Decomposition behavior of CaSO4 during potassium extraction from a potash feldspar-CaSO4 binary system by calcination☆
Li Lü,Chun Li,Guoquan Zhang*,Xiaowei Hu,Bin Liang
School of Chemical Engineering,Sichuan University,Chengdu 610065,China
1.Introduction
Potassium is an important chemical element for plant growth[1].Potash fertilizer has played a greatrole in increasing the yield ofagricultural products[2].With the increasing world population,the global demands for food are continuously growing[3].Therefore,it is very important to promote grain production growth,especially in China,India,and Brazil.However,hydrosoluble potassium resources are unevenly distributed worldwide.In China,there is a severe shortage of hydrosoluble potassium resources(only about 2%of the world's hydrosoluble potassium resources are present in China);therefore,nearly half of China's yearly potash fertilizer consumption relies on imports[4].Even so,China's potassium application proportion is only around one-third of that of other developed countries.In contrast,there is an extraordinary abundance of non-hydrosoluble potassium resources globally[5].According to estimates,there are 97 billion tons of proven reserves of K-feldspar ore that could be economically exploited in China.It is of great significance to utilize potassium-rich ores to produce potash fertilizer for potassium-de ficient countries.
It is difficult to extract potassium at room temperature and atmospheric pressure because of K-feldspar's very stable cage structure[6].Therefore,potassium-extraction processes usually break down the aluminosilicate structure to release potassium ions from KAlSi3O8.Recently,varieties of potassium-extraction processes were proposed.Ma et al.[7]investigated K-feldspar leaching by acid decomposition in the H2SO4-CaF2system with the assistance of ultrasound.Su et al.[8]studied the process of K-feldspar decomposition using a hydrothermal method in mixed alkaline solutions containing NaOH and KOH–H2O medium ata concentration of 8.5 mol?L-1from 240 °C to 280 °C.Bachani et al.[9]screened K-solubilizing bacteria that could increase the K availability from K-Feldspar.Zhang etal.[10]researched ultrasound-assisted extraction of potassium from potassium ore and obtained a potassium recovery rate of 94%.In fact,more researchers are interested in the process of high temperature roasting[6].Despite its higher energy consumption,the technological roasting method is relatively simple,and it does not face problems related to strong acid or alkali corrosion,or difficult wastewater treatments[11–13].
To reduce the reaction temperature,many kinds of additives have been selected for the K-feldspar roasting process,such as Na2CO3,Na2SO4,NaOH,CaCO3,Ca(OH)2,CaCl2,and CaSO4.Ofthese,CaSO4is preferred for use in China.China's output of phosphate fertilizer has steadily been the highest in the world;however,it is associated with an enormous amount of phosphogypsum by-products,which cause serious environmental problems[14].The extraction of potassium from K-feldspar by adding phosphogypsum can prepare potassium sulfate,a quality chloride-free potash fertilizer,to deal with the phosphogypsum[15].
Untilnow,minimalresearch on the extraction ofpotassiumfromthe potash feldspar-CaSO4binary system has been reported.More studies have concentrated on the extraction of potassium from the ternary system of potash feldspar-CaSO4-CaCO3[16–19].Compared with the binary system,adding extra calcium carbonate can reduce the reaction temperature,but the ternary system produces more low-value residues and releases enormous amounts of carbon dioxide.Besides,the understanding of the reaction mechanism is still immature because the reactions are complicated.
Liang et al.[20]proposed a novel K-feldspar technical utilization route of preparing potassium sulfate by roasting K-feldspar with phosphogypsum and mineralizing CO2using potassium-extracted residue.The new process was evaluated and was found to be economically feasible with good environmentalbene fits.In ourresearch,we noticed that the reaction of extracting potassium from K-feldspar with CaSO4roasted at high temperatures is accompanied by the decomposition of CaSO4,which is barely mentioned in other reports related to CaSO4additives.
To clarify the effects of CaSO4decomposition on potassiumextraction and the entire reaction mechanism,we investigated the potassium extraction process for the potash feldspar-CaSO4binary system at various roasting temperatures and CaSO4to K-feldspar ore mass ratios.The K-feldspar ore and CaSO4were pressed into tablets and roasted at various temperatures to obtain the optimized condition for potassium extraction.The reaction mechanism and the behavior of CaSO4decomposition were examined using X-ray diffraction(XRD),thermogravimetric(TG)analysis,and scanning electron microscopy(SEM).
