Non-contact tensile viscoelastic characterization of microscale biological materials
Yuhui Li·Yuan Hong·Guang-Kui Xu·Shaobao Liu·Qiang Shi·Deding Tang·Hui Yang·Guy M.Genin,5·Tian Jian Lu·Feng Xu
1 Introduction
Biological material shave properties that arise from processes that are coordinated across hierarchical scales from nanometers to centimeters[1–3].The mechanical performance of biological materials is of great interest from the viewpoint of biomedical applications,such as bioinspired engineering of innovative materials,reconstruction of functional tissue constructs and diagnosis of disease[3–6].For instance,in bone tissues,mechanics arises from processes beginning at the nanoscale of tropocollagen molecules, and rising through the scale of bioapatite crystals(with length in30–50nm)into mineralized collagen fibrils and fibers[7,8].Tendons and ligaments are built upon a similar structure[9,10].Hierarchical characterization of these and other tissues in tension poses a pressing challenge because key hierarchies involve combinations of length-scales and forces that are difficult to access in the context of a tensile test.Our focus here is a new technology that enables contact-free gripping of specimens across a broad range of spatial length-scales and forces.
Thus far,a large number of data have been reported with regard to mechanical properties of biomaterials under compression and shear[11,12].However,very little is known about the behaviors of such biomaterials under tension,especially for biomaterials with mechanical weakness,due to the limitations of existing tensile tests for biological specimens such as difficulties in the fabrication and handling of specimens without damage,or the accurate extraction of mechanical properties from the tensile tests of such biologi-cal samples.Moreover,most biological materials,especially for protein-based materials(e.g., fibrin,collagen,gelatin)and various natural tissues(e.g.,such as brain,liver,muscle and bone),are viscoelastic,exhibiting creep or stress relaxation behaviours[13,14].The viscoelasticity of biological materials can also dissipate cell traction forces,which provide a powerful cue to interacting cells.Indeed,recent studies have found an impact of altered material viscoelasticity,independent of the stiffness,on various cell behaviours using biological gels as substrates for cell culture[15,16].Thus,it is of great importance to characterize material viscoelasticity for understanding the time-dependent behaviour of biological materials and even their effect on cell fate.
Tools for nano-and micro-scale mechanical characterization of biological materials have varied little since the first atomic force microscope(AFM)like instrument was developed for indentation of living cells[17,18].These methods obtain information about the compressive properties on a surface,which is difficult to interpret in the context of the tensile properties of a tissue[19].Moreover,the perturbations of such contact testing method as induced by surface effect and geometry of indenter tip can hardly to avoid.Micropipette aspiration[20,21],in which mechanical properties are estimated from the suction pressure to compress a cell into a pipette tip,have similar limitations.Tensile testing methods are widely available at them illimeter to centimeter scales,but are a challenge to apply at the nano and microscales[22,23].Devices such as the Chasiotis MEMS tensile tester[24]and AFM systems for tensile testing of proteins[25,26]require elaborate ligand/receptor or adhesion systems for gripping.Free-standing film techniques require ultra-thin(400nm) films and smooth specimens,and the fabrication processes of such specimens are complex and limit their applications[27].A persistent challenge in dissecting the deformation and toughening mechanisms that give rise to this integrity is tensile measurement of viscoelastic properties at the micrometer-level“mesoscale”of these hierarchical materials.Additionally,a range of tissues such as elements of microvasculature that exist at this length scale continue to be a challenge to characterize under tension,making characterization of these tissues an elusive goal.
Recently,Mahadevan’s group in Harvard has developed a simple method to test the tensile properties of biological tissues based on a magnetic actuation process[28].In this technique,a steel bead was attached to the biological sample and a permanent was used to apply a tensile force.Although such method has been used to evaluate the elastic properties of embryonic tissues,it is still difficult to determine the viscoelastic properties of the biological samples.In addition,such method was applied to test soft biological tissue with moduli ranging from 5 to 6 kPa,which can be only applicable for a few in vivo tissue types,such as neural and adipose tissues.A test method with a broader testing range should be developed to evaluate he mechanical properties of other stiff tissues,such as skin,muscle,and vascular.From a mechanotransduction point of view,cells can sense and exert forces to the extracellular matrix(ECM)by adjusting the transmembrane molecules(e.g.,integrin complexes)and by actomyosin dynamics resulting in transmission of forces against the matrix.However,the contribution of cell remodelling and ECM to the matrix mechanics in a 3D microenvironment remains elusive.To address these challenges,we have improved such magnetic actuation method,extending recently developed 3D tissue culture systems to testing of microscale biomaterials[29,30].Magnetically-actuated culture systems available in existing literatures include patterned poly(dimethylsiloxane) (PDMS)cantilevers[31], cell-laden gels[32],and functional skeletal muscle fibers[33].We adapted these non-contact methods for measuring elastic and viscoelastic tensile properties of mesoscale biological materials.We termed these “magnetic mechanical testing”(MMT)devices.MMT devices allow the characterization of a broad range of mechanical properties from tens to thousands of KPa.After validating against data for poly(ethylene glycol)diacrylate(PEGDMA),gelatin methacrylate(GelMA), and PDMS gels in tensile and flexural tests,we demonstrated the MMT system by mesoscale characterization of cell and tissue construct mechanics,revealing for substantial differences between meso-and macroscale properties.
