Lead Induces Different Responses of Two Subpopulations of Phagocytes in the Holothurian Eupentacta fraudatrix
DOLMATOVA Lyudmila S., and DOLMATOV Igor Yu.
?
Lead Induces Different Responses of Two Subpopulations of Phagocytes in the Holothurian
DOLMATOVA Lyudmila S.1),*, and DOLMATOV Igor Yu.2),3)
1),,,,690041,2),,,,690041,3),,690922,
In view of increasing lead pollution (Pb2+) of coastal waters, the compensatory abilities of holothurians need to be assessed. The goal of the work is to clarify the functional and phenotypical differences between two types of phagocytes (P1 and P2) inexposed to Pb(NO3)2. It has been shown that 2mgL?1lead exposure for 48h increases the number of P2 phagocytes as compared to P1 cells, does not significantly affect cell viability in both P1 and P2 phagocyte fractions, and significantly enhances chromatin condensation in P2 but not in P1 phagocytes. A lead concentration of 4mgL?1increases the number of P1 phagocytes compared to that of P2 type, and does not change cell viability and chromatin condensation in P1 phagocytes.In the P2 type, it decreases cell viability and does not influence the level of apoptosis. The protection against lead-induced apoptosis is apparently mediated by the activities of antioxidant enzymes, especially glutathione S-transferase. The differences in labeling cell surface receptors of P1 and P2 phagocytes by plant lectins alsoindicate the specific phenotypic properties of these cells. The results clarify the potential and GSH-dependent mechanisms of immune adaptation in holothurians that have been shortly exposed to lead at concentrations close to the maximum environmentally relevant level in coastal waters. Additionally, P1 and P2 phagocytes are first shown to have different functions and phenotypes during the response to lead, which indicates the complexity of the phagocytic system in holothurians and contributes to understanding the immunity evolution.
amoebocytes; antioxidant capacity; apoptosis; cell adaptation; lead; plant lectin; con A; DBA; PNA; SBA
1 Introduction
Lead (Pb2+) is one of the most toxic heavy metals that affect the health of living organisms. Its impact on organs and organ systems of vertebrates, such as blood, reproductive and immune systems among others is well documented (Sharma., 2011). However, much less data are available about its effects on invertebrates.
Lead poisoning can induce a delay of the growth and development of marine invertebrates (Catarino, 2008). The invertebrates have been shown to have various mechanisms to reduce the potential toxicity of the metal. These mechanisms include an increase in metal excretion or sequestration (Chiarelli and Roccheri, 2014). The sup- posed mechanism of lead detoxificationits binding to cytoskeleton was not identified in all species studied (Ca- tarino., 2008), while some species (polychaete and bivalve mollusks) were able to regulate the concentration of essential metals in their tissues (Bryan and Langston, 1991).
Species of Echinodermata (the exclusively marine phy- lum) are known to accumulate Pb2+in their tissues (Ca- tarino, 2008). The reports about the ability of holo- thurians (or sea cucumbers) to accumulate lead describe both low (McAloon and Mason, 2003; Dolmatova.,2010b) and high (Wang., 2015; Mohammadizadeh., 2016) levels of accumulation. These differences are apparently related to the specific traits of these ani- mals and/or characteristics of their biotopes. It was shown that lead can be redistributed in their organs in tissue- and species-dependent ways. Thus, the highest level of lead, compared to its level in gonads and body wall, was re- gistered in the intestine of(Dol- matova., 2010a).The highest level of lead in the body wall, compared to that in the intestine and the res- piratory tree, was found in(Wang, 2015).In, the respiratory tree exhibited, on the contrary, a higher level of lead compared to the body wall (Mohammadizadeh., 2016). The mechanism of redistribution of Pb2+in tis- sues of the species is apparently very important for main- taining their essential functions, as it was shown that different echinoderms keep their reproductive function unchanged even in highly polluted areas (Catarino., 2008).
Cells of immune system form the first line of defense against xenobiotics (Kasten-Jolly and Lawrence, 2014), and, therefore, the immune system is considered to be the most sensitive indicator of toxicants’ impacts on organ- isms. Thus, monitoring the immune system may be in- formative even when other systems seem to be undam- aged (Taheri, 2015). In addition, the immune system is known to play an important role in detoxification in vertebrates exposed to heavy metals. A lead-induced damage to immunity in vertebrates occurs at different levels. In particular, lead affects the activity of Th cells, but not natural killers (Kasten-Jolly and Lawrence, 2014). A chronic lead nitrate ingestion caused a significant de- cline in the numbers of lymphocytes and monocytes, but not in neutrophil content (Sharma, 2010). In con- trast to vertebrates, invertebrates have only the innate immune system. In lead-exposed mollusks, hemocytes (phagocytes) constitute the system for metal transport between tissues, while their immune functions may be disturbed (Homa., 2005). As is known, lead can in- duce immunosuppressionapoptosis and inhibition of antioxidant defense in macrophages of vertebrates (Sha- bani and Rabbani, 2000). Similarly, its genotoxic effects and inhibition of some antioxidant enzymes were detected in coelomocytes of some marine invertebrates (Poromov., 2014; Singh., 2017).
Nevertheless, the immune cells of marine invertebrates can adapt to lead while they are exposed to lead at high concentrations (Soto., 2008; Poromov., 2014). In short-term experiments, marine invertebrates or their cells were exposed to different effective non-lethal or sublethal concentrations of lead in seawater, from 0.1–0.5mgL?1(polychaete, 1–5 days) (Singh., 2017) to 2mgL?1(sea star, 3–10 days) (Poromov., 2014), and even to 12.5mgL?1(Green mussel, 96–168h) (Tan and Lim, 1984). These con- centrations are much higher than those described for non- polluted areas (<0.1μgL?1) (Korshenko, 2016). However, high lead concentrations may be found in some rivers (up to about 2mgL?1) (Arbneshi., 2008),which indicates the possibility of short-term impact of high levels of this metal on invertebrates inhabiting estuaries and coastal areas (Huang and Onyx, 2004).
is a common inhabitant of coastal waters of the Peter the Great Bay, Sea of Japan. Its tissues are rich in biologically active substances that make the spe- cies promising for aquaculture (Dolmatova, 2007). In the Peter the Great Bay, lead concentration in the sea- water rarely exceeds the level of 1μgL?1(Korshenko, 2016). However, its concentration can reach 40μgL?1in some polluted areas (Naumov, 2007). Apparently, it can be even higher in waters adjacent to areas of high indus- trial activity. For instance, the concentration of lead in solids in Rudnaya River (flowing into Rudnaya Bay, with a lead refinery situated on the coast) approaches the level of 800 μgg?1(Shulkin and Bogdanova, 2003). Under the similar conditions, a high level of lead pollution of sea- water, up to 1.14mgL?1, was described by Jacob. (2013) near the Mangalor Refinery, India. Thus, in such coastal areas the lead impact on holothurians is high due to the intense pollution of the seawaters by lead from riv- ers and refinery. However, the limits of lead tolerance in holothurians remain unknown. In view of the current tendency ofincreasing lead pollution of seawater (Kor- shenko, 2016), it is important to understand the compen- satory immune mechanisms in holothurians exposed to lead at concentrations close to the maximum environ- mentally relevant level.
The immune cells of, amoebocytes (pha- gocytes), like those of other echinoderms, are analogous to macrophages of vertebrates. After a gradient centri- fugation, the phagocytes of echinoderms are separated into two fractions with poorly understood roles during the immune response (Chia and Xing, 1996). Phagocytes ofwere also divided by centrifugation into two fractions, referred to as P1 and P2 (Dolmatova., 2003).Their roles in case of lead exposure remain unknown.
The goal of the work was to clarify the functional and phenotypical differences of the two types of phagocytes induring the adaptive response to anexposure to lead nitrate [Pb(NO3)2].
2 Material and Methods
2.1 Animals
(Holothuroidea, Dendrochirotida) (60–70mm in length) were collected by divers from the waters of the marine reserve in Vostok Bay (Peter the Great Bay) in September. Immediately after collection, the specimens were transported to the laboratory of the Marine Biological Station (National Scientific Center of Marine Biology, FEB RAS) and placed into tanks with aerated seawater. Prior to experiments, they were kept at a seasonal temperature of 17℃ for 2 weeks. The mean value of lead concentration in seawater of this area was less than 0.1μgL?1(Korshenko, 2016).
2.2 Experimental Design
The individuals were distributed over 3-L aquaria (three groups, 6 animals each variant of exposure) containing seawater without (control) or with Pb(NO3)2(2 or 4mgL?1). The surface layer of the water column was aerated by producing air bubbles. During the experiment, the sea- water temperature was 17±0.4℃, and salinity was 34, corresponding to their natural seasonal values in this area. The animals were exposed to the seasonal light-to-dark period about 12h–12h. The holothurians were fed natural substances from the seawater column without additional food supply.
After a 48-h exposure, animals were dissected, and the coelomic fluid (one volume) was transferred into flasks containing two volumes of anticoagulant solution (30mmolL?1EDTA, 31gL?1NaCl, and 50mmolL?1Tris-HCl, pH 7.6) (Chia and Xing, 1996). The collected sam- ples of coelomic fluid from 3 animals were combined for further phagocyte separation.
2.3 Phagocyte Isolation
Aliquots of the coelomic fluid (2mL) were layered on top of a two-step gradient (ficoll-verografin in anticoa- gulant solution at the ratios (V:V) of 1:2 for step 1 and 1:1 for step 2). Centrifugation was carried out at 300×at 5℃ for 15min. Then the distinct bands at the interfaces between sample and step 1 (corresponding to the P1 phagocyte fraction), and step 1 and step 2 (the P2 phago- cyte fraction) (Dolmatova., 2004) were collected and washed twice with a phosphate-buffered saline supple- mented with 36gL?1NaCl and 0.1% glucose (PBS), pH 7.4 (Chia and Xing, 1996). The pellet was resuspended in 1mL of PBS. Cells were counted in a Goryaev chamber. Cell viability was estimated by trypan blue exclusion.