2.Experimental
2.1.Materials
The raw K-feldspar ore was obtained from Baoxing,Sichuan Province,China.After crushing and milling,the K-feldspar ore was ground to 74-μm granularity and dried at 110 °C for 24 h.The chemical composition of K-feldspar ore is shown in Table 1.The other reagents used for the analysis were pure reagents(CaSO4as anhydrous CaSO4).
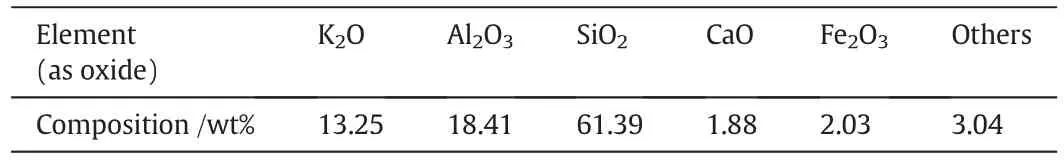
Table 1 Chemical composition of K-feldspar ore
Fig.1 presents the XRD analysis of the raw K-feldspar ore.According to the chemicalcomposition and XRDanalysis,itcan be inferred thatthe contents of KAlSi3O8and SiO2are 78.4%and 10.69%,respectively.
2.2.Methods
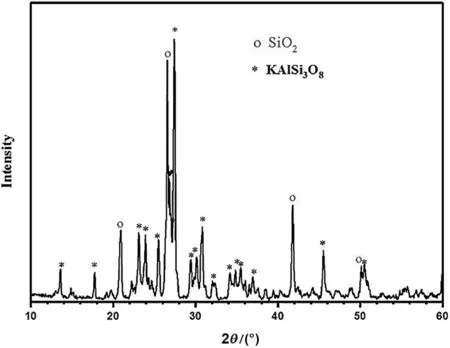
Fig.1.The XRD pattern of raw K-feldspar ore.
The raw K-feldsparore was mixed with CaSO4ata constantratio and then pressed into a tablet(diameter of 13 mm,thickness of 5 mm)at a pressure of 6 MPa using a powder tablet press(HW-01,Jinwei,Tianjin,Electronic Instrument Co.,Ltd.).After weighing 6 tablets,the samples were placed in a square porcelain boat and roasted in a high temperature tube furnace(GXL-1500X,Kejing,Hefei,Material Science Co.,Ltd.)for 2 h at different temperature.During the roasting process,the volatile SO2could be absorbed by the alkali solution.After the roasting process,the porcelain boat was removed,cooled,and weighed.Then,the roasted slag was crushed into a powder and leached at a 10:1 liquid to solid ratio at 80°C for 30 min.After filtration,the leachate was used to calculate the potassium extraction rate,and the residue was used to characterize the roasted slag.
The extraction rate ofpotassium(%)was estimated using the following equation:

where m1is the mass ofK-feldsparore in the reactants,αis the K2Ocontentin the K-feldspar ore(wt%),and m2is the mass ofhydrosoluble K2O in the calcined slag.
The decomposition rate of CaSO4can be calculated as follows:

where X is the decomposition rate of CaSO4,Wris the loss quality of the samples after burning,and Wpis the quality of raw CaSO4in the ingredients.
2.3.Analysis and characterization
The K-feldspar ore was mixed with sodium hydroxide and sodium peroxide in a Ni crucible,melted in a muf fle furnace at 750°C for 10 min,and then dissolved in a mixed acid(HCl and H2SO4)solution.The leaching rate of potassium was analyzed by the potassium tetraphenylborate gravimetric method.
XRD(DX-2007,Fangyuan Instrument Factory,China)was used to confirm the mineral phase of the samples using Cu Kαradiation(λ =0.154056 nm)and a scan rate of 1(°)·min-1from 20°to 60°.The surface morphologies of the samples were characterized by SEM(Hitachi S-4800,Japan).The elemental constitution of the different samples was analyzed by energy-dispersive X-ray spectroscopy(EDS;Oxford IE 250,England).TG(Hengjiu Science Instrument Thermogravimetric,China)was performed with N2(50 ml·min-1)at a heating rate of 10 °C·min-1.