2 Results and discussion
2.1 MMTsystem:setup and characterization
To enable the non-contact mechanical loading of biological specimens,a magnetically-actuated sample,an auto-linear stage and applied magnetic field were integrated(Fig.1a).Herein,soft fiber-shaped gel specimens (e.g.,polyacrylamide(PAM),polyacrylamide/Alginate,PEGDMA and GelMA)were used for tensile testing.Gel fiber each contained one stiff and strong magnetically-actuated layer of PEGDMA(20%w/v)encapsulating iron microbead with uniform size(~250μm),following photo-cross linking process.The magnetic force can be regulated by changing the separation between the magnetically-actuated layer and the end surface of permanent magnet(Fig.1b)and even the number of encapsulated microbeads.The deformation of fire-shaped samples can be recorded by a digital microscope(VHX-1000)(Fig.1c).To avoid the dehydration of biological samples,the mechanical testing was performed in a humid control system(~60%hum idity).To verify the accuracy of the MMT system,the elastic properties of tough PAM gel samples with different monomer fraction(8%,12%,16%w/v,respectively)were characterized by both magnetic sys-tem and commercial mechanical testing equipment(Bose Electroforce3200).The resulting force–displacement curves of tested gel samples from magnetic system were nearly linear within 10%strain,and the slope of these curves was calculated as the elastic modulus of gel samples.Figure 1d showed that the simulated results of three kinds of PAM gel fibers can be well- fitted with the experimental data,suggesting the robustness of MMT system.Similar results of elastic modulus were observed for the gel fibers tested by magnetic method to those of commercial tensile testing equipment,indicating the high accuracy of the developed MMT system(Fig.1e).To obtain amore accurate data, the inertial effects of microspheres should be considered in the further study.The large testing range of magnetic system has also been verified by testing various polymeric gels,whose elastic modulus are in the range of kPa to MPa[34,35].
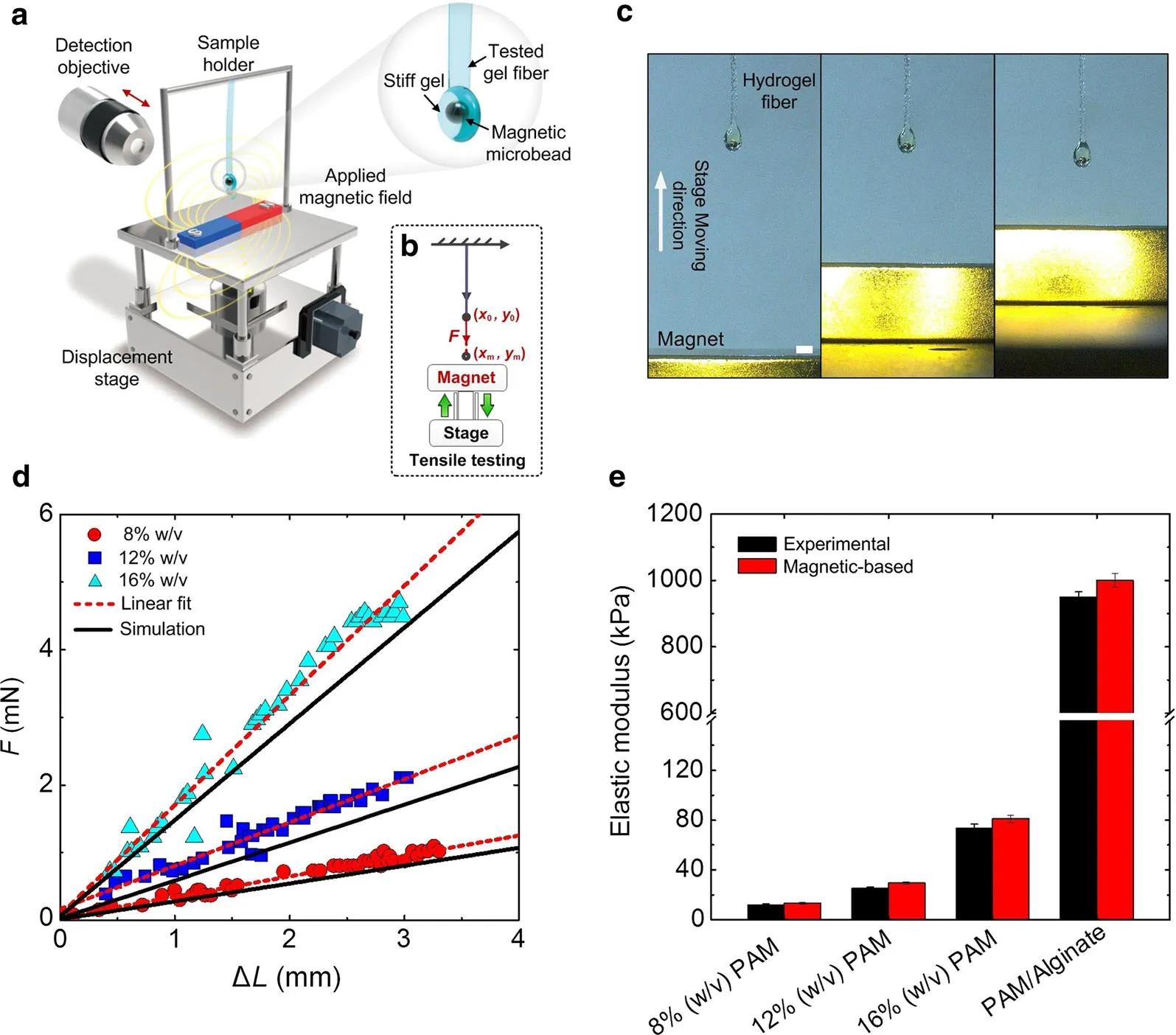
Fig.1 Magnetic tensile-testing system setup and accuracy verification.a,b Schematics of MMT system setup.MMT system contains a fiber shaped biological sample,a permanent magnet,an auto-linear stage,and a digital microscope.Each tested biological sample was composed a stiff“magnetically-actuated”PEGDMA layer encapsulating an iron microsphere.Strains were precisely controlled by using permanent magnet fixed on an auto-linear stage.c Side view of deformed gel samples by regulating the separation between magnet and “magnetically-actuated”layer.d The force–displacement curves of PAM gel samples with different monomer fraction(8%,12%,16%w/v,respectively).The numerical results can be well fitted with experimental data.e Verification of MMT system’accuracy by comparing the tested results with commercial mechanical testing equipment(Bose Electroforce 3200).The large testing range of magnetic system has also been verified by testing various polymeric gels,whose elastic modulus are on the order from~20kPa to 1MPa.Error bars,s.d.(n=10 samples).Scale bar:2 mm
2.2 MMT system overcomes the challenge of gripping soft biological materials
Gripping of soft biological materials is a perennial challenge for mechanical testing:adhesives,friction grips,suction grips,and inter penetrating fibrous mats all lead to stress concentrations or changes across material hierarchies that confound interpretation of results.MMT system,however,were able to sidestep this challenge for hydrogels that are difficult to manipulate and handle.Here,we tested two kinds of soft gels(PEGDMA and GelMA)due to their widespread applications from fundamental(e.g.,artificial ECM considered in the further study.The large testing range of magnetic[36])to applied research(e.g.,drug carrier[37])areas.The force–displacement curves of PEGDMA and GelMA gel fibers were nearly linear under 10%strain and the results suggested that PEGDMA is stiffer than GelMA with the same monomer fraction(10%,15%,20%w/v,respectively)(Fig.2a,b).We then characterized the effect of monomer fraction on gel elastic properties using MMT system.Figure 2c showed the elastic modulus profile of gel samples with different monomer fractions.The elastic modulus of PEGDMA were 12.75±1.79,47.20±3.41,71.2±5.41kPa,while the modulus of GelMA were 6.04±0.45,9.88±1.25,22.43±2.21kPa for monomer fraction 5%,10%,20%w/v,respectively.The results indicate that the increased monomer fraction significantly enhances the elastic properties of gels as a result of relatively high cross linking densities.
