Environmentally Safe Surfactants in Cosmetic/Detergent Formulations:Risk of Microbial Contamination and Possible Solutions
Gerardino D’Errico
Department of Chemical Sciences, University of Naples “Federico II”, Italy
Introduction
Environmental concerns has become one of the main factors in driving the design of new cosmetic and detergent formulations. In the last years, the market of green surfactants has steadily increased. However, besides the many advantages these surfactants bring, some problem can arise from an increased susceptibility of these formulations to degradation and microbial contamination during manufacturing and storage. Indeed, cosmetic/detergent products unable to suppress the growth of several microorganisms, represent a potential health hazard. Microbial contamination is one of the major causes for product recalls in the world, especially in developing countries. Particularly,a serious issue, which has to be considered, is the possible microbial contamination of cosmetic/detergent products during their use by consumers, with the consequent health risk.[1]
As an example, cationic surfactants, such as Cetrimonium Chloride (CTAC) and Behentrimonium Chloride (BTAC),have been massively used in hair conditioner formulations.These kind of surfactants are regarded as disinfectants and formulations in which they are included are often considered as “self-preserving”, i.e. not requiring additional preservatives.However, these species are characterized by a strong aquatic toxicity.[2]For this reason, alternative surfactants have been proposed; among them, Behenamidopropyldimethylamine(BAPDA) seems very promising. This substance consists of a C22alkyl chain, an amidopropyl functional group and a dimethyl tertiary amine group (Figure 1).[3]BAPDA is more compatible with the aquatic environment compared to other cationic surfactants and is characterized by a higher biodegradability. The present work aims at investigating the response of formulations based on this kind of surfactants to microbiological contamination, and the effect of preservatives.

Figure 1. Molecular structure of Behenamidopropyldimethylamine(BAPDA)
Particularly, EPR analysis were performed on all formulations to highlight microstructural differences induced by the presence of preservatives; furthermore, microbiologic analysis and a specific biologic test, called Challenge Test, were performed in order to analyze the effectiveness of preservatives, used in both formulations. Our work offers a perspective on preservative efficacy, being not exhaustive on microbiological safety of a cosmetic product. Overall, this depends on a combination of several factors, not only based on the results of the Challenge Test, but also on the formulation characteristics, production conditions and final packaging in accordance with good manufacturing practice (GMP).[4]
Materials and methods
Formulation
We designed a conditioner, hereafter named Formulation I,using the components listed in Table 1.
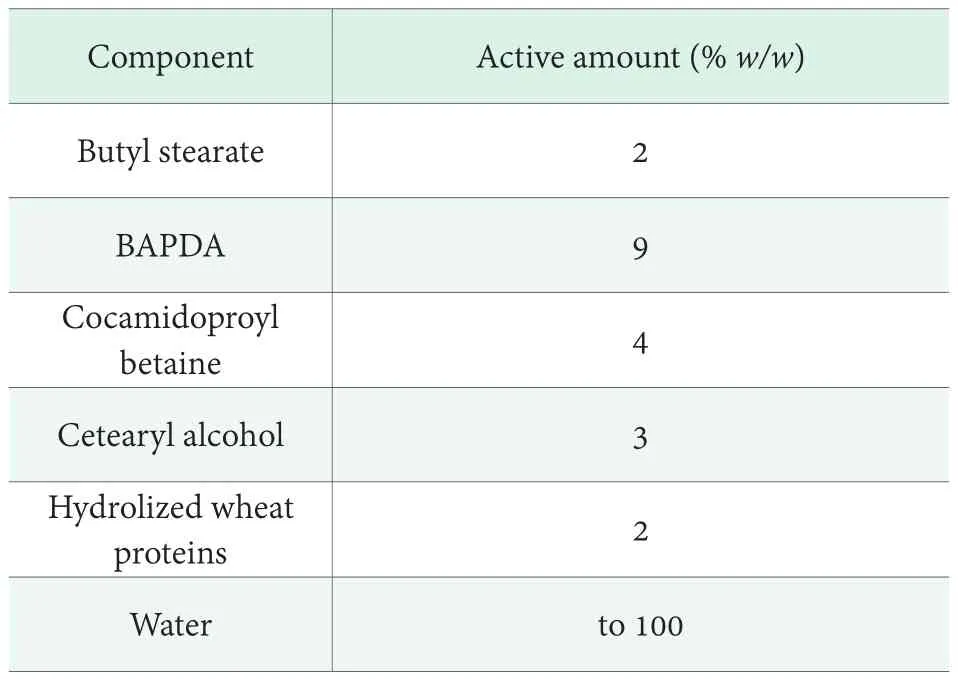
Table 1. Main components of Formulation I
All components were obtained from ACEF (Fiorenzuola d’Arda, PC, Italy) as commercial grade products and were used without further purification. The water amount reported in Table 1 includes water from components supplied as aqueous mixtures and deionized water purposely added to adjust total active concentration.