Fig.2.Morphology of the roasted samples at a mass ratio of K-feldspar ore to CaSO4 of 1:2(a-800 °C,b-900 °C,c-1000 °C,d-1100 °C,e-1150 °C,f-1175 °C,g-1200 °C,h-1225 °C,i-1250 °C,and j-1300°C).
3.Result and Discussion
3.1.Characterization of the roasting products
Fig.2 presents the micromorphology of the roasted samples at different roasting temperatures.From 800 °C to 1100 °C,there are no differences between the raw material and roasted samples,which confirms that K-feldspar ore and CaSO4cannotreactin this temperature range.However,the roasted samples decompose from 1150°C to 1200°C.The decomposition of CaSO4releases gas;therefore,it can cause volume expansion and cracks on the surface.At a roasting temperature of 1200°C,a white shell is visible on the cracked profile material.At 1250°C,the local area of the product is smelting and the shape gradually disappears.Above 1250°C,the tablets melt,and the molten product is clearly layered.Because of different densities,the white product is on the surface and the yellow-green product sinters on the bottom of the porcelain boat.We infer that the eutectic temperature of K-feldspar ore and CaSO4 is 1200°C.A higher roasting temperature provides smelting conditions,which are not conducive to the reaction.

Fig.3.SEM analysis of the samples(a)roasted residues at 1200 °C,(b)leaching residues at 1200 °C,(c)product on the upper layer of the roasted samples at 1300 °C,and(d)lower layer of the roasted samples at 1300°C.

Fig.4.TG curves of raw K-feldspar ore,CaSO4,and K-feldspar ore-CaSO4.
Fig.3(a)and(b)shows the SEM analysis of the roasted samples and leaching residue at 1200°C.Fig.3(c)and(d)shows the different products of the roasted samples at 1300°C.Fig.3(a)shows three different morphological substances in the roasted samples at 1200°C.EDS analysis confirms that the white striped aggregates are K2Ca2(SO4)3,the layered particles are silicate,and the other may be CaSO4.Fig.3(b)shows that the white striped aggregates disappear after leaching.Fig.3(c)shows the white product on the upper layer,which is in the shape of a strip or powdery small particles,and is confirmed as K2Ca2(SO4)3by EDS analysis.The yellow-green product on the bottom layer is very hard.Fig.3(d)indicates that it is a sintered melt containing Si,Al,and Ca.
3.2.TG analysis of K-feldspar ore and CaSO4
Fig.4 shows the TG curve of raw K-feldspar ore,CaSO4,and K-feldspar ore-CaSO4.The TGcurves of K-feldsparore and CaSO4show that no mass loss occurs from room temperature to 1200°C,which indicates that neither decomposes in this temperature range.When the mass ratio of K-feldspar ore to CaSO4is 1:2,the TG curve shows an obvious mass loss near 1000°C,which reaches a mass-loss ratio of approximately 55%at 1400°C.The reaction temperature of the K-feldspar ore-CaSO4system is inferred as 1000°C,and the decomposition rate of CaSO4increases with increasing temperature.
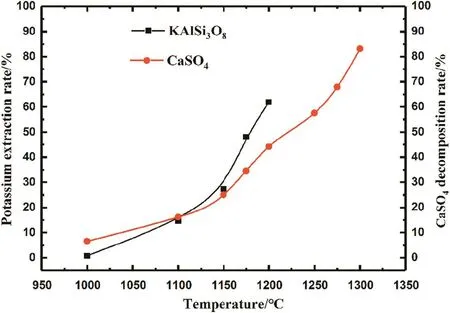
Fig.5.Effect of roasting temperature on the potassium extraction rate and CaSO4 decomposition rate.
3.3.Effect of roasting temperature on the reaction of the K-feldspar ore-CaSO4 system
The in fluence of roasting temperature on the potassium extraction rate and CaSO4decomposition rate was examined from 800°C to 1300°C(Fig.5).The potassium extraction rate is zero from approximately 800–1000 °C;therefore,Fig.5 only shows the reaction range of 1000–1300 °C.The reaction of the K-feldspar ore-CaSO4system begins at 1000°C;with increasing temperature,the extraction rate of K increases.At 1200°C,the potassium extraction rate is 62%.However,the decomposition ofCaSO4accompanies the K-feldspar ore-CaSO4reaction system.Approximately half of CaSO4is decomposed at 1200°C.Above 1200°C,the reaction product is sintered,and we cannot calculate the extraction rate of K.Meanwhile,CaSO4continues to decompose with increasing temperature and reaches a decomposition rate of 83.1%at 1300°C.