2.4 Chromatin Condensation Measurement
A portion of cell suspension ((0.3–0.5)×106cellsmL?1) of P1 or P2 phagocytes was centrifuged at 1000×for 5min.The pellet obtained was fixed with 4% parafor- maldehyde and stored before performing the assay at 4℃. For chromatin condensation measurement, cells were washed twice with PBS, and the pellet was stained with Hoechst 33342 (Sigma-Aldrich, St. Louis, MO, USA) (1μgmL?1in PBS) as described by Komatsu. (1998). Percentage of cells with changes in nuclear morphology was calculated using fluorescent microscope technique (microscope Leica DM 4500, Weltzlar, Germany).
2.5 Assays for Antioxidant Enzyme Activities
The cell suspensions were frozen in liquid nitrogen and stored at –40℃ before enzyme assays. After defrosting at room temperature, the cells were destroyed by sonication (22kHz; 25×6s; 0℃) in the presence of 0.1mmol?1phenylmethylsulphonyl fluoride (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany). Enzyme activities were measured in nuclear-free supernatants using a spectro- photometer SF-56 (LOMO,Russia). Glutathione reductase (GR; EC 1.6.4.2) activity was de- termined by the rate of decrease in NADPH (Sigma-Al- drich, St. Louis, MO, USA) absorbance (Yusupova, 1989) at 340nm (ε=6.22mmol?1cm?1) and pH 6.4 as described early (Dolmatova., 2004).Catalase (EC 1.11.1.6) activity was determined by the rate of decrease in H2O2absorbance at 240nm (ε=0.04mmol?1cm?1) and pH 7.4 (Dolmatova., 2004). Activity of glutathione S- transferase family (GST; EC 2.5.1.18) was measured us- ing 1-chloro, 2,4-dinitrobenzene (Sigma-Aldrich, St. Louis, MO, USA) as the substrate (Habig., 2004) at 340nm (ε=9.6mmol?1cm?1) and pH 6.8 which was optimal as was found in our preliminary experiments. Each assay was performed in triplicate.
Protein was quantified using abrilliant blue(Sigma-Aldrich Chemie GmbH, Taufkirchen, Ger- many)method with fetal bovine serum albumin (Greiner Labortechnik GmbH, Frickenhausen, Germany) as the standard.
2.6 Detection of Lectin Binding to Cell Surface
Cells were fixed with 1% glutaraldehyde in PBS and stored at 4℃ before FITC-conjugated lectin staining. Prior to assay, the cells were washed 3 times with PBS.
FITC-conjugated lectins (ICN Biomedicals, Eschwege, Germany) from(PNA),(SBA),(DBA) and(con A), which bind primarily to Galβ1-3GalNAc, GalNAcα1-3GalR, GalNAc and α-D-mannopyranosides, respectively (Gnedkova., 2015), were used accord- ing to the protocol of cell staining with lectin as described by McKenzie and Preston (1992). Each of FITC-conju- gated lectins (1mgL?1) was added to a cell suspension at a ratio of 1:10 (V:V). After incubation at 24℃ for 1h, the cells were centrifuged (200, 5℃, 10min) and washed 3 times with PBS. Cell fluorescence was assessed under a microscope (Leica DM 4500, Weltzlar, Germany). The level of lectin binding to cell surface was evaluated as the percentage of the cells having zones of bright green fluo- rescence compared to the total cell count. In each repli- cate, at least 100 cells were analyzed.
2.7 Statistical Analysis
The data are presented as the mean±standard error(SEM) (=6). The Kolmogorov-Smirnov test to ensure the normal distribution in the continuous data and Bart- lett’s test for detecting the homogeneity of variances were performed using GraphPad InStat, v. 3.01 (GraphPad Soft- ware, Inc.). The two-way analysis of variance (ANOVA) to evaluate the main effects and interaction of the two factors (lead concentration and cell type) (Table 1) and the following Holm-Sidak multiple-comparison test to contrast differences between groups were performed us- ing GraphPad Prism v. 6.01 (GraphPad Software, Inc.). In all comparisons, the level of significance was<0.05.
3 Results
To analyze the main effects and interaction of lead and phagocyte type on the functional and phenotypical mar- kers under study, two-way ANOVA was performed. As Table 1 shows, lead had significant effects on most pa- rameters except for cell viability. Type of cells also sig- nificantly affected most parameters but not chromatin condensation and binding plant lectins con A and DBA. In addition, the interaction between the two factors was significant in most cases with the exception of their ef- fects on cell viability and activities of GR and GT.
3.1 Cell Number
As shown in Fig.1, the mean cell numbers in both con- trol fractions were similar to each other. A lead concen- tration of 2mgL?1induced a significant decrease in num- ber of cells in both fractions compared to the control, while the content of cells in P2 fraction was 1.6 times higher than that in P1. In contrast, a lead concentration of 4mgL?1significantly increased the cell number in both fractions compared to the control levels, and the number of P1 phagocytes was 1.7 times higher than that of P2 cells.
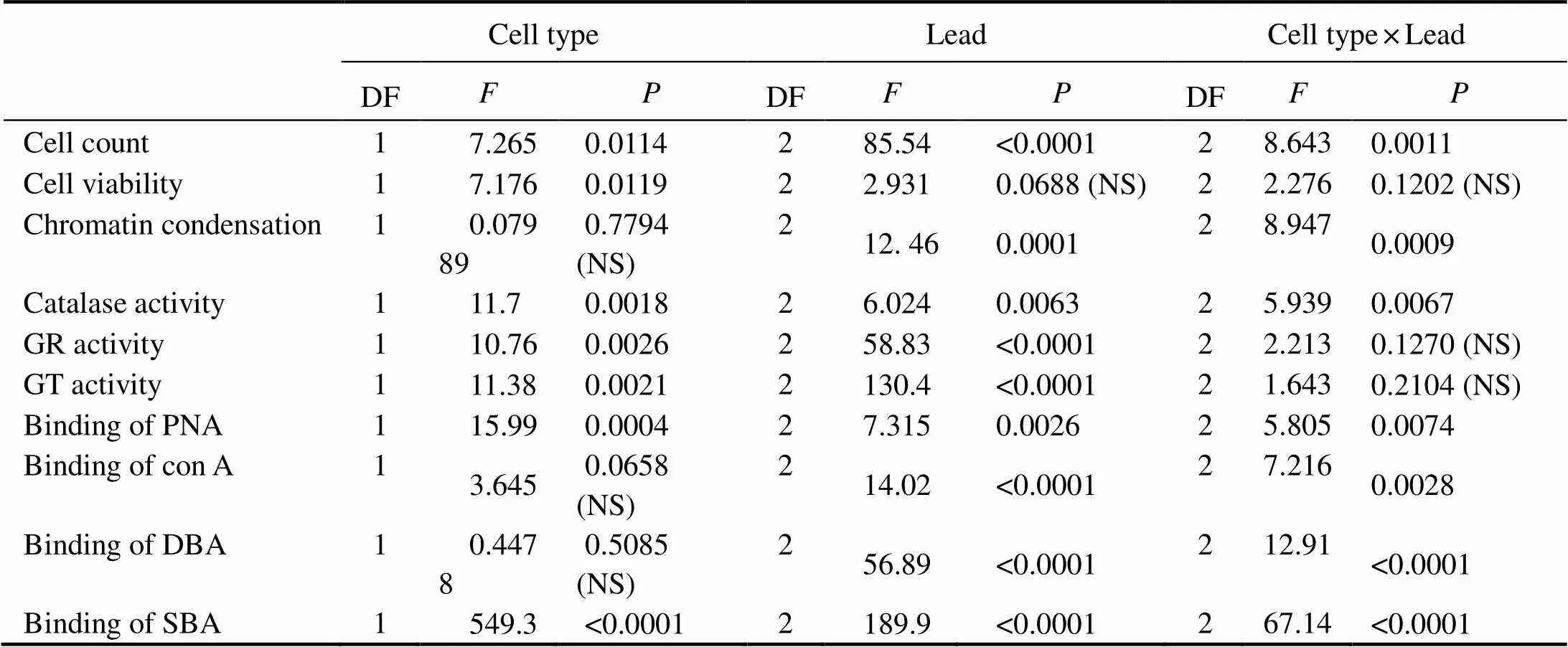
Table 1 Summary of the results of the two-way ANOVA performed to analyze the effects of the lead concentration, the type of phagocytes, and the interaction between these two factors on the parameters studied
Notes: DF, degrees of freedom;, Fisher’s-ratio; NS, not significant at<0.05.