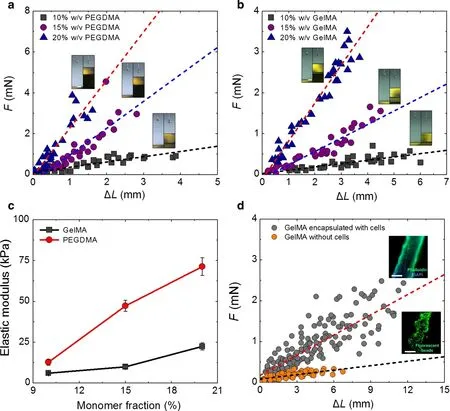
Fig.2 The measurement of the tensile properties of soft biological gels.a,b The force–displacement curves of PEGDMA and GelMA gel fibers were nearly linear under 10%strain with varying monomer fraction(10%,15%,20%w/v,respectively).c The elastic modulus of PEGDMA gel with different monomer fraction(10%,15%,20%w/v)is 12.75±1.79,47.20±3.41,71.2±5.41 kPa,respectively.For GelMA gel,the elastic modulus for different monomer fraction is 6.04±0.45,9.88±1.25,22.43±2.21kPa,respectively.Error bars,s.d.(n=10 samples).d The force–displacement curves of GelMA gel samples(encapsulating with and without cells)tested at day 14 of culture.The F-actin of cells was stained by phalloidin(Green)and nuclei were stained by DAPI(Blue).To characterize the gel(without cells)morphology, fluorescent microbeads(Green)were embedded.(n=20 samples).Scale bar:a,b 2mm;d 500μm
2.3 MMT system enables characterization of cell and tissue construct mechanics
To answer the question how cell remodelling process contribute to their mechanical microenvironment,we used GelMA gel,a bioactive gel derived from natural gelatin that is effective for cell attachment and proliferation,to encapsulate NIH/3T3 cell populations at an initial cell density of 1×105cells/m l.MMT system was applied to measure the elastic modulus of cell-laden GelMA gels(monomer fraction:10%w/v)after 2 weeks culture,Fig.2d.We observed that the slope of cell-laden gels is larger than gel without encapsulated cells.The elastic modulus of cell-laden gels is 8.41kPa(increasing 39.2%compared to initial GelMA gel without encapsulated cells),suggesting that proliferated cells and cell secreted matrix may contribute to the gel mechanical properties.Inversely,the elastic modulus of control group(gel without encapsulated cells)is 1.13kPa(decreasing 81.3%compared to initial samples),which is mainly due to the hydrolysis of GelMA gel in the culture medium after 2 weeks.These results indicate that MMT system is capable of identifying the mechanical properties frequently without sac-rifice of samples.From a mechanotransduction view,the key issue is the mechanical characterization of cell and material interactions.This is important because the mechanotransduction occurs through the focal contact between the cells and materials.Therefore,the measurement of the focal contact by using cell adhesion moiety by using nanoscale mechanotools,such as AFM,is also important.