3.4.XRD analysis of the roasted samples
Fig.6 shows the XRD analysis of the roasted residues at different temperatures.At 1000°C,the roasted residues contain KAlSi3O8and CaSO4,which is the same as the raw material and indicates that the reaction is not obvious.At 1100°C,new phases of K2Ca2(SO4)3,CaSiO3,and CaAl2Si2O8are visible.With increasing temperature,the diffraction peak of CaSO4decreases.At 1300°C,the XRD analysis shows that the white product on the roasted sample surface is K2Ca2(SO4)3.
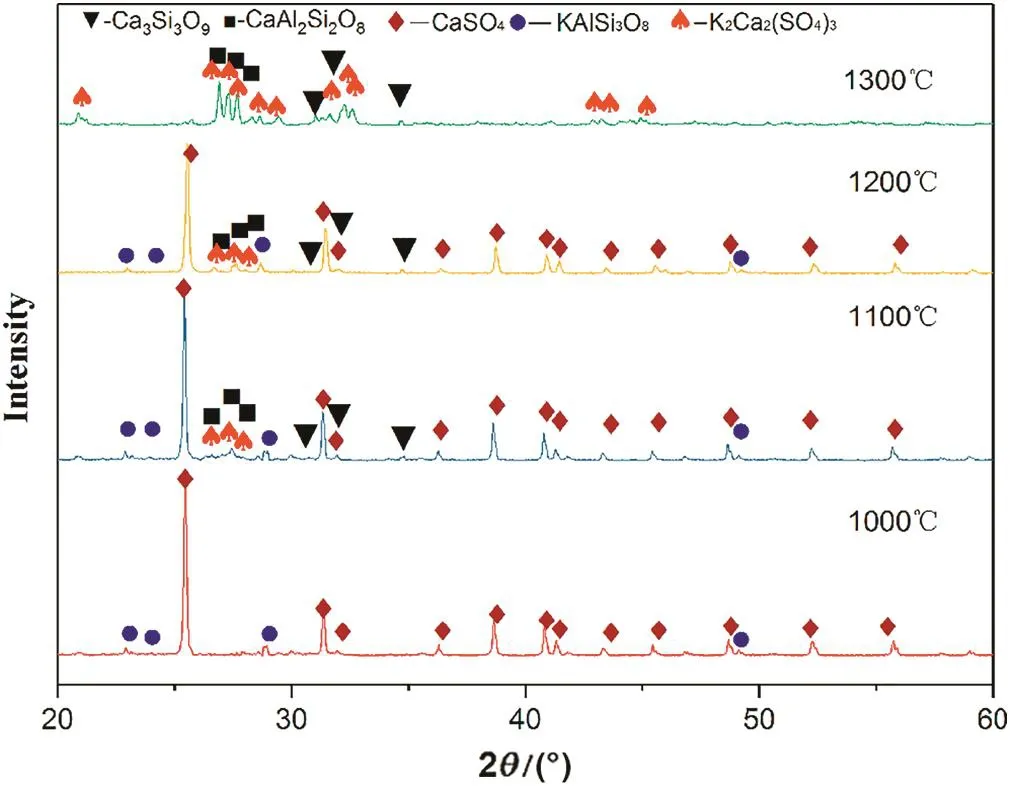
Fig.6.XRD analysis of the roasted residues at different temperatures.
According to the reaction products,it can be inferred that the composition of the Si-Al-O structure in K-feldspar is not destroyed during the roasting process;rather,ion exchange of Ca2+in CaSO4and K+in KAlSi3O8occurs and generates CaAl2Si2O8and K2SO4,respectively.Because the melting point of K2SO4is 1069°C,the product K2SO4may react with the CaSO4and form a K2Ca2(SO4)3solid solution above 1100°C.Wang et al.[21]proposed the following chemical reaction:
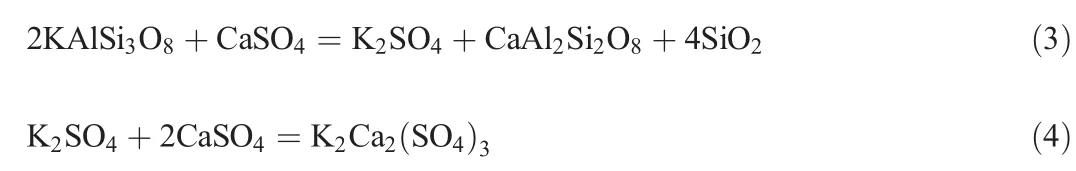
Fig.7 shows the XRD analysis of roasted residue and leaching residue.Diffraction peaks of K2Ca2(SO4)3are presentforthe roasted residue but not for the leaching residue,which indicates that K2Ca2(SO4)3shows a good solubility.Additionally,the potassiumsaltcan be obtained directly by water leaching.