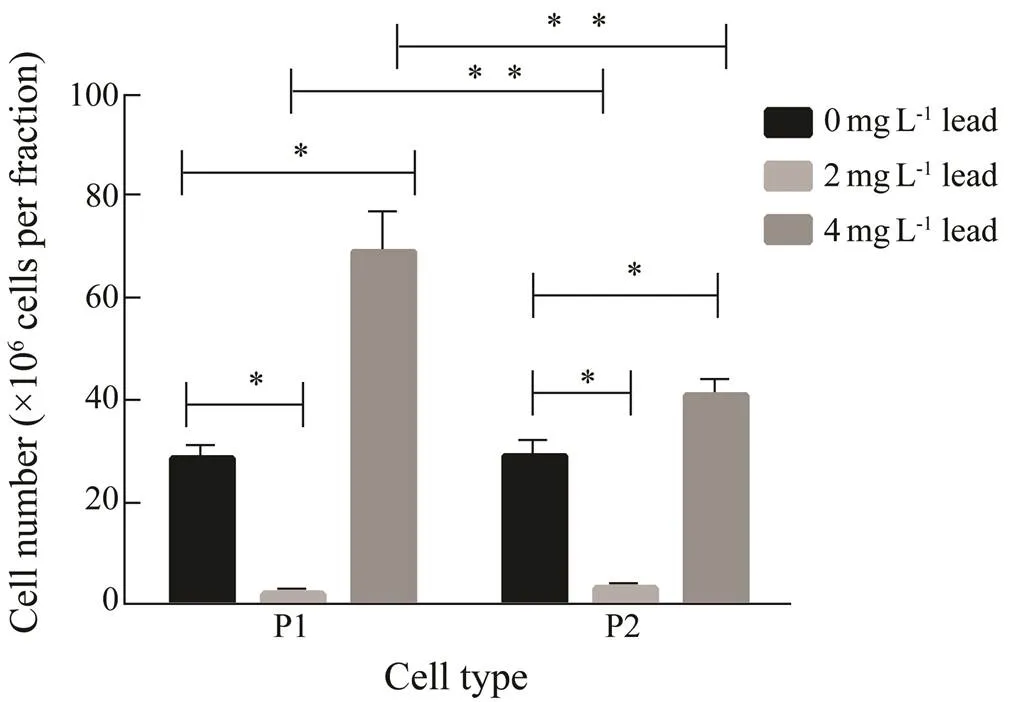
Fig.1 The influence of lead on cell counts in the P1 and P2 phagocyte fractions (isolated from the coelomic fluid combined from three animals) from E. fraudatrix after a 48-h exposure to lead. Each value represents the mean±s.e.m. (n=6); * P<0.05 vs. control; ** P<0.05 vs. the corresponding value.
3.2 Viability of Phagocytes
The two-way ANOVA (Table 1) did not show any sig- nificant effect of lead concentration on cell viability. The analysis of the lead effect on each of the two types of phagocytes revealed that after a 48-h exposure to both concentrations of lead, the viability of P1 phagocytes did not change significantly (Fig.2). For P2 phagocytes, 2mgL?1lead also insignificantly reduced viability (by 14%), but the effect became significant at 4mgL?1lead (21%,<0.05).
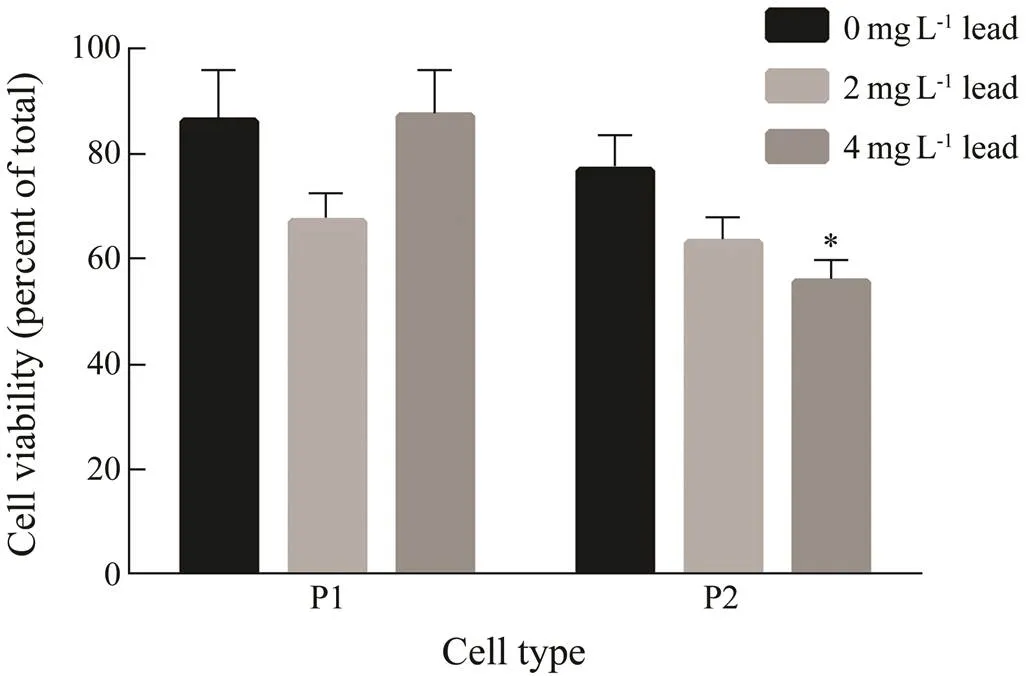
Fig.2 The viability of P1 and P2 phagocytes of E. frau- datrix after a 48-h exposure to lead. Each value represents the mean±s.e.m. (n=6); * P<0.05 vs. control.
3.3 Chromatin Condensation
The fluorescence microscopy analysis revealed that chromatin condensation had similar values in both control P1 and P2 phagocytes (22%±1.8% and 23.4%±1.1%, respectively) (Fig.3). The chromatin condensation level in P1 cells was not affected by both concentrations of lead significantly, although there was a 14% increase at a concentration of 2mgL?1. In P2 phagocytes, 2mgL?1lead significantly elevated the chromatin condensation (by 30%), but 4mgL?1lead, on the contrary, significantly reduced its level (by 20%).
3.4 Activity of Antioxidant Enzymes
In P1 phagocytes, leadinduced a significant down- regulation of the catalase activity (by 45% compared to the control) only at a 4mgL?1concentration.In contrast, 2mgL?1lead, but not 4mgL?1, caused an increase in catalase activity by 50% in P2 phagocytes (Fig.4A).
The GR activity was downregulated by 2mgL?1lead in P1 phagocytes by 34% and increased by 84% compared to the control at a 4mgL?1lead concentration. In the P2 fraction, 2mgL?1lead also significantly downregulated the activity of enzyme (by 20%) and the concentration of 4mgL?1increased the activity by 74% (Fig.4B).
Lead also induced a concentration-dependent increase of the GST activity in P1 phagocytes, insignificant at 2mgL?1, but 4.3 fold of the control at 4mgL?1. Similarly, the GST activity in P2 was not significantly affected by 2mgL?1lead, but was 8-fold of the control at 4mgL?1(Fig.4C).
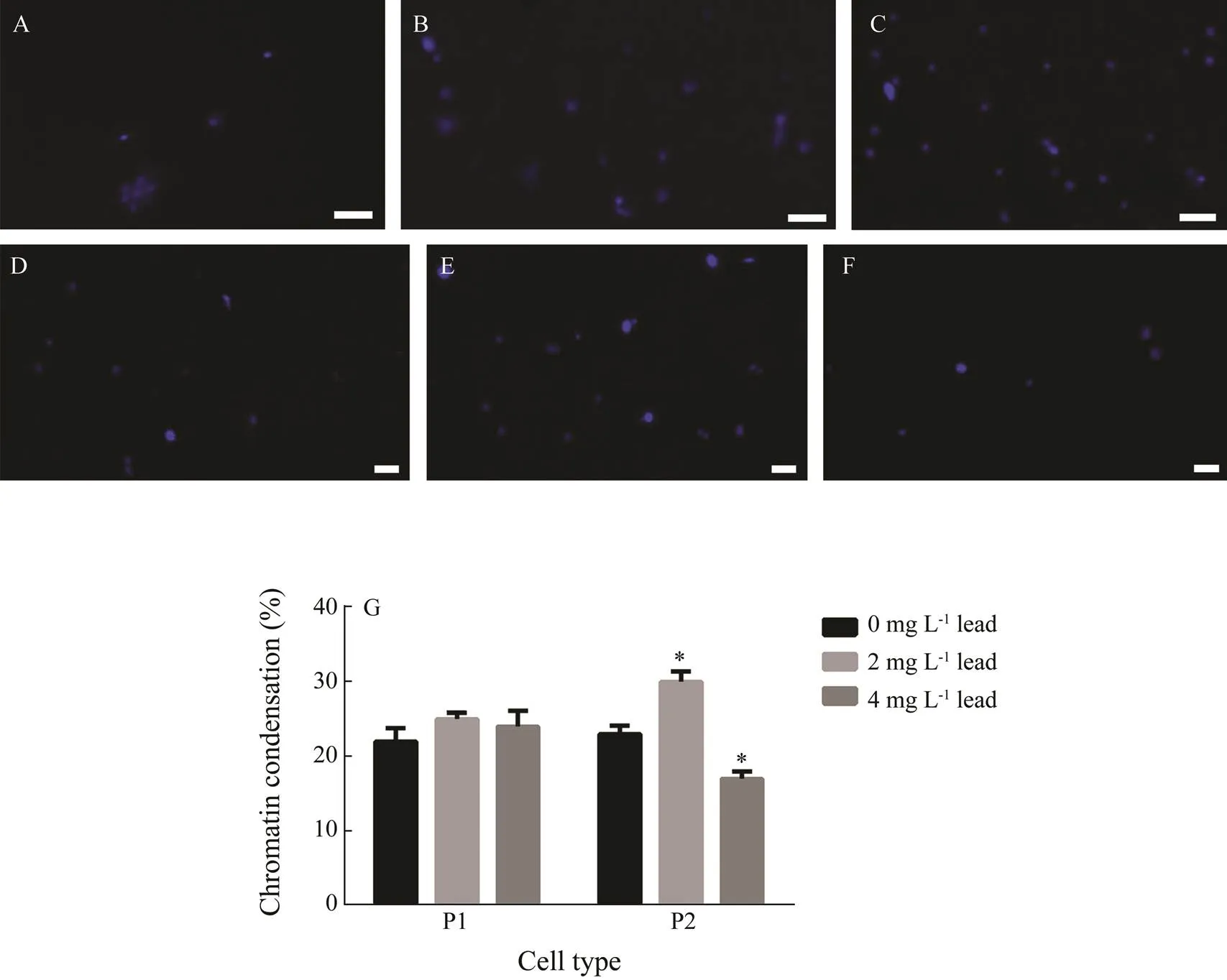
Fig.3 Chromatin condensation in P1 and P2 phagocytes of E. fraudatrix in the control and after a 48-h lead exposure. (A–C) Micrographs of P1 phagocyte nuclei stained withHoechst 33342; magnification 200×. (D–F) Micrographs of P2 phagocyte nuclei; magnification 100×. Scale bar is 10μm. A and D represent the control; B and E, lead exposure, 2mgmL?1; C and F, lead exposure, 4mgmL?1. (G) Changes in percentage of cells with chromatin condensation. Each value represents the mean±s.e.m. (n=6); * P<0.05 vs. control.