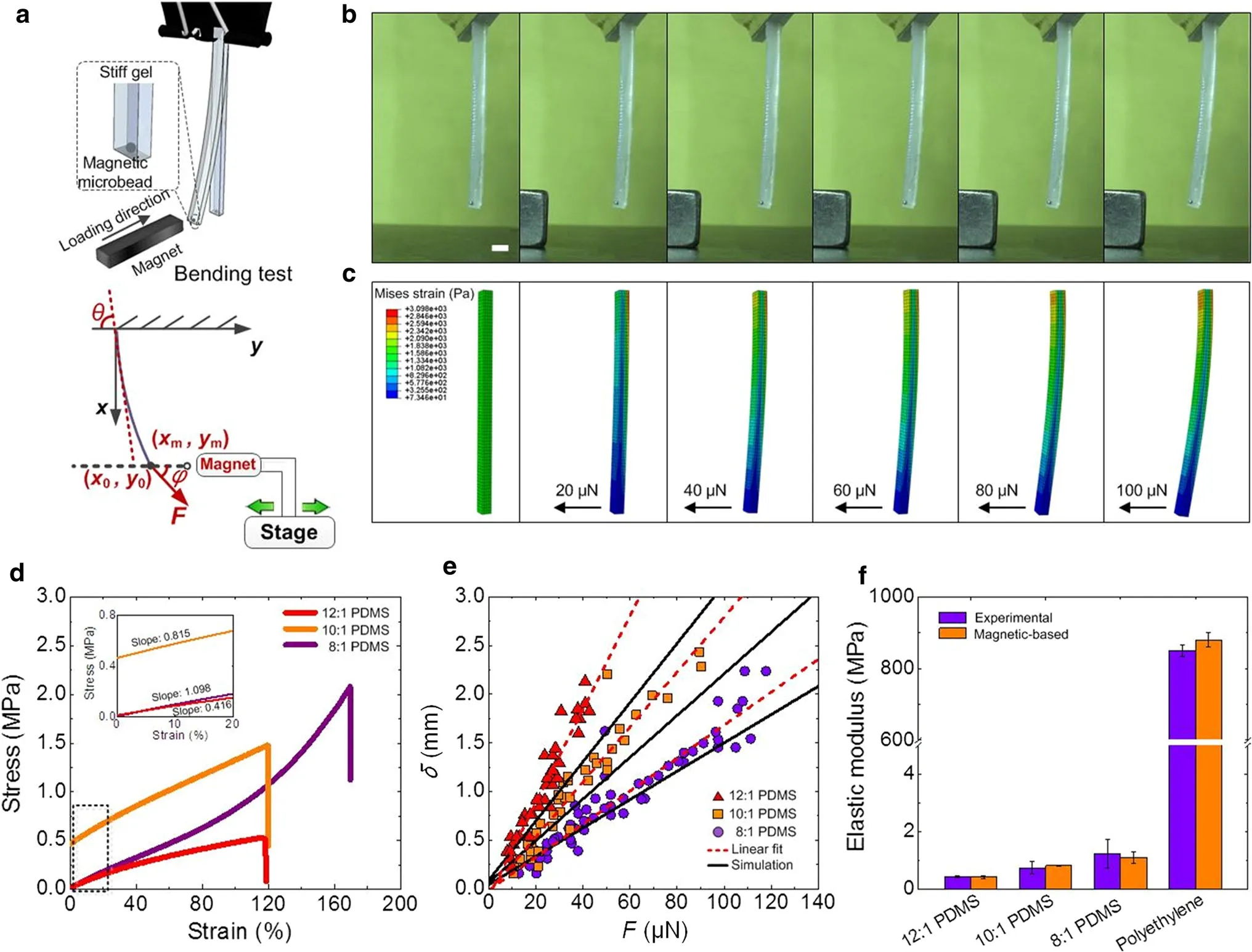
Fig.3 Flexural properties of biological materials tested using MMT system.a Schematics of magnetic bending test of PDMS flexural properties.b Images of deformed PDMS samples under magnetic field.c Numerical results of sample deformation under varying mechanical force.d Stress–strain curves of PDMS gels with different curing ratios(12:1,10:1,8:1,respectively)tested using conventional equipment(n=5 samples).e The bending curves of PDMS tested by MMT system(n=10 samples).f The large testing range of MMT system has also been verified by testing polyethylene film,whose elastic modulus can achieve~800 MPa.(n=10 samples).Scale bar:2 mm
2.4 MMT system enables flexural characterization of microscale specimens
Flexural properties of biological materials also play an important role in maintaining the material functionality.For instance, flexure represents a major mode of deformation of native heart valve leaflets,which regulates the physiological valve closure during the heart pumping.Biological gels provide a useful tool for mimicking native functional tissues,attributing to their tunablebio chemical and biophysical properties.While biological gels have been well studied in compression and tension,the flexural properties of such gels are still rarely known.Here,we used MMT system to identify the flexural properties of stiff PDMS gels through magnetic bending(Fig.3a,b).We also performed finite element simulation using ABAQUS software(Fig.3b).The simulating results of PDMS bending deformation can be well fitted with experimental results(Fig.3c).Here,we tested PDMS gels with different curing ratios(12:1,10:1,8:1)with Bose mechanical equipment(Fig.3d)and MMT system(Fig.3e),respectively.Both the experimental and theoretical results showed that the flexural properties can be enhanced with increasing monomer fraction.To identify the robustness of the magnetic system,we test the flexural properties of a stiff material-polyethylene,which is a kind of thermoplastic resin with widespread applications in plastic films,geomembranes and containers.The elastic modulus of tested polyethylene film can achieve~800 MPa,suggesting the wide testing range of the MMT system(Fig.3f).
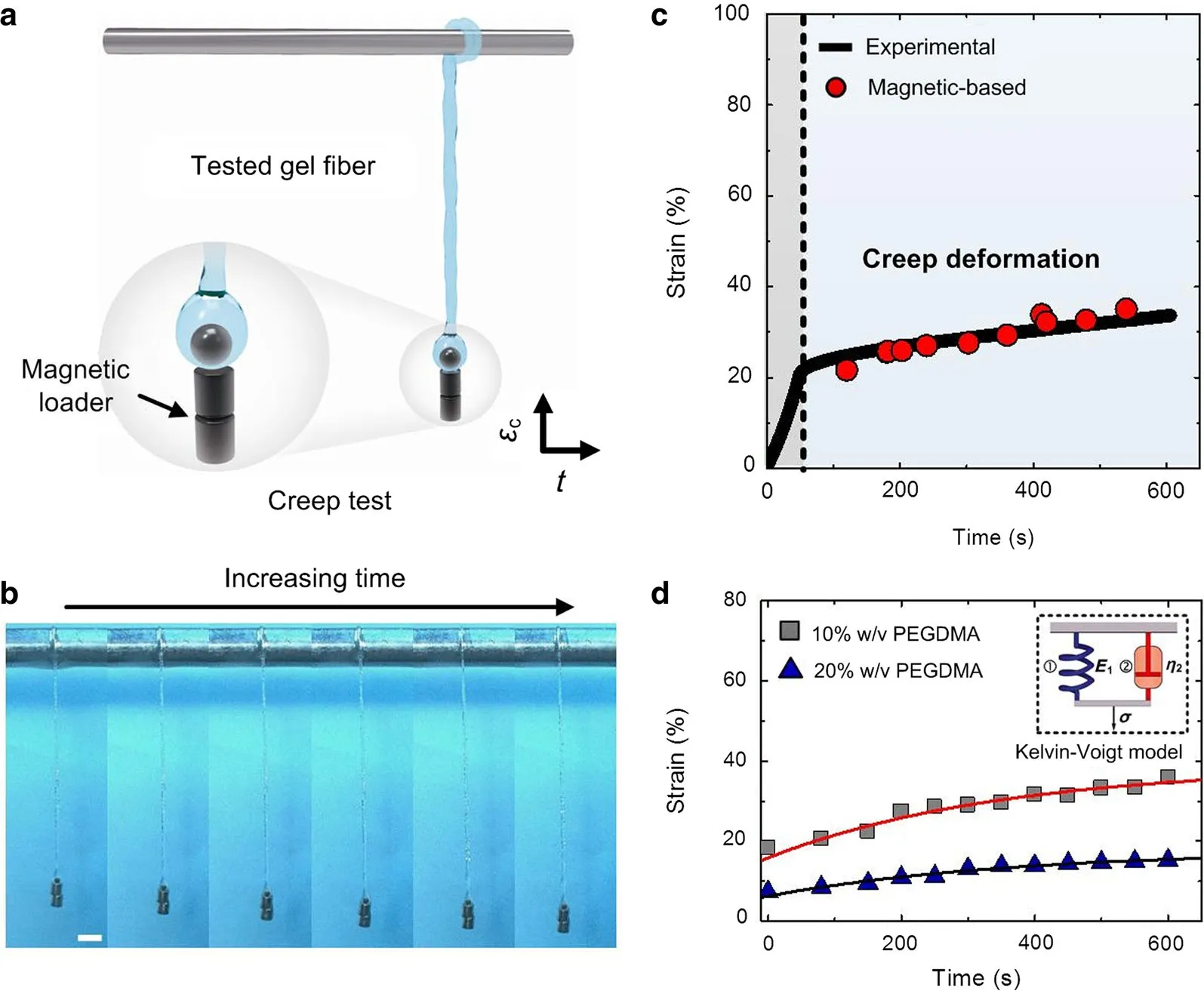
Fig.4 Viscoelasticity of biological materials characterized by MMT system.a Schematics of creep test of gel fibers based on magnetic loader.b The creep data of PAM gel fibers(16%w/v)tested by both MMT system,which can match with the creep curves tested from commercial equipment(n=3 samples).c Side view of deformed gel samples by fixing the loading force with magnetic loader.d Creep deformation of PEGDMA gels(10%and 20%w/v,respectively)tested by MMT system,which was fitted with a Kelvin–Voige model.Scale bar:5 mm