Fig.7.XRD analysis of roasted residue and leaching residue at 1200°C(a-roasted residue and b-leaching residue).
According to Eq.(3),partial SiO2is dissociated from K-feldspar structure during the ion exchange process.However,SiO2diffraction peaks are not observed in Fig.6,but Ca3Si3O9peaks are seen.It is inferred that the generated SiO2reacts with CaSO4,which is shown as Eq.(5).

A white precipitate,which cannot be dissolved by HNO3,is observed when the released gas at 1200°C is absorbed by the H2O2solution and Ba(NO3)2solution is added.is inferred to be present in the solution.It is manifested that SO2gas is released from the decomposition of CaSO4.A thermodynamics calculation confirms that pure CaSO4can only decompose above 1700°C[22].Apparently,the addition SiO2to CaSO4can reduce the decomposition temperature of CaSO4.According to Eq.(3),the reaction of generated SiO2with CaSO4is conducive to extract potassium from K-feldspar ore.However,the decomposition of CaSO4reduces the concentration ofwhich leads to a lower extraction rate of K.
Fig.8.Morphology of CaSO4-SiO2 at different temperatures(a-1200 °C and b-1300 °C).

Fig.9.SEM analysis of roasted CaSO4-SiO2 at different temperatures(a-1200 °C and b-1300 °C).
3.5.Effect of SiO2 and CaSO4 on K extraction
To investigate the effect of SiO2and CaSO4on the potassium extraction process,an experiment was conducted with a 2:1 mass ratio of CaSO4to SiO2in a mixed tablet with a roasting time of 2 h and at 1200 °C and 1300 °C.Fig.8 shows that the tablets maintain their shapes at 1200 °C and 1300 °C.
Fig.9 presents the SEM analysis of the roasted samples of the SiO2and CaSO4mixture.At 1200°C,the SEM image shows that the roasted samples are mainly composed of relatively dense particles,and the EDS analysis indicates that the particles are composed of CaSO4.The SEM results show that some blowholes emerge on the surface of the CaSO4particles upon reacting with SiO2at 1200°C,which indicates that SO2and O2gases are released from CaSO4.
At 1300°C,the roasted samples show a relatively loose structure of the layered material,and the EDS analysis confirms that it is calcium silicate.At a mass ratio of CaSO4to SiO2of 2:1,CaSO4is already decomposing at 1200°C,and up to 98%of CaSO4is decomposed at 1300°C.
Fig.10 shows the XRD analysis of the roasted samples.Relatively weak diffraction peaks of Ca3Si3O9are observed at 1200°C.The composition of CaSO4disappears at 1300°C.The XRD analysis shows the reaction product at 1300°C is composed of SiO2,Ca3Si3O9,and Ca2SiO4.The reaction can be expressed as follows:

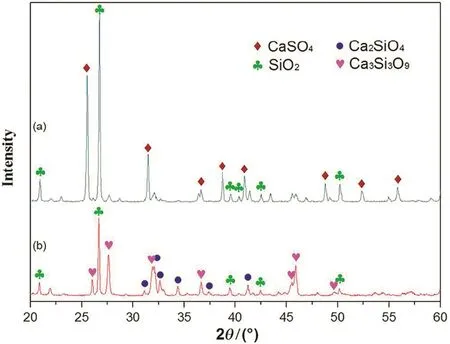
Fig.10.XRD analysis of roasted CaSO4-SiO2 at different temperatures(a-1200°C and b-1300°C).

According to the loss on ignition,the decomposition rate of CaSO4is 23.8%at 1200 °C and 98.3%at 1300 °C,which indicates the CaSO4is completely decomposed.