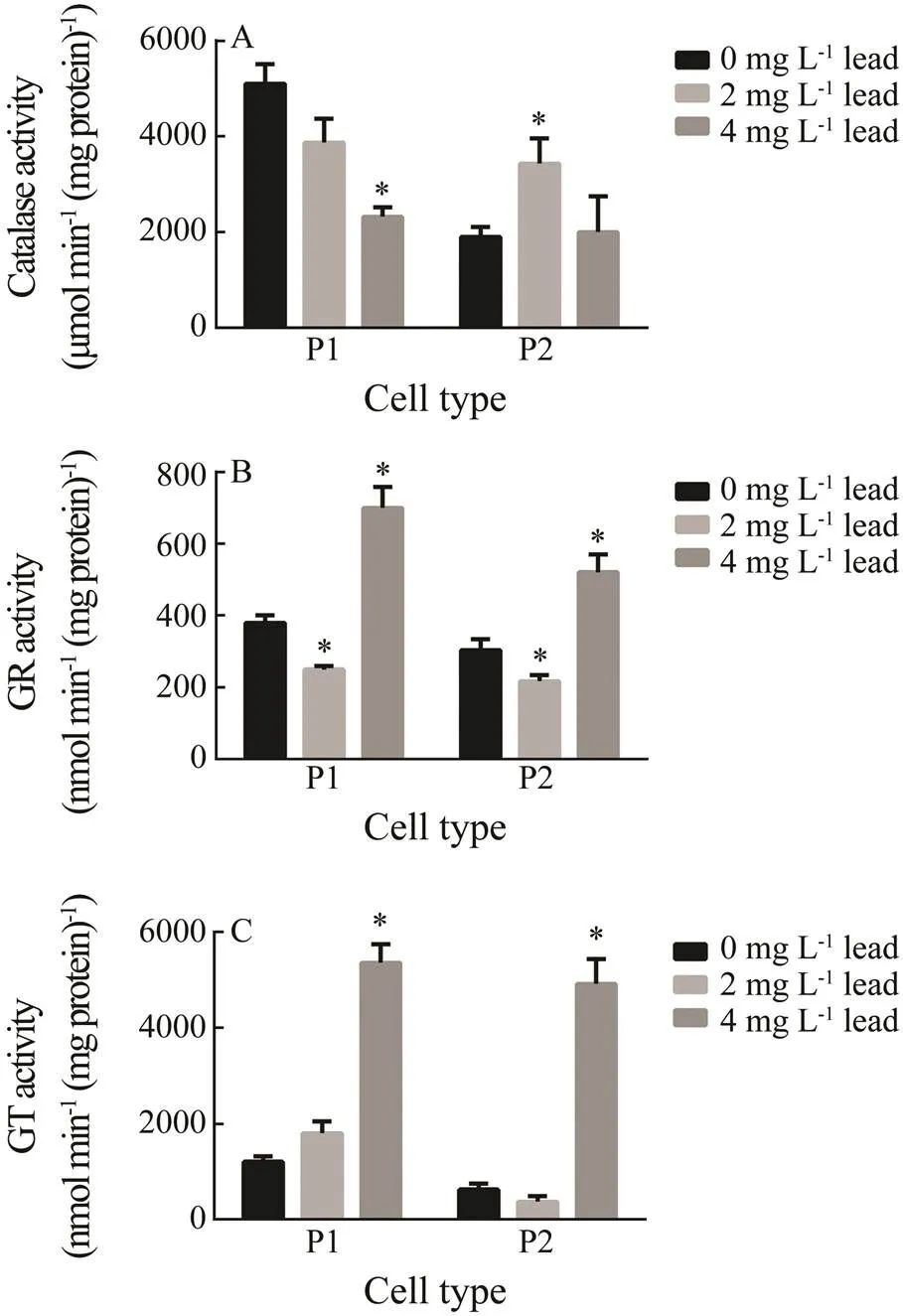
Fig.4 Activities of antioxidant enzymes in P1 and P2 phagocytes of E. fraudatrix after a 48-h lead exposure: (A) catalase; (B) glutathione reductase; (C) glutathione S- transferase. Each value represents the mean±s.e.m. (n=6); * P<0.05 vs. control.
3.5 Lectin Binding to the Surface Receptorsof Phagocytes
In the control, different plant lectins bound to P1 and P2 phagocytes at different rates (Figs.5A–D). Thus, con A and PNA labelled the largest and the least numbers of phagocytes, respectively. These two lectins bound to P1 and P2 phagocytes with similar intensities (Fig.5E). However, DBA and SBA labeled a significantly higher proportion of P1 cells as compared to P2 (1.9-fold and 4.8-fold, respectively).
Lead concentration-dependently decreased binding of PNA to surface receptors of P1 phagocytes by 18% and 68% at concentrations of 2 and 4mgL?1, respectively. Nevertheless, lead did not influence PNA binding to P2 phagocytes.
Also, lead concentration-dependently decreased con A binding to P1 cells, by a maximum of 37% at a concen- tration of 4mgL?1. In P2 type, 2mgL?1lead increased con A binding by 70%; however, 4mgL?1lead did not influence it.
Lead at 2mgL?1also affected DBA labelling of both types of phagocytes in a similar manner: it increased by 30% (insignificant) and 70% (<0.05) in P1 and P2 phagocytes, respectively. The concentration of 4mgL?1lead decreased binding of lectin to P1 phagocytes by 50% compared to the control, and, in contrast, its binding to P2 remained by 25% higher than in the control (<0.05).
Variations in SBA binding to the cellsurface receptors were opposite, depending on lead concentration in both types of phagocytes. Lead at 2mgL?1decreased this bind- ing to P1 and P2 phagocytes by 50% and 80%, res- pectively;however lead at 4mgL?1elevated labeling P1 and P2 phagocytes by 90% and 100%, respectively.
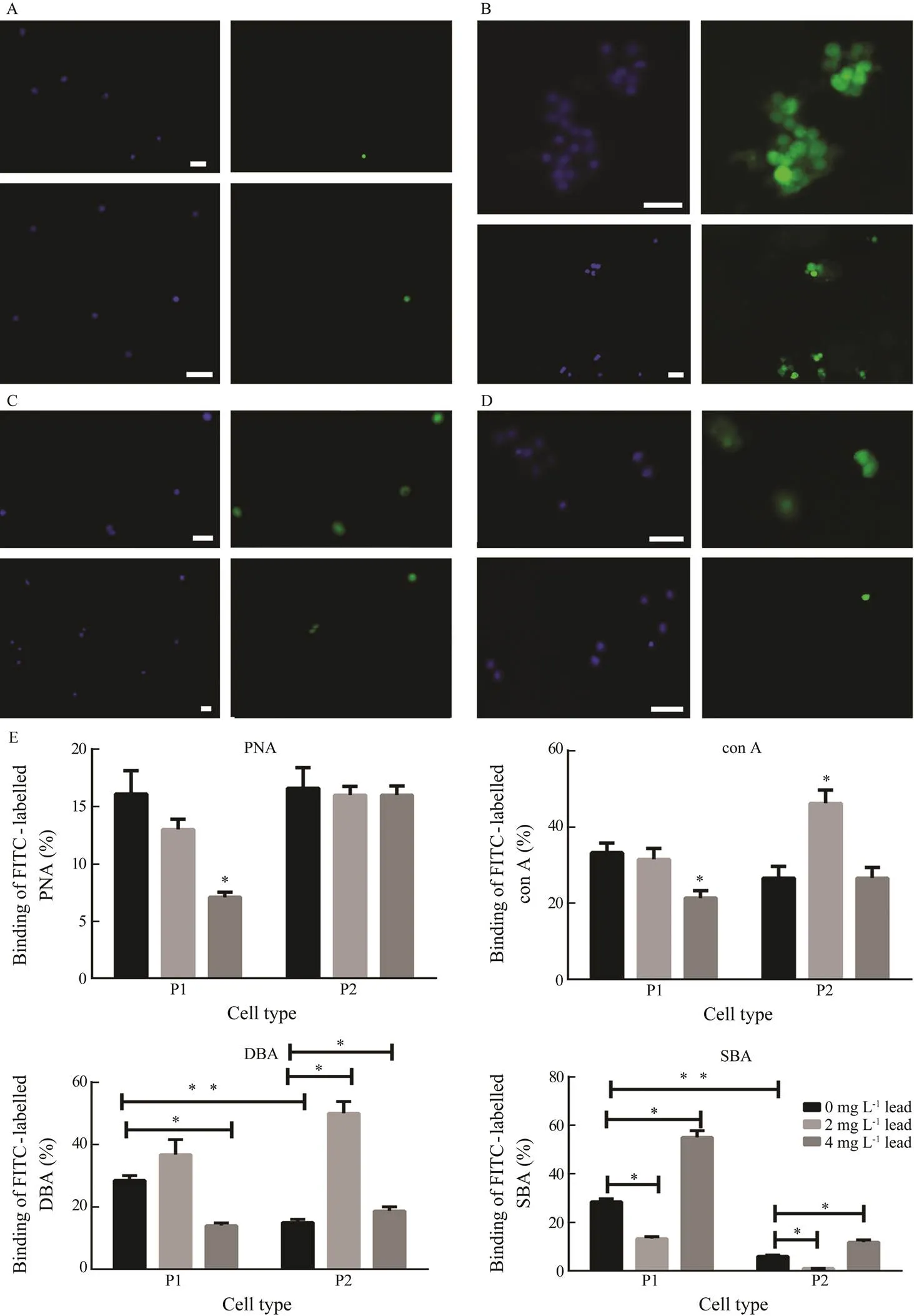
Fig.5 Binding of FITC-conjugated plant lectins to P1 and P2 phagocytes in the control and after a 48-h lead exposure. (A–D) Micrographs of the control P1 (top panel) and P2 (bottom panel) phagocytes with bound (A) PNA, (B) con A, (C) DBA, and (D) SBA (FITC, green) and their nuclei stained with DAPI (blue). Magnification is as follows: (A) 100× in top panel and 200× in bottom panel; (B–C) 200×in top panel and 100× in bottom panel; (D) top and bottom panel, 200×. Scale bar is 10μm. (E) The changes in percentage of phagocytes binding lectins after exposure to lead. Each value represents the mean±s.e.m. (n=6); * P<0.05 vs. control; ** P<0.05 vs. the corresponding value.