2.5 Viscoelastic testing of biologicalgels
Another important mechanical property of biological materials(e.g.,gels)and tissues(e.g.,skin,muscle and tendon)is the viscoelasticity.For instance,collagen gel exhibits stress relaxation or a decrease in the elastic modulus over time when a constant strain is applied.This is mainly because of the configurational rearrangements,disposition and interaction among the polymeric network in both their short-and long-range interrelations.Under stress,the response to local re-distribution is rapid,and the response to long-range interactions is slow before a new assortment of configurations is obtained.The viscoelasticity of biological materials can also dissipate cell traction forces,which provide a powerful cue to interacting cells.Indeed,recent studies have found an impact of altered material viscoelasticity,independent of the stiffness,on various cell behaviours using biological gels as substrates for cell culture.Thus,characterization of material viscoelasticity can enhance our understanding of the time dependent behaviour of biological materials and even their effect on cell fate.Here,we characterized the viscoelasticity of biological gels via creep test.For this,we applied constant stress on tested gel fibers by fixing two magnetic loaders on the tip of fibers through magnetic force(Fig.4a,b).The constant stress can be easily tuned by changing the number of magnetic loaders.The deformation of tested gel fibers with loading time can be recorded by a digital microscope.To verify the accuracy of MMT system on creep test of biological gels,we compared the creep strain of PAM gel fibers obtained by MMT system and commercial Bose equipment.Both of these results showed that the sample exhibited an elastic deformation step and then deformed with time under a constant stress(15kPa).Figure 4c showed that the creep strainεcof tested gel fibers slow ly grew with timet(10 min).Then,the creep behaviours of PEGDMA gel fibers(10%and 20%w/v)were studied using MMT system.For tracking the nominal strain for the creep process,we reset the recorded time at the beginning of creep process to 0.The creep strain of both 10%and 20%PEGDMA gel fibers grew smoothly within10m in and these results can be well fitted by a Kelvin–Voigt model.Such results indicated that MMT system can serve as a powerful tool for studying the viscoelastic properties of biological soft materials.