3.6.Effect of CaSO4 decomposition on potassium extraction from the K-feldspar ore
Fig.11 shows the potassium extraction rate at 1200°C and 2 h at different mass ratios of K-feldspar ore to CaSO4.As the mass ratio of CaSO4increases,the K extraction rate increases.During the roasting process,CaSO4releases Ca2+and exchanges K+from the K-feldspar ore;however,it may also react with SiO2and form Ca3Si3O9.Itcould occupy some Ca2+,but this would cause the loss of SO42-,which would hinder K2SO4generation.
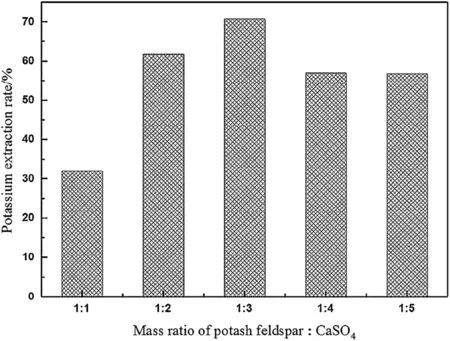
Fig.11.Potassium extraction rate at different mass ratios of K-feldspar ore to CaSO4.
Based on the above discussion,the decomposition mechanism of CaSO4during potassium extraction from K-feldspar ore could be described in Fig.12.Therefore,the extraction rate of potassium can increase when the amount of CaSO4increases by an appropriate amount.However,when the ratio of CaSO4is too large,it may release more SO2and decrease the reactant contact area,which would negatively affect the reaction.
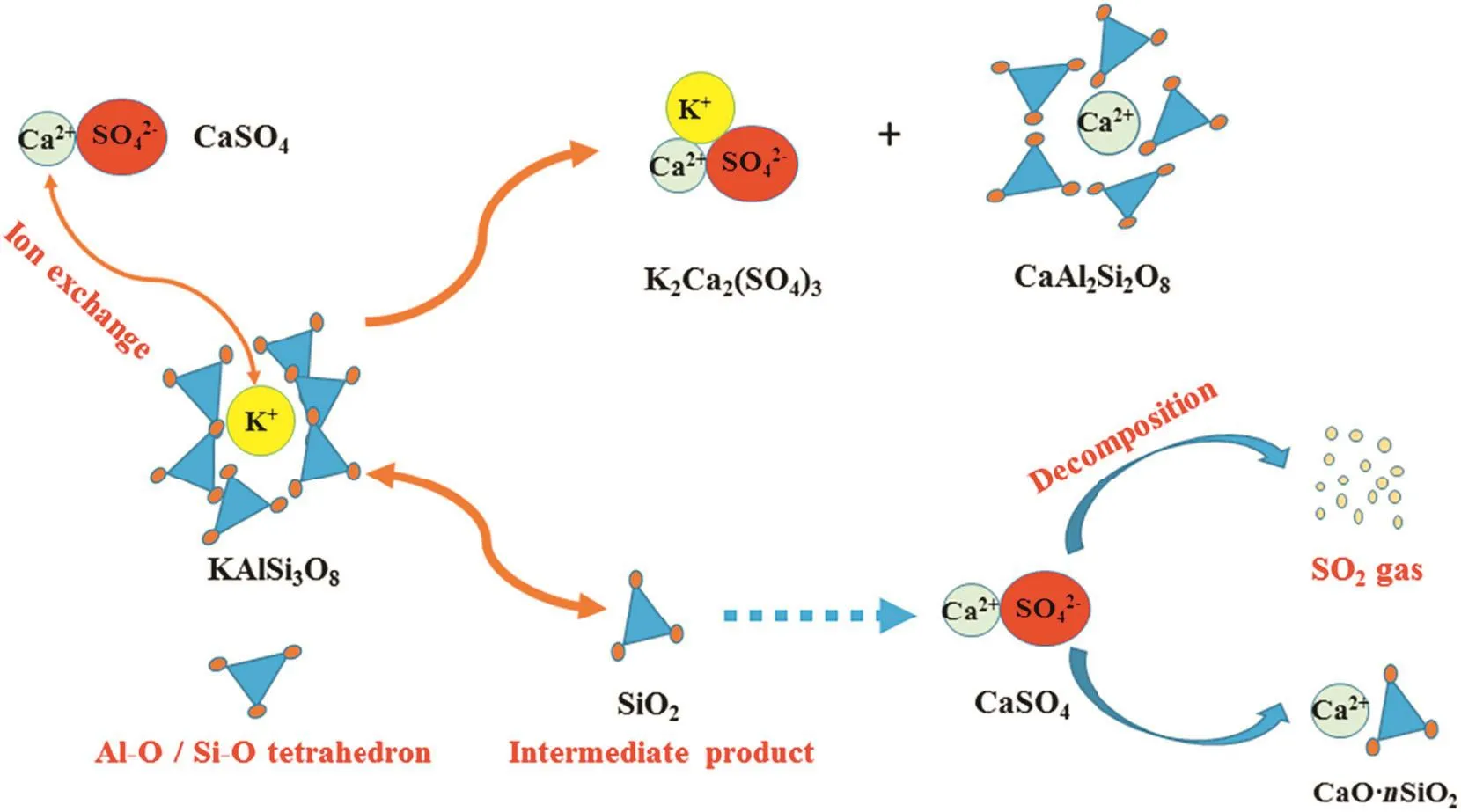
Fig.12.Decomposition mechanism of CaSO4 during potassium extraction.