4 Discussion
4.1 Lead Affected the Ratio of P1 and P2Phagocyte Numbers
Several researchers have previously reported that lead can induce different effects on the number of immune cells in vertebrates. Thus, according to Rocke and Samuel (1991), a chronic exposure to leaddecreases the total count of leukocytes. Other authors claimed that lead does not significantly increase the total macrophage number (Aldahmash and El-Nagar, 2016), or exclusively increases the number of some leukocyte types (Rosenberg., 2003), or even strongly stimulates leukocytosis(Hogan and Adams, 2004). These discrepancies in- dicate that lead can apparently modulate the cell number in vertebrates depending on dose and time of exposure. In invertebrates, a decrease in the number of coelomocytes in earthworm was shown after a 3-day dermal exposure to lead(Homa., 2005). However, no experi- mental data on the effects of lead on the phagocyte num- ber in holothurians are available.
In the present work, the numbers of P1 and P2 pha- gocytes in coelomic fluid were similar in the control. At a lead concentration of 2mgL?1, the numbers of phagocytes in both fractions significantly reduced, apparently as a consequence of their recruiting to tissues. This indicates that an increased immune protection is required in the case of a lead exposure. The number of P1 phagocytes especially decreased as compared to the P2 type,which demonstrates the importance of this type of cells in pro- tection against the toxic effects of lead. In the individuals exposed to a higher concentration of lead, there was an increase in the phagocyte numbers in both fractions ap- parently due to a compensation of recruited cells by in- crease in total phagocyte number. Moreover, there was even a shift in the ratio of the numbers of the two phago- cyte types, with enhanced cell number in P1 fraction compared to that in P2, indicating the increasing impor- tance of recruitment of P2 phagocytes. Apparently, P1 and P2 phagocytes played different roles in the protection of holothurian exposed to different concentrations of lead.
4.2 Toxic Effects of Lead Were Lower in the P1 Phagocyte Than Those in the P2 Phagocyte
The viability of P1 phagocytes was not significantly affected by lead at both concentrations. In contrast, the viability of P2 cells was depressed in a concentration- dependent manner. Thus, P1 phagocytes seem to be more resistant to the damaging effect of lead. Apparently, an increase in lead concentration to a certain threshold can cause a reduction in the toxic effect of lead (adaptation) in this type of cells. These findings are similar to those de- scribed for the liver cell lines of vertebrates, where lead, depending on the type of cell lines, could decrease its toxic effect when concentration increased (Sa?di., 2013). As two-way ANOVA indicated that neither lead concentration nor interaction between lead and cell type significantly influenced cell viability (Table 1), the pha- gocyte type was apparently a key factor inducing differences in variations of this parameter.
Two-way ANOVA also showed that lead effects on chromatin condensation depended on the cell type. Thus, studies on the mechanisms of cell death revealed an in- significantly (P1) or significantly (P2) higher level of such apoptotic hallmark as chromatin condensation (Kroe- mer, 2009) at a 2mgL?1concentration.At a 4mgL?1con- centration, its level in the P1 type was unchanged com- pared to the control, and significantly decreased in P2.
A comparison of the results of lead effects on the cell viability and chromatin condensation in P1 phagocytes indicates that the cell viability is apoptosis-dependent. In P2 phagocytes, 2mgL?1lead induced a decrease (insig- nificant) in cell viability, apparently, due to the increased apoptosis. Nevertheless, a high level of cell mortality remained in P2 cells after exposure to 4mgL?1lead which induced even significant reduction in apoptosis compared to the control. The leading role of necrosis in lead-in- duced death was found earlier for mouse macrophages (Gargioni, 2006). Probably, the higher lead concen- tration also could stimulate necrosis in the P2 cells.
The key role of phagocyte type in cell viability, along with the different effects of lead on chromatin conden- sation depending on the cell type, indicate that the me- chanisms underlying the response of cells to lead differ from each other.
It is well known that a lower level of oxidative stress provokes apoptosis, whereas a higher level promotes ne- crosis (Colussi., 2000). Consideringthat lead can inhibit the activities of antioxidant enzymes in cells of vertebrates, which causes an increase in their sensitivity to stress (Hande and Naran, 2000), we studied the in- fluence of lead on the activity of antioxidant enzymes in the two types of phagocytes in.
4.3 Antioxidant Enzyme Defense is More Effective in the P1 Type Compared to the P2 Typeof Phagocytes
Catalase is known to be a key antioxidant enzyme in leukocytes of vertebrates (Curi, 1998).It also plays an important role in modulating apoptosis in holothurian phagocytes (Dolmatov., 2005). The lead-induced inhibition of catalase was shown for different organs and tissues of vertebrates and invertebrates, including holo- thurians (Sharma., 2010; Wang, 2015). This agrees with the results of the present work, in which lead downregulated the catalase activity in P1 phagocytes at a higher concentration. However, in P2 phagocytes, 2mgL?1lead even stimulated catalase activity, while 4mgL?1lead did not influence it.
These differences in catalase activity variation between the two types of phagocytes may be related to the differ- ent levels of the oxidant-antioxidant balance of the con- sidered cells in normal conditions. In fact the catalase activity was more than twice as high in the control P1 phagocytes as in the control P2 in the present work. As is known, lead can inhibit catalase activity indirectlyits oxidative effects (Sharma., 2010). Thus, the higher level of basal catalase activity in P1 is apparently a con- sequence of increased level of reactive oxygen species (ROS) in these cells, and may be a cause of its faster de- cline when exposed to lead. The report that catalase activ- ity in lead-exposed juvenile fish increased only on the 10th day, along with an increase in ROS level, is of inter- est to this study (Cherkesova., 2012). Similarly, an increase in antioxidant activity in vertebrates was re- ported at lower levels of lead, while a decrease was shown at higher concentrations over long period of expo- sure (Patrick, 2006).This indicates that different effects of lead on the catalase activity depend on the level of oxidative stress. It also confirms there is higher tension of catalase activity in the P1 phagocytes compared to that in the P2 type.
In spite of the decrease in catalase activity in P1 phagocytes of lead-exposed holothurians, the level of the enzyme activity remained higher compared to that in the P2 type. However, taking into consideration the fact that apoptosis in phagocytes of both vertebrates (Bryan and Langston, 1991; Colussi., 2000) and invertebrates (Homa., 2016) often develops in conditions of re- duced catalase activity, these data indicate that other mechanisms could play a role in anti-apoptotic protection in P1 cells.
Another group of detoxifying enzymes belongs to the glutathione system. Reduced glutathione (GSH) is one of the strongest antioxidants inactivating free radicals, and plays a major role in detoxification of heavy metals (Sharma, 2011). The GSH system alsoincludes such enzymes as GR responsible for reducing the oxidized form of glutathione (Yusupova, 1989) and GST that cata- lyzes the reduction of organic hydroper-oxides and lipo- proteins in the presence of GSH (Habig., 2004).
Previously, it was shown that lead decreases the ratio of reduced and oxidized glutathione, as well as GR ac- tivity, in concentration-dependent manner both in terrestrial and marine vertebrates (Flora., 2008; Taheri., 2015). Additionally, lead was found to bind to sulfhydryl groups present in GSH-dependent enzymes and to sup- press the GSH level (Ahamed and Siddiqui, 2007). How- ever, the data on the impact of lead on the GR activity are often controversial (Tátrai.,2001; Cherkesova., 2012) and apparentlydepend on type of cells and tissues studied, time of treatment, and concentration (Cherkesova, 2012).
In the present work, the finding of the inhibiting effect of lead on the GR activity in phagocytes at a lower con- centration (2mgL?1) and the increasing effects at a higher concentration (4mgL?1) are consistent with data de- scribed for some cells of vertebrates (Sandhir,1994). The inhibiting effect of lead canpossibly result from its direct effect on the enzyme and its substrate GSH. In the latter case, lead binds to –SH residues (Wu and Wei, 2012), resulting in a decrease in GSH level. At the same time, the increase in the GR activity, induced by the higher concentration of lead, is related apparently to switching for the compensation mechanisms. A stimula- tion of enzyme activity or its synthesis in response to the decrease in GSH was described earlier by some authors, and the concentration of the reduced form of glutathione may be even increased by chronic lead nitrate administra- tion (Roomi, 1986). A decreased catalase activity is often followed by a higher demand for GSH (Sharma, 2010; Spolarics and Wu, 1997), and, therefore, the de- crease in the GSH level in the P1 type apparently induced an increase in the GR activity in the present work. GR could compensate the reduction in the GSH level and protect P1 cells at a lead concentration of 4mgL?1. How- ever, the fact that the catalase activity increased and the GR activity had a tendency to decrease in P2 phagocytes at a 2mgL?1lead may indicate that the increase in the ROS level was followed by a GSH depletion; however, the maintenance of catalase activity at the control level in P2 cells after exposure to 4mgL?1lead could be related to preventing the increase in generation of ROS which re- sulted from the increased activity of GR (possibly,the mechanisms similar to those in P1 phagocytes).