Fig.5 Elasticity of biological tissues characterized by MMT system at mesoscale.a–f Tensile properties of different rabbit tissues,including artery,vein,nerve,skin,and muscle,characterized by MMT system.The force–displacement curves of these tissues tested by MMT system exhibit a nonlinear and parabolic relationship(n=10 samples).g Comparison of elastic modulus of native tissues between magnetic-based and standard method.(n=10 samples).Scale bar:(a,top right)2mm;(a,lower left)500μm
2.6 Mechanical testing of biological tissues at mesoscale
The mechanical properties of native tissues are important for maintaining tissue functionality.The architectural features of tissues may be presented to cells across multiple length scales,from nanometers to millimeters in size,as a result of different levels of mechanical performance.However,the determination of mechanical properties of biological tissues at mesoscale is largely unknown.Here,we applied MMT system to applied MMT system to test the tensile properties of different biological tissues including artery,vein,nerve,skin,and muscle(Fig.5).The force–displacement curves of these tissues tested by MMT system exhibit a nonlinear and parabolic relationship.Our results also revealed the scale effect on mechanical properties of native tissues.For instance,the microscale biological samples tested by commercial equipment show different tensile modulus compared with the MMT testing results(Fig.5g).The vascular system is composed of arteries and veins,which play an important role in the transport of nutrients,oxygen,and other chemical signals to various parts of the body.The artery has the functionality to carry blood from the heart to other parts of the body,whereas the vein mainly collects blood back to the heart.The main protein composition of artery and vein ECM is the elastin and collagen.Elastin has an outstanding elastic property and is responsible for the small deformation of the vascular tissue and helps it to recover to the original position.Collagen is the main source of the mechanical properties of vascular tissue,which exhibits viscoelastic properties.For both artery and vein,they exhibit significant nonlinear elastic properties.The force–displacement curve of artery tested by MMT exhibits linear relationship at strain level of<10%whereas the vein exhibits linear elastic properties under 3%strain.Such difference may be caused by the different elastin composition of artery and vein.The artery approximately contains twice the amount of elastic fibers than vein[38].The nerve tissue is softer than artery and vein,which exhibits similar mechanical properties.The skin is a three-layer composition of epidermis,dermis and subcutaneous tissue,which has multifunctionalities including thermoregulation,barrier between organism and environment,and host defense.The derm is makes major contribution to the mechanical properties of skin tissues which is composed of elastin and collagen.The modulus of single collagen fiber as estimated by AFM is around 50–100MPa,whereas the modulus of whole piece of skin varies ranging from 5 to 100kPa.Interestingly,the skin tissues at mesoscale exhibit a medium tensile modulus around 600kPa.The force–displacement curve of the skin tested by MMT exhibits a nonlinear relationship,which is the result of the interaction between elastic elastin fibers and the viscoelastic collagen fibers.At low strains(up to about 5%),less stiff elastic fibers dominate and the crimp of the collagen fibers straightens,requiring very little force to stretch the skin.The skin becomes stiffer when the crimp is straightened.For skeletal muscles,the tensile modulus of single filament at microscale is around 10–80kPa,whereas the tensile elasticity of muscle fiber at mesoscale significantly increases up to MPa levels.Such diversity in mechanical performance at different scale levels:nano,micro,meso and structural,may relate to the anisotropic and hierarchical structure of biological materials,starting at the nanometer level and continuing up to the structural dimensions.
3 Conclusion
Taken together,we developed a simple and facile method(i.e.,MMT system)to measure the mechanical properties of biological materials through a magnetic actuation system without contacting the samples.The approach is broadly applicable,and enables simple magnetic gripping of specimens for both elastic and viscoelastic testing at mesoscale.The accuracy of MMT system was also verified by comparing the tested results with those obtained from commercial mechanical testing equipment.MMT system is able to produce reliable and reproducible data for pure gels,cell embedded gel constructs and native tissues in a non-destructive and real-time manner.MMT system also holds great potential for probing more detailed mechanical information of biological materials,such as tensile strength,fatigue life,and energy dissipation.We expected that these unique features offer a powerful tool for revealing the mechanics of biological materials at different scale levels.
3.1 Experimental section
Fabrication of the biological samples:For polyacrylamide gel fabrication,a polymer solution containing acrylamide(monomer)(MP Biomedicals,Aurora,Ohio),N,Nmethylene-bis-acrylamide(crosslinker)(MBA)(Sigma Aldrich,St.Louis,USA),1/100 volume of 10%aluminium persulfate(APS)(Sigma Aldrich,St.Louis,USA)and 1/1000 volume ofN,N,N′,N′-tetramethylethylenediamine(TEMED)(Sigma Aldrich,St.Louis,USA)was prepared.This solution was sandwiched between a DCMS-treated slide and a functionalized coverslip,and allowed to polymerize at room temperature for 5m in.Following polymerization,polyacrylamide gel was incubated in 1mg/m lN-sulphosuccinimidyl-6-(4′-azido-2′-nitrophenylamino)hexanoate(sulfo-SANPAH)(Pierce,Rockford,IL)activated with ultraviolet(UV)light for 10 min,washed with 50 m MHEPES at pH 8.5(Sigma Aldrich,St.Louis,USA),and then located in deionized water overnight at room temperature.The PEGDMA and Gel MA gel can be fabricated as described previously.Briefly,the PEGDMA or GelMA precursor solution and photoinitiator(2-hydoxy-2-methylpropiophenone;TCI,Shanghai,China)were mixed and then exposed to 365nm UV light at a power of2.9mW×cm?2(modelXLE-1000A/F,Spectroline)for25–30s.The fiber-shaped samples were generated by pressing the cylindrical gels through scientific sieves and were collected in a water- filled petri dish and swelling for 24h before mechanical testing.The diameter of each fiber is~900μm and the length is~35mm.To fabricate a magnetic layer of gel fibers,a PEGDMA droplet(20%w/v,200μL)encapsulating one iron microsphere(K&J Magnetics)was added to the end of each gel fiber,which subsequently underwent a photocrosslinking process.The native tissues were isolated from rabbit(New Zealand rabbit,350–400g)and cut into fiberd-shaped samples and used for mechanical testing by MTT system.For commercial equipment,the native tissue was moulded as a brick sample(20mm×10mm×5mm).For bending test,the PDMSs ample(2mm×4mm×20mm)was prepared using a designed mold.
Mechanical testingof the fiber-shapedsamples:To experimentally characterize magnetic force applied to iron microspheres in magnetically-actuated layers,the Stokes drag method was used. The deformation fiber-shaped samples can be obtained through a digital microscope(VHX-1000)and analysed using Image-Pro Plus(Media Cybernetics).Nominal strain,ε,was calculated in each layer asε=λ?1,λis the ratio of the current to initial length of the sample.The average strain over the synthetic tissue layers was calculated by aver-aging the strain of10–20samples.To predict the deformation of biological samples,we performed the numerical simulation by using a typical Neo–Hookean model with elastic modulus derived from experimental results described below.The strain-energy function can be described as following:
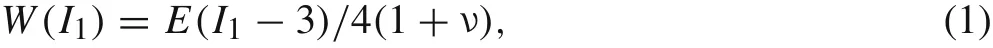
whereI1is Green–Lagrangian strain tensor,Eis the elastic modulus,andνis the Poisson’s ratio of gels.To experimentally estimate the magnetic force that we applied to the iron microsphere,the Stokes drag method was used here.Assuming the particle gravity is negligible,the sum of magnetic force,the drag force and the inertial force is balanced.The magnetic force on their on microsphere can then be estimated as

whereFmagandFinerrepresent the Stokes drag force and inertial force,respectively.Here,we assumed that the inertia forceFineris negligible.Since the magnetic bead is spherical,the drag force can be calculated from Stokes’law,

where theFrepresents viscous drag,vis the dynamic viscosity of the poly(ethylene oxide)(PEO)solution,Ris the radius of the iron microsphere,andUis the speed of the iron microsphere.To precisely test the Stokes drag force,an iron microsphere was placed in PEO solution of defined high viscosity,then subjected to a magnetic field.The speed of each iron microsphere was estimated as a function he separation between the end of the magnetic permanent from video taken through a 20×objective,acquired using a high-speed camera(Phantom Cine stream v.711,Vision Research,Co.,Ltd.)at 3000 images per second.The acceleration of the microsphere can be estimated from the camera images.The tensile modulus of the biological samples was derived from these relationships.For creep test,two magnetic loaders were fixed on the tip of gel fibers by magnetic force.The deformation of gel fibers with loading time can be obtained by analysing the microscopy images.