4.Conclusions
A novel process of roasting and leaching a tablet mixture of K-feldspar ore and CaSO4was proposed,and the effect of the decomposition behavior of CaSO4on the potassium extraction was discussed in depth.K-feldspar ore and CaSO4were pressed into tablets and roasted at various temperatures to obtain an optimized process for potassium extraction.The results show thatthe reaction of K-feldspar ore-CaSO4occurred above 1000°C.With increasing temperature,the decomposition rate of CaSO4shows an increasing trend.At a 1:3 mass ratio of K-feldspar ore to CaSO4at 6 MPa,2 h,and 1200°C,the extraction rate of potassium reached 62%and the decomposition rate of CaSO4was 44%.When the temperature was above 1200°C,samples were sintered,which had a negative effect on potassium extraction.Additionally,CaSO4continued to decompose,and a decomposition rate of 83.1%was obtained at 1300°C.At a 2:1 mass ratio of CaSO4to SiO2,CaSO4had already decomposed at 1200°C,and up to 98%of CaSO4was decomposed at 1300°C.When K-feldspar ore-CaSO4was roasted for potassium extraction,hydrosoluble mischcrystal K2Ca2(SO4)3was produced by ion exchange of K+and Ca2+.Intermediate SiO2could reactwith CaSO4and release SO2.The decomposed CaSO4would decrease the potassiumextraction rate.The extraction rate ofpotassium could be increased when the amountofCaSO4increased appropriately.However,when the ratio of CaSO4was too large,it could release more SO2and decrease the reactant contact area,which negatively affected the reaction.The SO2released from CaSO4decomposition is effectively recycled.
References
[1]D.Cordell,J.O.Drangert,S.White,The story of phosphorus:global food security and food for thought,Glob.Environ.Chang.19(2009)292–305.
[2]A.Dixit,The map-based sequence of the rice genome,Nature 436(2005)793–800.
[3]N.D.Mueller,J.S.Gerber,M.Johnston,D.K.Ray,N.Ramankutty,J.A.Foley,Closing yield gaps through nutrient and water management,Nature 490(2012)254–257.
[4]W.Shangguan,J.Song,H.Yue,S.Tang,C.Liu,C.Li,B.Liang,H.Xie,An efficient milling-assisted technology for K-feldspar processing,industrial waste treatment and CO2mineralization,Chem.Eng.J.292(2016)255–263.
[5]B.Yuan,C.Li,B.Liang,L.Lu,H.Yue,H.Sheng,L.Ye,H.Xie,Extraction of potassium from K-feldspar via the CaCl2calcination route,Chin.J.Chem.Eng.23(2015)1557–1564.
[6]S.K.Jena,P.K.Misra,B.Das,Studies on extraction of potassium from feldspar by roast-leach method using phosphogypsum and sodium chloride,Miner.Process.Extr.Metall.Rev.37(2016)323–332.
[7]J.Ma,Y.Zhang,Y.Qin,Z.Wu,T.Wang,C.Wang,The leaching kinetics of K-feldspar in sulfuric acid with the aid of ultrasound,Ultrason.Sonochem.35(2016)304–312.
[8]S.Su,H.Ma,X.Chuan,Hydrothermal decomposition of K-feldspar in KOH-NaOH-H2O medium,Hydrometallurgy 156(2015)47–52.