The changes in the GST activities in both phagocyte types were opposite to those described above for catalase. Lead elevated the GST activity in a direct concentration- dependent manner in P1 phagocytes. These changes in the GST activity confirm that the catalase inhibition in P1 phagocytes was not followed by a significant decrease in GSH, the concentration of which remained sufficient for maintaining the GST activity. The enzyme activity was not significantly influenced by a 2mgL?1lead in P2 cells, although a tendency to inhibit its activity was detected. Probably the activity of GST was not increased in re- sponse to 2mgL?1lead exposure due to a lower ability of these cells to compensate the low level of GSH. However, the GSH level was apparently elevated enough to support the increase in the GST activity at a 4mgL?1lead due to the increased GR activity.
It was shown previously that the compensation of the GSH decrease may be possible in some types of cells even under conditions of inhibiting superoxide dismu- tase, which is responsible for dismutation of superoxide radical into Н2O2(Pershyn, 2009), and catalase ac- tivities (Spolarics and Wu, 1997). In lymphocytes, it re- sults from a fixed level of glutathione-defense enzymes, and is considered a cause of the lower sensitivity of lym- phocytes to lead-induced oxidative stress, compared to other blood cells (Pershyn, 2009). The lack of in- teraction between the effects of lead and cell type on both GR and GT activities, shown by two-way ANOVA, also indicates the presence of certain difference between glu- tathione-dependent antioxidant systems of the two types of phagocytes. Hence, the higher activity of GSH-de- pendent enzymes in P1 cells apparently results in a higher resistance against oxidative stress of the P1 phagocytes as compared to the P2 type. This conclusion is supported by the report about the dependence of tolerance to lead ex- hibited by macrophages on their high antioxidative ca- pacity (Shabani and Rabbani, 2000).
4.4 Phagocytes Can Acquire Different Phenotypes During the Response to Lead
The first evidence of a receptor modulation during lead-induced apoptosis was shown by Dini. (1993) in liver cells. Further studies confirmed this finding and revealed that the number and distribution of binding sites were receptor- and cell-type dependent (Dini.,1993; Ruzittu, 1999).
In the present work, cell type did not influence significantly the con A level and lectin identically labeled P1 and P2 cells in the control, which indicates that the expression of surface receptors containing mannose residue is similar for both phagocyte types. However, the effect of lead on con A binding depended on the type of phago- cytes. Thus, lead concentration-dependently decreased the con A binding to P1, in contrast to P2 phagocytes, where lead increased the con A binding to cell surface receptors at a concentration of 2mgL?1, but did not influence it at a higher concentration.
The analysis of data demonstrates that the different effects of lead on the cell viability and apoptosis in P1 and P2 phagocytes were found against the background of the opposite variations in con A binding to these cells. In vertebrates, con A binds mainly to apoptotic cells (Seco- Rovira., 2013). In holothurians, the distinct propor- tions of con A binding to mannose residues of cell surface receptors reflect different lead-induced changes in the phenotypes of P1 and P2 phagocytes and, as in vertebrates, seem to be markers of the apoptotic status of the cells.
According to the two-way ANOVA, the effect of cell type on the DBA binding to the surface of phagocytes also was insignificant. It is apparently resulted from the most similar tendencies of variation of this parameter in P1 and P2 cells, except for the 4mgL?1lead exposure, when the variations were opposite. These variations were indirectly associated with the opposite changes in via- bility of the cells of both types. However, lead effects on the proportion of cells labeled by DBA significantly de- pended on the cell type: it differed between cell types at a concentration of 2mgL?1and became even opposite at 4mgL?1. Apparently, this effect is related to the differences in cell viability between the phagocyte fractions after exposure to lead. This agrees with data of Dini. (1993) on the expression of a great amount of N-acetyl- galactosamine residues on the surface of dead cells (he- patocytes).
At the same time, the intensity of SBA binding to re- ceptors of P1 phagocytes was higher compared to that in P2 in the control, and lead effect also depended on the cell type. However, similar tendencies were observed in the variations of SBA affinity sites in P1 and P2 cells when the cells were exposed to lead, and there was a cer- tain reverse relationship between the proportion of SBA binding and the chromatin condensation variation, which suggests a positive role of galactose-containing receptors in the cell defense against lead. These data are in accor- dance with the observations of Kim. (2014) who reported that the cell survival increased after stimulation of cell surface receptors containing galactoside residues. Nevertheless, the variations in the binding of DBA and SBA to phagocyte surface were opposite, which indicates that stimulation of different galactoside residues (Gal- NAcα1-3GalR and GalNAc) may have the opposite ef- fects.
In addition, changes in expression of galactoside re- sidues may also play a role in migration of phagocytes from the coelomic cavity, as there were similar tendencies of variations in the SBA affinity and the cell number in both cell types. This agrees with the results described by Delbos. (1983) on inhibition of migration of primor- dial germ cells with SBA in.
The proportions of the phagocytes labeled by PNA were identical in the controls for both phagocyte fractions, but also depended on the cell type at lead exposure. This lectin is mainly known to modulate cell adhesiveness (Gnedkova., 2015), closely related to anti-apoptotic protection. Therefore, decrease in its binding to P1, but not to P2, phagocytes, associated with reduced catalase activity, may indicate the participation of receptors with Galβ1-3GalNAc specificity in the protection against lead- induced apoptosis.
These data indicate that different phenotypes of the two types of phagocytes during lead exposure associates with different adaptive abilities of these cells. This is in accor- dance with the results of Martins-Souza. (2006) who showed in freshwater mollusks that species-resistance to infection correlated with the differences in the binding plant lectins to their hemocytes.
As is known, vertebrate macrophages can undergo ac- tivation to pro-inflammatory (M1, classically activated) or anti-inflammatory (M2, alternatively activated) pheno- types in response to different pathophysiologic conditions. Moreover, macrophages were reported to acquire distinct functional phenotypesundergoing different pheno- typic polarization (Italiani andBoraschi, 2014). Classi- cally activated M1 macrophages are the key players of the first line of defense against bacterial infections (Mills and O’Neill, 2016), and alternatively activated M2 macro- phages are involved in tissue repair and use the oxidative metabolism for their long-term functions (Orihuela., 2016). Nevertheless, for echinoderms, the presence of ‘a(chǎn)lternative’ subpopulations of phagocytes has not yet been described. The data of the present study on the dif- ferent phenotypical and functional variations of P1 and P2 phagocyte responses to lead highlight the possible analogy between them and macrophages of the M1 and M2 types.
The similarity between the two types of holothurian phagocytes and the two types of macrophages can be un- derstood in more details when comparing their functional properties. As has been mentioned above, the different effects of lead on the two types of phagocytes depended on the level of their antioxidant activity, mainly on the level of glutathione-dependent enzyme activity. The spe- cial role of GSH in regulation of macrophage polarization was described earlier. M1 type has a higher ratio of reduced-to-oxidized glutathione (GSH/GSSG), and a GSH depletion can switch macrophages from the M1 pheno- type towards M2 (Peterson., 1998). An analysis of the dynamics of glutathione-dependent enzymes in holo- thurian phagocytes indicates that the GSH maintaining ability was higher in P1 cells compared to that in the P2 cells.
In addition, a tight relationship exists between the me- tabolic state and the phenotype of macrophages, as GSH level in cells is closely coupled to the NADPH/NADP balance depending on the pentose phosphate pathway, in turn, related to the glycolytic pathway (Wu and Wei, 2012). Thus, M1 macrophages are known to obtain en- ergy through glycolysis (Mills and O’Neill, 2016), while M2 macrophages use oxidative metabolism for their long-term functions (Orihuela, 2016). In the present work, the concentration-dependent decrease in the cata- lase activity in P1 phagocytes under lead exposure is ap- parently followed by an increase in H2O2generation, as the function of catalase is to destroy this molecule. In some cases, it was shown that treatment with H2O2results in the blockage of glycolytic flux and protection against apoptosis (Colussi., 2000). Therefore, it is possible that adaptation to apoptosis in lead-exposed P1 phago- cytes may occurthis glycolytic-dependent way. How- ever, the data on the dynamics of catalase activity in P2 phagocytes indicate a lower level of ROS in these cells and suggest rather the aerobic way of metabolism in those cells. Therefore, it is suggested that the differences in functional activities between P1 and P2 phagocytes may be related to the different metabolic pathways in these cells similar to those in M1 and M2 macrophages of ver- tebrates. As is known, the balance between the mitochon- drial respiration and glycolysis level is a major determi- nant of cell survival (Michaeloudes., 2015). It may be a cause of different levels of lead cytotoxicity in the P1 and P2 phagocytes.
Based on the data of distinct variations in the response of holothurian P1 and P2 phagocytes to lead, as well as on the similarity between antioxidant capacities of P1 phagocytes and M1 macrophages on the one hand, and P2 phagocytes and M2 macrophages on the other hand, we suggest that the P1 and P2 phagocytes may be functional analogs of M1 and M2 macrophages, respectively.
Little is known on the exclusive effect of lead on macrophage polarization in vertebrates. Thus, lead can induce a preferential generation of the Th1 type of cyto- kines in macrophages (Krocova., 2000), which means their polarization towards the M1 type. However, another study has shown that lead stimulates polarization of macrophages towards the M2 type (Muraille, 2000). At the same time, vertebrate macrophages are suggested to present in tissues as a mixture of M1 and M2 phenotypes, and a shift in the balance of these phenotypes may result in a pathological state (Tan., 2016). As for holothurians, the decrease in the number of P1 phagocytes as compared to the P2 cells in coelomic fluid at a lower concentration of lead may be considered that mostly P1 phagocytes are present in tissues. It is likely that P1 phagocytes, similar to M1 macrophages, may promote the inflammatory process. In contrast, apparently mostly P2 phagocytes are in tissues at a higher concentra- tion of lead.Their higher sensitivity to the toxic effect of lead, compared to that of the P1 type, might result in an increased level of their mortality. Further studies are required to verify these suggestions.