To avoid microbial contamination of the formulation,different types of preservatives were added. In particular, two kinds of preservatives were considered: the first was a mixture of methylchloroisothiazolinone and methylisothiazolinone(supplied by ACEF), whose exploitation in commercial products is very well assessed; the second was electrolytic colloidal silver,recently patented by Italian researchers, and kindly supplied by the inventors.[5]In order to evaluate if perservatives are actually needed in hair conditioner formulations, Formulation I was prepared both without preservatives and with above mentioned preservatives. Hereafter, the formulation including methylchloroisothiazolinone and methylisothiazolinone is named Formulation I(a); the formulation including colloidal silver is denoted as Formulation I(b); Formulation I without any preservative, used as control, is named Formulation I(c).
Because of their strong aquatic toxicity, the two kinds of preservatives were used in very low concentrations: 0.1% w/w for the mixture of methylchloroisothiazolinone and methylisothiazolinone and 0.05~0.5 mg L-1 for electrolytic colloidal silver with respect to the weight of the cosmetic product.
The formulative procedure is based on the separate preparation of an oil phase and an aqueous phase. The oil phase was preliminarily obtained by mixing butyl stearate,BAPDA and cetearyl alcohol under stirring at 70 °C. Hot distilled water (~70 °C) was slowly added to the oil phase so to have an emulsion O/W. Subsequently the temperature of the mixture was decreased at 40 °C and cocamidopropyl betaine, hydrolyzed wheat proteins and the fragrance were added. The mixture pH was then adjusted to 4.5 with lactic acid.
EPR measurements
The spin probes used for EPR analysis, namely 5-DSA(5-Doxyl-stearic acid, purchased from Sigma—Aldrich) and TEMPOl (4-Hydroxy-TEMPO, Sigma—Aldrich, >97%),were used without further purification. EPR spectra were recorded with a 9 GHz Bruker Elexys E500 spectrometer(Bruker, Rheinstetten, Germany). Samples were placed in 25μ l glass capillaries and flame sealed. The capillaries were placed in a standard 4 mm quartz sample tube containing light silicone oil for thermal stability. All the measurements were performed at 25 °C.
Challenge Test
The evaluation of the effectiveness of a preservative is one of the most critical stages in the formulation process of cosmetic products. We evaluated the in vitro microbiological stability in accordance with the method ISO (International Organization for Standardization) 11930:2012, according to the European Regulation 1223/2009.[6,7]
This ISO method comprises:
— a preservative efficacy test;
— a procedure for evaluating the overall antimicrobial protection of a cosmetic product, based on a risk assessment described in ISO 29621.[8]
The preservative efficacy test, also named Challenge Test,represents one of the most suitable and reliable methods to evaluate the microbiological stability of a cosmetic/detergent product. This test is carried out by contaminating the product with microorganisms from several species and by subsequently assessing the change in the microbial load by plate counting the number of surviving microorganisms at defined intervals during a period of 28 days.
The Challenge Test steps can be summarized as follows:
— preparation of microorganisms for the inoculum;
— inoculation of samples;
— check of surviving microorganisms at pre-determined intervals (7, 14 and 28 days);
— evaluation of the results.
The test is performed using the strains listed in Table 2 as test microorganisms.