Numerical simulation:To design the magnetic field focusers and characterize the mechanical fields within biological samples,we performed a series of numerical simulations using commercially available software(COMSOL Multiphysics4.0a,Comsol Inc.).First,we predicted the magnetic field gradients experienced by the iron microspheres within the magnetically-actuated layers as a function of the separations between the ends of magnetic field focusers and the centers of iron microspheres.The magnetic force vector applied to each iron microsphere was estimated according to:

whereAis the surface of the iron microsphere,nis the outward normal of dA,μ0is the magnetic permeability of a vacuum,Msatis the saturation moment of an individual microsphere,andis the external magnetic field gradient tensor at positionx.For bending stimulation,images of the gel deformation and microbead movement were captured at 10 frames per second by the VHX-1000 microscope.The deflection of gel samples was determined from the images by Image-Pro Plus software.From the force-deflection spot,the bending stiffness can be calculated and applied in the Bernoulli–Euler beam bending equation(Eq.(3))to determine the flexural deformation:

whereEbmis the bending modulus,Iis the moment of inertia,ΔKis curvature,bis the width,whereasais the length of the sample cross-area.To simulate the creep deformation of gel fibers,a Kelvin–Voigt model was used here(Fig.4d).It is the simplest model that can be used to represent the behaviour of a polymer component at the beginning of loading.In the Kelvin model the stress does not relax and remains constant at:

The time-dependent strain in a creep test was estimated according to:

whereλ,(η/E),is the relaxation time.
Cell encapsulation:NIH/3T3 fibroblasts were cultured in DMEM(Sigma-Aldrich,St.Louis,MO,USA)supplemented with 10%fetal bovine serum(GIBCO)in a 5%CO2-humidified incubator at 37°C.To collect and encapsulate cells,the cells were first trypsinized with 0.25%trypsin(Sigma-Aldrich,St.Louis,MO,USA)and centrifuged at 800 rpm for 5 min.The cells were suspended in GelMA gel precursor solution at a density of 1 × 105m l?1,and then mixed with 0.5%w/v of photoinitiator.Cell-encapsulating gel fibers were then photocrosslinked by exposing to 365 nm UV light at a power of 2.9mW ·cm?2(model XLE-1000 A/F,Spectroline)for 25 s.The samples were then washed with DPBS three times and incubated.Culture medium is exchanged twice a day during culturing.
Immunostaining:F-actin stress fibers and the nuclei of cells were stained by fluorescein isothiocyanate(FITC)conjugated phalloidin(Acti-stain 488 phalloidin,Cytoskeleton,Inc.)and 4′,6-diamidino-2-Phenylindole(DAPI;Invitrogen TM,Life Technologies,Inc.),respectively.Briefly,for stress fiber staining, fibroblasts in gel fibers were fixed by 4%formaldehyde(Sigma-A ldrich,St.Louis,MO,USA)for 15 min,permeabilized with 0.5%Triton X-100(Sigma-Aldrich,St.Louis,MO,USA)for 5 min,and then incubated with 200μL of 100 nM FITC phalloidin solution in the dark at room temperature for 30–40 min.For nuclear staining,cells were counterstained with 200μL of 100 nM DAPI in DPBS for 30 s.The samples were then imaged using an Olympus X81 microscope(Olympus,Tokyo,Japan).
AcknowledgementsThis project was partially supported by the National Natural Science Foundation of China(Grants11532009,11372243,and 11522219) and the China Postdoctoral Science Foundation(Grant2016M 602810).This project was also supported by the Initiative Postdocs Supporting Program(Grant BX201600121).