5 Conclusions
Until now, the roles of different phagocyte subpopu- lations of holothurians in response to lead exposure have not been studied. This paper describes the variations in some markers of functional activities of P1 and P2 pha- gocytes inexposed to high concentrations of lead for a short time (48h). To summarize, the number of cells in the coelomic fluid of lead-exposed individuals has been shown to vary between phagocyte types depending on the lead concentration. This indicates that P1 and P2 phagocytes may play different roles in the protection against lead toxicity in holothurians. Additionally, the evaluation of lead toxicity in the two phagocyte types has revealed that P1 phagocytes are apparently more resistant to the damaging effect of lead compared to P2 cells. The antioxidant enzyme activities in the two types of phago- cytes inwere also shown to vary after a short-term lead-induced stress.The higher activities of catalase and GST in the P1 type of phagocytes, compared to the P2 type, may indicate a higher level of GSH/GSSG ratio in the P1 phagocytes than in the P2 type. This ap- parently results in different sensitivity of P1 and P2 phagocytes to the toxic effect of lead, and an increase in lead concentration to a certain threshold can cause a re- duction in the toxic effect of lead (adaptation) in P1 phagocytes rather than in P2.
The different expressions of cell surface carbohydrate residues alsoindicate a phenotypic difference between these cells. The increase in the expression of mannose- and GalNAc-containing receptors may indicate a rise in apoptosis, while the increasing expression of GalNAcα1- 3GalR-cell surface receptor residues associates with anti- apoptotic protection.
Due to these functional and phenotypic differences between the P1 and P2 types of phagocytes, P1 and P2phagocytes might play different roles in immune response, which is similar to those of M1 and M2 macrophages.
The data also indicate the capability of immune cells of holothurianto adapt to high lead concentra- tions, even close to the maximum environmentally rela- tive level,during short-term exposure, and the different roles of each of the phagocyte types in adaptation to lead are supposed. These results are helpful both for under- standing the immunity evolution and for risk assessment studies concerning the holothurian mariculture in areas under the increasing pressure of lead pollution.Addition- ally, these results can be a baseline information for de- velopment new technique targeting a certain type of phagocytes to increase the resistance of holothurians to toxic effects of lead.
Acknowledgements
The authors thank the Far Eastern Center of Electron Microscopy, National Scientific Center of Marine Bio- logy,for facilities. They also thank anonymous reviewers for their constructive comments.
Aldahmash, B. A., and El-Nagar, D. M.,2016. Antioxidant ef- fects of captopril against lead acetate-induced hepatic and splenic tissue toxicity in Swiss albino mice.,23 (6):667-673, DOI: 10.1016/j.sjbs. 2016.05.005.
Ahamed, M., and Siddiqui, M. K., 2007. Low level lead expo- sure and oxidative stress: Current opinions.383 (1-2): 57-64, DOI: 10.1016/j.cca.2007.04.024.
Arbneshi, T., Rugova, M., and Berisha, L., 2008. The level concentration of lead, cadmium, copper, zinc and phenols in the water river of Sitnica.3 (2): 66-73.
Bryan, G. W., and Langston, W. J., 1991. Bioavailability, accu- mulation and effects of heavy metal in sediments with special reference to United Kingdom estuaries: A review.76 (2): 89-131.
Catarino, A., Cabral, H. N., Peeters, K., Pernet, P., Punjabi, U., and Dubois, P., 2008. Metal concentrations, sperm motility, and RNA/DNA ratio in two echinoderm species from a highly contaminated fjord (the S?rfjord, Norway).27 (7): 1553-1560, DOI: 10.1897/ 07-402.
Cherkesova, D. U., Rabadanova, A. I., Muradova, G. R., and Gabibov, M. M., 2012. Dose and time-dependent lead in- fluence on survival rate and condition of oxidizing and anti- oxidatic system of russian sturgeon (Brant) young fishes., 14:1937-1940 (in Russian with English abstract).
Chia, F-S., and Xing, J., 1996. Echinoderm coelomocytes., 35 (4): 231-254.
Chiarelli, R., and Roccheri, M. C., 2014. Marine invertebrates as bioindicators of heavy metal pollution., 4: 93-106, DOI: 10.4236/ojmetal.2014.44011.
Colussi, C.,Albertini, M. C., Coppola, S., Rovidati, S., Galli, F., and Ghibelli, L., 2000.H2O2-induced block of glycolysis as an active ADP ribosylation reaction protecting cells from apoptosis.,14 (4):2266-2276, DOI: 10.1096/ fj.00-0074com.
Curi, T., Demelo, M., Palanca, A., Miyasaka, C., and Curi, R., 1998. Percentage of phagocytosis, production of O2?, H2O2and NO, and antioxidant enzyme activities of rat neutrophils in culture., 16 (1): 43-48, DOI: 10.1002/(SICI)1099-0844(199803)16:1<43::AID-CBF761 >3.0.CO;2-5.
Delbos, M., Sa?di, N., and Gipouloux, J. D., 1983. Inhibitory effect of lectins extracted fromand soy- beans on germ cell migration in the embryo of(Amphibia, Anura)., 296(14):645-650 (in French).
Dini, L., Falasca, L., Lentini, A., Mattioli, P., Piacentini, M., Piredda, L., and Autuori, F., 1993. Galactose-specific receptor modulation related to the onset of apoptosis in rat liver.,61 (2): 329-337.
I.and2005. Dexamethasone-induced apoptosis of holothurianphagocytes. In:. Taylor & Francis, London, 105-111.
Dolmatova, L. S., Eliseykina, M. G., Timchenko, N. F., Ko- valeva, A. L., and Shitkova, O. A., 2003. Generation of reac- tive oxygen species in the different fractions of the coelomo- cytes of holothurianin response to the thermostable toxin of., 21 (4): 293- 304, DOI: 10.1007/BF02860423.
Dolmatova, L. S., Romashina, V. V., and Eliseikina, M. G., 2004. Antioxidant enzymatic activity of coelomocytes of the Far East sea cucumber, 40:126-135, DOI: 10. 1023/B:JOEY.0000033803.35634.46.
Dolmatova, L. S., Slinko, E. N., and Kolosova, L. F., 2010a. The contents of heavy metals in tissues of holothuriansin Peter the Great Gulf.,12: 1287-1291 (in Russian with English abstract).
Dolmatova, L. S., Timchenko, N. F., and Stasenko, N. J., 2007. Characteristics of content and medico-biological studies of the complex of biologically active substances from the Far Eastern species of sea cucumbers. In:.Nauka, Moscow, 684-694 (in Russian).
Dolmatova, L., Zaika, O., Slinko, E., and Kolosova, L., 2010b. Antioxidant enzyme defense and heavy metal accumulation in tissues of holothuriansand: Characteristics of body-length depend- ences during spring-summer period., 5 (1): 96-105.
Flora, S. J. S., Mittal, M., and Mehta, A., 2008. Heavy metal induced oxidative stress and its possible reversal by chelation therapy., 128 (4): 501- 523.
Gargioni, R., Filipak, N. F., Buchi, D. F., Randi, M. A., Franco, C. R., Paludo, K. S., Pelletier, E., Ferraro, M. V.,Cestari, M. M., Bussolaro,D.,and Oliveira, R.C. A., 2006. Cell death and DNA damage in peritoneal macrophages of mice () exposed to inorganic lead.,30 (7):615-623, DOI: 10.1016/j.cellbi.2006.03.010.
Gnedkova, I. A., Lisyany, N. I., Stanetskaya, D. N., Rozumenko, V. D., Glavatskiy, A. Y., Shmeleva, A. A., Malysheva, T. A., Chernenko, O. G., and Gnedkova, M. A., 2015. Properties of glioma C6 cells., 17 (1): 4-11 (in Russian with English abstract).
Habig, W. H., Pabst, M. J., and Jackoby, W. B., 1974. Glu- tathione S-transferase. The first enzymatic step in mercapturic acid formation.,249 (22): 7130-7139.
Hande, G., and Naran, E., 2000.Can antioxidants be beneficial in the treatment of lead poisoning., 29 (10):927-945, DOI: 10.1016/S0891-5849(00) 00413-5.
Hochachka, P. W., and Somero, G. N., 1984.. Princeton University Press, Princeton, 560pp.
Hogan, G. R., and Adams, D. P., 2004. Lead-induced leukocyto- sis in female mice.,41 (4):295-300.
Homa, J., Olchawa, E., Stürzenbaum, S. R., Morgan, A. J., and Plytycz, B., 2005. Early-phase immunodetection of metal- lothionein and heat shock proteins in extruded earthworm coelomocytes after dermal exposure to metal ions.,135 (2): 275-280, DOI: 10.1016/j.envpol. 2004.10.019.
Homa, J., Stalmach, M., Wilczek, G., and Kolaczkowska, E., 2016. Effective activation of antioxidant system by immune- relevant factors reversely correlates with apoptosis ofcoelomocytes., 186:417-430, DOI:10.1007/s00360-016-0973-5.
Huang, S. L., and Onyx, W. H. W., 2004. A review of heavy metal pollution in the Pearl River Estuary., 164: 367-378.
Italiani, P., andBoraschi, D., 2014. From monocytes to M1/M2 macrophages: Phenotypical. functional differentiation., 5: 514, DOI: 10.3389/fimmu.2014.00514.
Jacob, J. M., Kumar, B. S., and Mohan,B. R., 2013. Selenium and lead tolerance in fungi isolated from sea water., 2(7): 2975-2982.
Kasten-Jolly, J., and Lawrence, D. A., 2014. Lead modulation of macrophages causes multiorgan detrimental health effects.,28 (8): 355-372,DOI:10.1002/jbt.21572.
Kim, S-M., Fujihara, M., Sahare, M., Minami, N., Yamada, M., and Imai, H., 2014. Effects of extracellular matrices and lectinagglutinin on cell adhesion and self- renewal of bovine gonocytes cultured,26 (2): 268-281,DOI: 10.1071/ RD12214.
Komatsu, N., Oda, T., and Muramatsu, T., 1998. Involvement of both caspase-like proteases and serine proteases in apoptotic cell death induced by ricin, modeccin, diphtheria toxin, and Pseudomonas toxin., 124 (5):1038- 1044.