[9]P.Bachani,S.Bhattacharya,D.Jain,S.K.Patidar,R.Soundarya,S.R.Tirkey,B.Ranawat,S.V.V.Bharadwaj,S.Mishra,Bioprospecting of halotolerant bacterial isolates for potassium recovery from K-feldspar,Chem.Eng.Technol.39(2016)1645–1652.
[10]Y.Zhang,J.Ma,Y.Qin,J.Zhou,L.Yang,Z.Wu,T.Wang,W.Wang,C.Wang,Ultrasound-assisted leaching of potassium from phosphorus-potassium associated ore,Hydrometallurgy 166(2016)237–242.
[11]S.Bhattacharya,P.Bachani,D.Jain,S.K.Patidar,S.Mishra,Extraction of potassium from K-feldspar through potassium solubilization in the halophilic Acinetobacter soli(MTCC 5918)isolated from the experimental salt farm,Int.J.Miner.Process.152(2016)53–57.
[12]S.K.Liu,C.Han,J.M.Liu,H.Li,Hydrothermal decomposition of potassium feldspar under alkaline conditions,RSC Adv.5(2015)93301–93309.
[13]S.Yin,G.Lin,S.Li,J.Peng,L.Zhang,Application of response surface methodology for optimization of parameters for microwave heating of rare earth carbonates,High Temp.Mat.Pr-Isr 35(2016)813–820.
[14]Z.Duan,J.Li,T.Li,S.Zheng,W.Han,Q.Geng,H.Guo,In fluence of crystal modi fier on the preparation of α-hemihydrate gypsum from phosphogypsum,Constr.Build.Mater.133(2017)323–329.
[15]Y.Zhang,E.Asselin,Z.Li,Laboratory and pilot scale studies of potassium extraction from K-feldspar decomposition with CaCl2and CaCO3,J.Chem.Eng.Jpn 49(2016)111–119.
[16]X.Ma,H.Ma,J.Yang,P.Zhang,M.Liu,F.Lin,Review on preparation of potassium sulfate in system of KAlSi3O8-CaSO4-CaCO3by calcination process,Chem.Miner.Process.44(4)(2015)55–61.
[17]M.Y.Bakr,A.A.Zatout,M.A.Mouhamed,Orthoclase,Gypsum and Limestone for Production of Aluminum Salt and Potassium Salt,1979 34–35.
[18]R.Nayak,J.R.Rao,A.Suryanarayana,B.B.Nayak,Alkaline Roasting as a Route for Extraction of Potassium from Feldspar,1997 173–175.
[19]H.Tayibi,M.Choura,F.A.López,F.J.Alguacil,A.Lópezdelgado,Environmental impact and management of phosphogypsum,J.Environ.Manag.90(2009)2377–2386.
[20]B.Liang,W.Chao,H.Yue,L.I.Chun,H.Xie,Evaluation for the process of mineralization of CO2using natural K-feldspar and Phosphogypsum to produce sulfate potassium,J.Sichuan Univ.46(2014)168–174.
[21]C.Wang,H.R.Yue,C.Li,B.Liang,J.H.Zhu,H.P.Xie,Mineralization of CO2using natural K-feldspar and industrial solid waste to produce soluble potassium,Ind.Eng.Chem.Res.53(2014)7971–7978.
[22]R.Li,C.Li,H.Yue,B.Liang,L.Lv,Y.Wang,Effect of temperature schedule on extracting potassium by calcining,reductive deculturation and CO2mineralization in the system of potash feldspar-CaSO4,J.Chem.Miner.Process.(2017)20–24.
 Chinese Journal of Chemical Engineering2018年4期
Chinese Journal of Chemical Engineering2018年4期
- Chinese Journal of Chemical Engineering的其它文章
- An innovative design of septic tank for wastewater treatment and its performance evaluation:An applicable model for developing countries
- Oil field produced water treatment in internal-loop airlift reactor using electrocoagulation/ flotation technique
- From pollutant to solution of wastewater pollution:Synthesis of activated carbon from textile sludge for dye adsorption
- 17α-Ethinylestradiol removal from water by magnetic ion exchange resin☆
- Transesteri fication of sun flower oil in microchannels with circular obstructions
- The extraction of potassium from K-feldspar ore by low temperature molten salt method☆