1.Meyers,M.A.,McKittrick,J.,Chen,P.Y.:Structural biological materials:critical mechanics-materials connections.Science 339,773–779(2013)
2.Chen,J.,W right,K.E.,Birch,M.A.:Nanoscale viscoelastic properties and adhesion of polydimethylsiloxane for tissue engineering.Acta Mech.Sin.30,2–6(2013)
3.Ji,B.,Gao,H.:Mechanical properties of nanostructure of biological materials.Mech.Phys.Solids 52,1963–1990(2004)
4.Lin,S.Z.,Li,B.,Feng,X.Q.:A dynamic cellular vertex model of growing epithelial tissues.Acta Mech.Sin.33,250–259(2017)
5.Hollister,S.J.:Porous scaffold design for tissue engineering.Nat.Mater.4,518–524(2006)
6.Li,Y.,Huang,G.,Gao,B.,et al.:Magnetically actuated cell-laden microscale hydrogels for probing strain-induced cell responses in three dimensions.NPG Asia Mater.8,e238(2016)
7.Reznikov,N.,Shahar,R.,Weiner,S.:Bone hierarchical structure in three dimensions.Acta Biomater.10,3815–3826(2014)
8.Alexander,B.,Daulton,T.L.,Genin,G.M.,et al.:The nanometrescale physiology of bone:steric modelling and scanning transmission electron microscopy of collagen-m ineral structure.J.R.Soc.Interface 9,1774–1786(2012)
9.Screen,H.R.C.,Leem,D.A.,Baderm,D.L.,et al.:An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties.Proc.IME H J.Eng.Med.218,109–119(2004)
10.Svensson,R.B.,Hansen,P.,Hassenkam,T.,et al.:Mechanical properties of human patellar tendon at the hierarchical levels of tendon and fibril.J.Appl.Physiol.112,419–426(2012)
11.Long,R.,Hui,C.Y.:Crackbuckling in soft gels under compression.Acta Mech.Sin.28,1098–1105(2012)
12.Stammen,J.A.,Williams,S.,Ku,D.N.,et al.:Mechanical properties of anovel PVA hydrogel in shear and unconfined compression.Biomaterials 22,799–806(2001)
13.Galford,J.E.,McElhaney,J.H.:A viscoelastic study of scalp,brain,and dura.J.Biomech.3,211–221(1970)
14.Zhang,T.,Yuk,H.,Lin,S.,et al.:Tough and tunable adhesion of hydrogels:experiments and models.Acta Mech.Sin.3,1–12(2017)
15.Chaudhuri,O.,Gu,L.,Klumpers,D.,et al.:Hydrogels with tunable stress relaxation regulate stem cell fate and activity.Nat.Mater.15,326(2016)
16.Chaudhuri,O.,Gu,L.,Darnell,M.,et al.:Substrate stress relaxation regulates cell spreading.Nat.Commun.6,6365(2015)
17.Henderson,E.,Haydon,P.G.,Sakaguchi,D.S.:Actin filament dynamics in living glial cells imaged by atomic force microscopy.Science 257,1944–1947(1992)
18.Radmacher,M.,Tillmann,R.W.,Fritz,M.,et al.:From molecules to cells:imaging soft samples with the atomic force microscope.Science 257,1900–1906(1992)
19.Yang,H.:Atomic Force Microscopy(AFM),Principles,Modes of Operation and Limitations.NOVA,New York(2014)
20.Hochmuth,R.M.:Micropipette aspiration of living cells.J.Biomech.33,15–22(2000)
21.Hogan,B.,Babataheri,A.,Hwang,Y.,et al.:Characterizing cell adhesion by using micropipette aspiration.Biophys.J.109,209–219(2015)
22.Dow ling,N.E.:Mechanical Behavior of Materials:Engineering Methods for Deformation,Fracture,and Fatigue.Prentice Hall,Englewood Cliffs(1993)
23.Drury,J.L.,Dennis,R.G.,Mooney,D.J.:The tensile properties of alginate hydrogels.Biomaterials 25,3187–3199(2004)
24.Chasiotis,I.,Knauss,W.G.:A new microtensile tester for the study of MEMS materials with the aid of atomic force microscopy.Exp.Mech.42,51–57(2002)
25.Thomson,N.H.,Fritz,M.,Radmacher,M.,et al.:Protein tracking and detection of protein motion using atomic force microscopy.Biophys.J.70,2421–2431(1996)
26.Schitter,G.,Astrom,K.J.,DeMartini,B.E.,et al.:Design and modeling of a high-speed AFM-scanner.IEEE Trans.Control Syst.Technol.15,906–915(2007)
27.Kim,J.H.,Nizam i,A.,Hwangbo,Y.,et al.:Tensile testing of ultrathin films on water surface.Nat.Commun.4,2520(2013)
28.Savin,T.,Shyer,A.E.,Mahadevan,L.:A method for tensile tests of biological tissues at the mesoscale.J.Appl.Phys.111,074704(2012)
29.Souza,G.R.,Molina,J.R.,Raphael,R.M.,et al.:Three-dimensional tissue culture based on magnetic cell levitation.Nat.Nanotechnol.5,291–296(2010)
30.Sakar,M.S.,Eyckmans,J.,Pieters,R.,et al.:Nat.Commun.7,11036(2016)
31.Zhao,R.,Boudou,T.,Wang,W.G.,et al.:Decoupling cell and matrix mechanics in engineered microtissues using magnetically actuated microcantilevers.Adv.Mater.25,1699–1705(2013)
32.Li,Y.,Huang,G.,Zhang,X.,et al.:Magnetic hydrogels and their potential biomedical applications.Adv.Funct.Mater.23,660–672(2013)
33.Li,Y.,Poon,C.T.,Li,M.,et al.:Chinese-noodle-inspired muscle myofiber fabrication.Adv.Funct.Mater.25,5999–6008(2015)
34.Sun,J.Y.,Zhao,X.,Illeperuma,W.R.K.,et al.:Highly stretchable and tough hydrogels.Nature 489,133–136(2012)
35.Zhao,X.:Multi-scalemulti-mechanism design of tough hydrogels:building dissipation into stretchy networks.Soft Matter 10,672–687(2014)
36.Yue,K.,Trujillo-de Santiago,G.,Alvarez,M.M.,et al.:Synthesis,properties,and biomedical applications of gelatin methacryloyl(GelMA)hydrogels.Biomaterials 73,254–271(2015)
37.Lai,T.C.,Yu,J.,Tsai,W.B.,et al.:Gelatin methacrylate/carboxybetaine methacrylate hydrogels with tunable cross linking for controlled drug release.J.Mater.Chem.B 4,2304–2313(2016)
38.Marc,A.M.,Po,Y.C.,Albert,M.L.,et al.:Biological materials:structure and mechanical properties.Prog.Mater.Sci.53,1–206(2008)
- Acta Mechanica Sinica的其它文章
- Instability waves and low-frequency noise radiation in the subsonic chevron jet
- Density enhancement mechanism of upwind schemes for low Mach number flows
- Hydrodynamic studies on two wiggling hydrofoils in an oblique arrangement
- The spanwise spectra in wall-bounded turbulence
- The dimension split element-free Galerkin method for three-dimensional potential problems
- Zero group velocity longitudinal modes in an isotropic cylinder