Korshenko, A. (ed.), 2016.. Nauka, Moscow, 184pp (in Russian with English abstract).
Krocova, Z., Macela, A., Kroca, M., and Hernychova, L., 2000. The immunomodulatory effect(s) of lead and cadmium on the cells of immune system.,14 (1):33-40.
Kroemer, G., Galluzzi, L., Vandenabeele, P., Abrams, J., Alnemri, E. S., Baehrecke, E. H.,Blagosklonny, M. V., El-Deiry, W. S.,Golstein, P., Green, D. R., Hengartner, M., Knight, R. A., Kumar, S., Lipton, S. A., Malorni, W., Nu?ez, G., Peter, M. E., Tschopp, J., Yuan, J., Piacentini, M., Zhivotovsky, B., and Melino, G., 2009. Classification of cell death: Recommen- dations of the nomenclature committee on cell death.,16 (1):3-11, DOI: 10.1038/cdd. 2008.150.
Martins-Souza, R. L., Pereira, C. A. J., Martins Filho, O. A., Coelho, P. M. Z., Corrêa, A., and Negr?o-Corrêa, D., 2006. Differential lectin labelling of circulating hemocytes fromandre- sistant or susceptible toinfection.,101 (Suppl. I): 185- 192.
McAloon, K. M., and Mason, R. P., 2003. Investigations into the bioavailability and bioaccumulation of mercury and other trace metals to the sea cucumber,, us- ingsolubilization., 46 (12): 1600-1608, DOI: 10.1016/S0025-326X(03)00326-6.
McKenzie, A. N. J., and Preston, T. M., 1992. Functional studies onhaemocyte subpopulations defined by lectin staining and density centrifugation.,16 (1): 19-30.
Michaeloudes, C., Kirkham, P., Adcock, I. M., and Chung, K. F., 2015. Mitochondrial reactive oxygen species and glycolysis in airway smooth muscle cell proliferation in COPD., 46 (S59):OA488, DOI: 10.1183/ 13993003.congress-2015.OA488.
Mills, E. L., and O’Neill, L. A., 2016. Reprogramming mito- chondrial metabolism in macrophages as an anti-inflamma- tory signal.,46 (1): 13-21, DOI: 10.1002/eji.201445427.
Mohammadizadeh, M., Bastami, K. D., Ehsanpour, M., Afk- hami, M., Mohammadizadeh, F., and Esmaeilzadeh, M., 2016. Heavy metal accumulation in tissues of two sea cucumbers,andin the north- ern part of Qeshm Island, Persian Gulf.,103 (1-2): 354-359, DOI: 10.1016/j.marpolbul.2015. 12.033.
Muraille, E., Leo, O., and Moser, M., 2014. TH1/TH2 paradigm extended: Macrophage polarization as an unappreciated patho- gen-driven escape mechanism.,5:603, DOI:10.3389/fimmu.2014.00603.
Naumov, Y. A., 2007. The anthropogenic impact on the coastal shelf zone of Russia’s Far-Eastern seas (a case study of the Peter the Great Bay)., 1: 106-114.
Orihuela, R., McPherson, C. A., and Harry, G. J., 2016. Micro- glial M1/M2 polarization and metabolic states., 173(4):649-665, DOI: 10.1111/bph.13139.
Patrick, L., 2006. Lead toxicity part II: The role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity., 11 (2): 114-127.
Pershyn, O., Kocheshkova, N., Vorobets, Z., and Antonjak, H., 2009. Effect of Pb2+upon antioxidant enzymes activity of rat lymphocytes., XXII: 23-26.
Peterson, J. D., Herzenberg, L. A., Vasquez, K., and Walten- baugh, C., 1998. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns., 95 (6): 3071- 3076.
Poromov, A. A., Peretykin, A. A., and Smurov, A. V., 2014. Influence of salinity on bioconcentration and genotoxicity of heavy metals forL. sea stars., 3 (21): 43-49 (in Russian with English abstract).
Rocke, T. E., and Samuel, M. D., 1991. Effects of lead shot ingestion on selected cells of the mallard immune system.,27 (1): 1-9, DOI: 10.7589/0090- 3558-27.1.1.
Roomi, M. W., Columbano, A., Ledda-Columbano, G. M., and Sarma, D. S., 1986. Lead nitrate induces certain biochemical properties characteristic of hepatocyte nodules., 7 (10):1643-1646.
Rosenberg, C. E., Fink, N. E., Arrieta, M. A., and Salibian, A., 2003. Effect of lead acetate on theengulfment and killing capability of toad () neutrophils., 136 (3): 225- 233.
Ruzittu, M., Carla, E. C., Montinari, M. R., Maietta, G., and Dini, L., 1999. Modulation of cell surface expression of liver carbohydrate receptors duringinduction of apoptosis with lead nitrate.298 (1): 105-112.
Sa?di, S. A., Salah, A. M., Windmolders, P., and El Feki, A., 2013. Cytotoxicity evaluation and antioxidant enzyme ex- pression related to heavy metals found in tuna by-products meal: An in vitro study in human and rat liver cell lines.65 (7-8):1025-1033, DOI: 10.1016/j.etp.2013.03.001.
Sandhir, R., Julka, D., and Gill, K. D., 1994. Lipoperoxidative damage on lead exposure in rat brain and its implications on membrane bound enzymes.74 (2): 66-71.
Seco-Rovira, V., Beltrán-Frutos, E., Ferrer, C., Sánchez-Huertas, M. M., Madrid, J. F., Saez, F. J., and Pastor, L. M., 2013. Lectin histochemistry as a tool to identify apoptotic cells in the seminiferous epithelium of Syrian hamster () subjected to short photoperiod.48 (6):974-983, DOI: 10.1111/rda.12196.
Shabani, A., and Rabbani, A.,2000. Lead nitrate induced apo- ptosis in alveolar macrophages from rat lung., 149 (2-3):109-114.
Sharma, S., Sharma, V., Paliwal, R., and Pracheta, D., 2011. Lead toxicity, oxidative damage and health implications. A review.2 (13): 215-221, DOI: 10.5897/IJBMBRX11. 002.
Sharma, V., Sharma, A., and Kansal, L., 2010. The effect of oral administration ofextracts on lead nitrate in- duced toxicity in male mice., 48 (3): 928-936, DOI: 10.1016/j.fct.2010.01.002.
Shulkin, V. M., and Bogdanova, N. N., 2003. Mobilization of metals from riverine suspended matter in seawater., 83: 157-167, DOI: 101016/S0304-4203(03)001 09-9.
Singh, N., Bhagat, J., and Ingole, B. S., 2017.189 (7): 308, DOI: 10.1007/s10661-017- 5993.
Spolarics, Z., and Wu, J. X., 1997. Role of glutathione and catalase in H2O2 detoxification in LPS-activated hepatic en- dothelial and Kupffer cells.273 (6 Pt 1): G1304-G1311.
Taheri, R., Salamat, N., and Movahedinia, A., 2015. Using im- mune responses inandfrom Persian Gulf as indicators of environmental health.,98 (1-2): 47-57, DOI: 10.1016/j.marpolbul.2015.07.014.
Tan, H., Wang, N., Li, S., Hong, M., Wang, X., and Feng, Y., 2016.The reactive oxygen species in macrophage polariza- tion: Reflecting its dual role in progression and treatment of human diseases.Oxidative , 2016 (4): 1-16,DOI: 10.1155/2016/2795090.
Tan, W. H., and Lim, L. H., 1984.The tolerance to and uptake of lead in the green mussel,(L.)., 42 (3-4): 317-332, DOI: 10.1016/0044-8486(84)90110-8.
Tátrai, E., Kováciková, Z., Hudák, A., Adamis, Z., and Ungváry, G., 2001. Comparativetoxicity of cadmium and lead on redox cycling in type II pneumocytes., 21 (6): 479-483.
Wang, J., Ren, T., Han, Y., Zhao, Y., Liao, M., Wang, F., and Jiang, Z., 2015. The effects of dietary lead on growth, bioac- cumulation and antioxidant capacity in sea cucumber,., 40 (2): 535-540, DOI: 10.1016/j.etap.2015.08.012.
Wu, S. B., and Wei, Y. H., 2012. AMPK-mediated increase of glycolysis as an adaptive response to oxidative stress in hu- man cells: Implication of the cell survival in mitochondrial diseases.1822 (2):233-247, DOI: 10.1016/j.bbadis.2011.09.014.
Yusupova, L. B., 1989. Increasing the accuracy of determining the glutathione reductase activity of erythrocytes., 4:19-21 (in Russsian with English abstract).
January 17, 2018;
May3, 2018;
July 3, 2018
? Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2018
. Tel: 007-423-2135273 E-mail: dolmatova@poi.dvo.ru
(Edited by Qiu Yantao)
 Journal of Ocean University of China2018年6期
Journal of Ocean University of China2018年6期
- Journal of Ocean University of China的其它文章
- Comparative Evaluation of Toleration to Heating and Hypoxia of Three Kinds of Salmonids
- Optimization of Hydrolysis Conditions for the Isolation of Angiotensin-I Converting Enzyme (ACE) Inhibitory Peptides from Rhopilema hispidum
- Trophic Interaction in a Portunus rituberculatus Polyculture Ecosystem Based on Carbon and Nitrogen Stable Isotope Analysis
- The Effect of Hydrolysis with Neutrase on Molecular Weight, Functional Properties, and Antioxidant Activities of Alaska Pollock Protein Isolate
- An Evaluation on the Ratio of Plant to Animal Protein in the Diet of Juvenile Sea Cucumber (Apostichopus japonicus): Growth, Nutrient Digestibility and Nonspecific Immunity
- Weighted Correlation Network Analysis (WGCNA) of Japanese Flounder (Paralichthys olivaceus) Embryo Transcriptome Provides Crucial Gene Sets for Understanding Haploid Syndrome and Rescue by Diploidization