In order to perform the test, calibrated microbial suspensions (inocula) having determined concentration at t0were prepared. In more details, the microbial count of inoculum was 107~108CFU/mL for bacteria and 106~107CFU/mL for fungi. Subsequently, the Formulation I samples were contaminated with these inocula and incubated for 28 days; the check of possible reduction of microorganisms by the preserving system were performed after 7, 14 and 28 days from the inoculation.
The method ISO 11930:2012 provides two criteria for the evaluation of the efficacy of the preserving system,based on the reduction of surviving microbes in the product, as summarized in Table 3.
If at the end of the Challenge test, the sample is in accordance with criterion A, the microbiological risk isacceptable and the cosmetic product is considered protect against the microbial proliferation and it is not necessary to consider other factors that are independent from the formulation.

Table 2. Test microorganisms used in the Challenge Test according to the method (ISO 11930:2012)
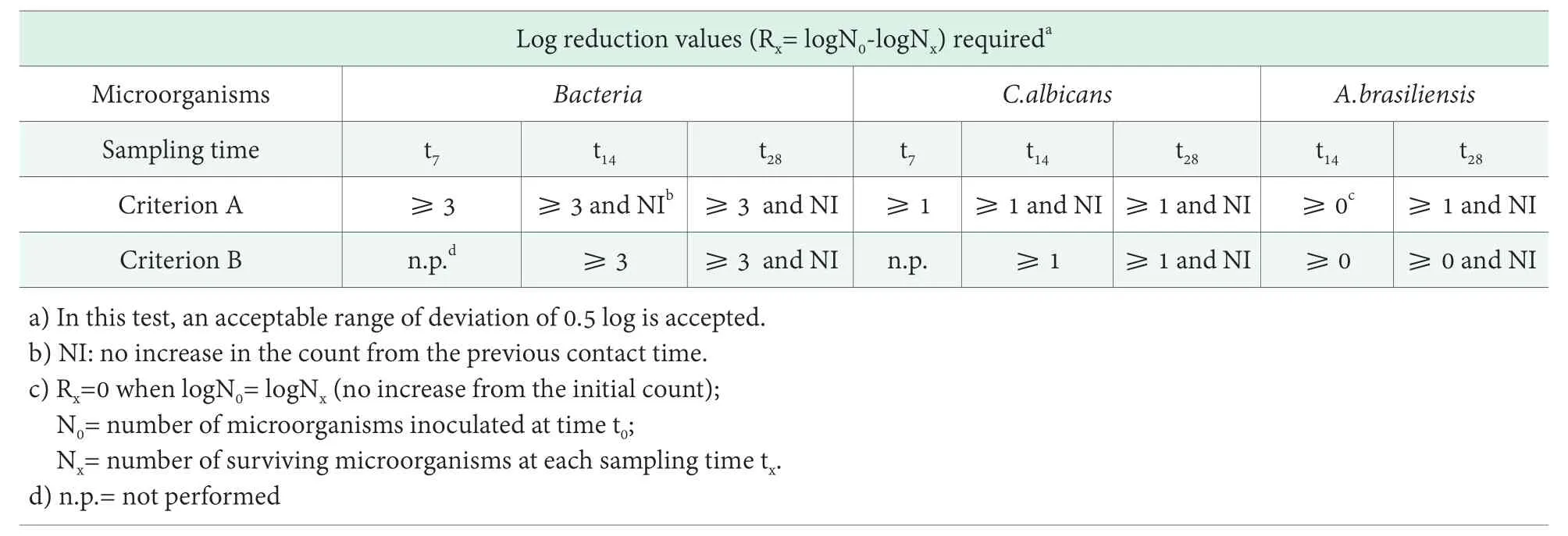
Table 3. Evaluation criteria for the preservation efficacy test (ISO 11930:2012)
On the other hand, if the sample is in accordance with criterion B, the microbiological risk is acceptable,thereby the level of protection is acceptable; if the risk analysis demonstrates, the existence of control factors not related to the formulation, for example, a protective packaging in order to reduce the microbiological risk.
Results and discussion
EPR results
The EPR analysis was performed by using two probes as spin labels: 5-DSA (5-Doxyl-stearic acid) and TEMPOl (4-Hydroxy-TEMPO) (Figure 2).
