydroxamic acid(AHA),a potent urease inhibitor,decreases the risk of struvite stone formation by preventing bacterial-induced urease from altering the urinary milieu.AHA has been shown in a number of RCTs to reduce stone growth and prolong the time interval to stone growth[143-145].However,adverse events are common,leading to high attrition rates.Side effects include tremor,palpitations,edema,proteinuria,headache,rash,alopecia,anemia,gastrointestinal discomfort,and thromboembolic phenomena.In light of these limitations,acetohydroxamic acid,along with suppressive antibiotics,are reserved for patients at high risk of recurrent struvite stone formation and/or those unable to undergo surgical stone removal.Using a lower dose of 250 mg twice daily has been proposed to reduce the overall adverse events,although deep vein thrombosis/pulmonary embolus was still prevalent[146].
6.Follow-up
The success of a medical prophylactic program hinges on improvement in urinary stone risk factors and ultimately on reduction in stone recurrence rate.By monitoring 24 h urine parameters,a change in stone risk might be anticipated and corrected before stones recur.The frequency of follow-up depends on the metabolic activity of the individual.Periodic imaging can detect failure of medical therapy and prompt early surgical treatment and modulation of the medical regimen.In addition,follow-up should include blood and urine testing for adverse effects of treatment,such as hypokalemia with thiazides or proteinuria with α-mercaptopropionyl glycine(tiopronin).
7.Conclusion
A thorough evaluation to identify the underlying causes of stone disease is necessary to direct medical therapy.A screening evaluation to identify those at risk and further metabolic testing in those at higher risk optimizes the diagnostic algorithm.While imperfect,24 h urine testing remains the best available tool to identify urinary stone risk factors and direct medical and dietary therapy.Pharmacotherapy is reserved for patients who fail dietary therapy or who have demonstrable metabolic abnormalities that are not amenable to dietary therapy.Treatment plans should be designed to minimize cost and maximize compliance and effectiveness.Finally,follow-up is essential to assess response to treatment and to modify treatment plans to maximize therapeutic benefit.
Author contributions
Study concept:Igor Sorokin.
Search for articles:Igor Sorokin,Margaret S.Pearle.
Review and inclusion of articles:Igor Sorokin.
Drafting of manuscript:Igor Sorokin.
Critical revision of the manuscript:Margaret S.Pearle.
Conflicts of interest
The authors declare no conflict of interest.
 Asian Journal of Urology2018年4期
Asian Journal of Urology2018年4期
- Asian Journal of Urology的其它文章
- Miniaturised percutaneous nephrolithotomy:Its role in the treatment of urolithiasis and our experience
- Present indications and techniques of percutaneous nephrolithotomy:What the future holds?
- Ureteral stents in urolithiasis
- Retrograde intrarenal surgery:An expanding role in treatment of urolithiasis
- Indications and contraindications for shock wave lithotripsy and how to improve outcomes
- Defining metabolic activity of nephrolithiasis-Appropriate evaluation and follow-up of stone formers