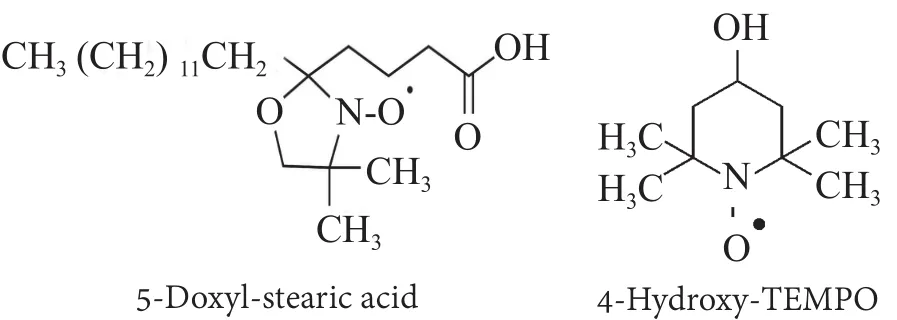
Figure 2. The chemical structure of two nitroxides used as EPR probes
Cyclic nitroxides are often used as spin-probes due to their remarkable stability and to the localization of the spin density on the NO moiety, which allows several information on the probe solubilization site to be extracted from the EPR spectrum. In particular, 5-DSA probe gives information about the structure of the surfactants molecular aggregates in the formulations whereas TEMPOl furnishes information about the aqueous environment, in which these aggregates are located. Both probes were used at a concentration 10-4M and were added to the formulation samples without preservatives and with preservatives.
The comparison among the spectra of 5DSA in Formulation I(a), (b) and (c) is shown in Figure 3. The spectra are similar and the lineshape indicates that in the conditioner, the surfactants form large micellar aggregates.
In particular, the motion of probe is slow and isotropic as indicated by the peaks at low and high field that are broadened.
Finally, the comparison among spectra of TEMPOl probe in conditioner without preservatives and with preservatives is shown in Figure 4.
The motion of probe is isotropic and narrow lines are observed. This result indicates that the aqueous environment in which the probe is located presents low viscosity.
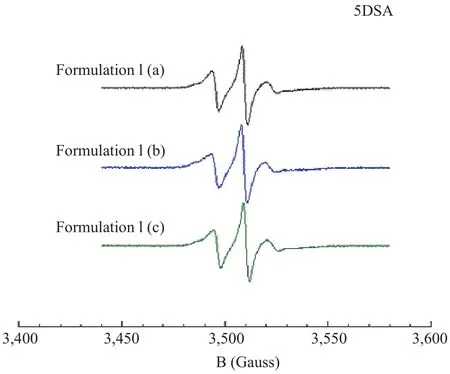
Figure 3. EPR Spectra of 5DSA probe in conditioner Formulation I in the presence, (a) and (b), and in the absence, (c), of preservatives
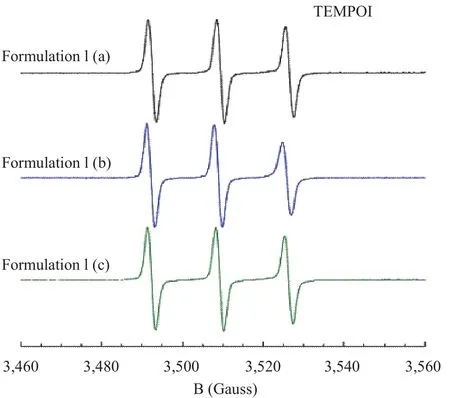
Figure 4. EPR Spectra of TEMPOl probe in conditioner Formulation I in the presence, (a) and (b), and in the absence, (c), of preservatives
The motion of probe is isotropic and narrow lines are observed. This result indicates that the aqueous environment in which the probe is located presents low viscosity.
All these results highlight that both in conditioner formulations, the preservatives do not induce microstructural differences with respect to the samples not preserved.
Microbiologic analysis
Microbiological analysis were performed on the same formulations at the Department of Biological Science of the University “Federico II” in collaboration with research group of Prof. Marco Guida, in order to compare microbial concentration overtime and through the Challenge Test.
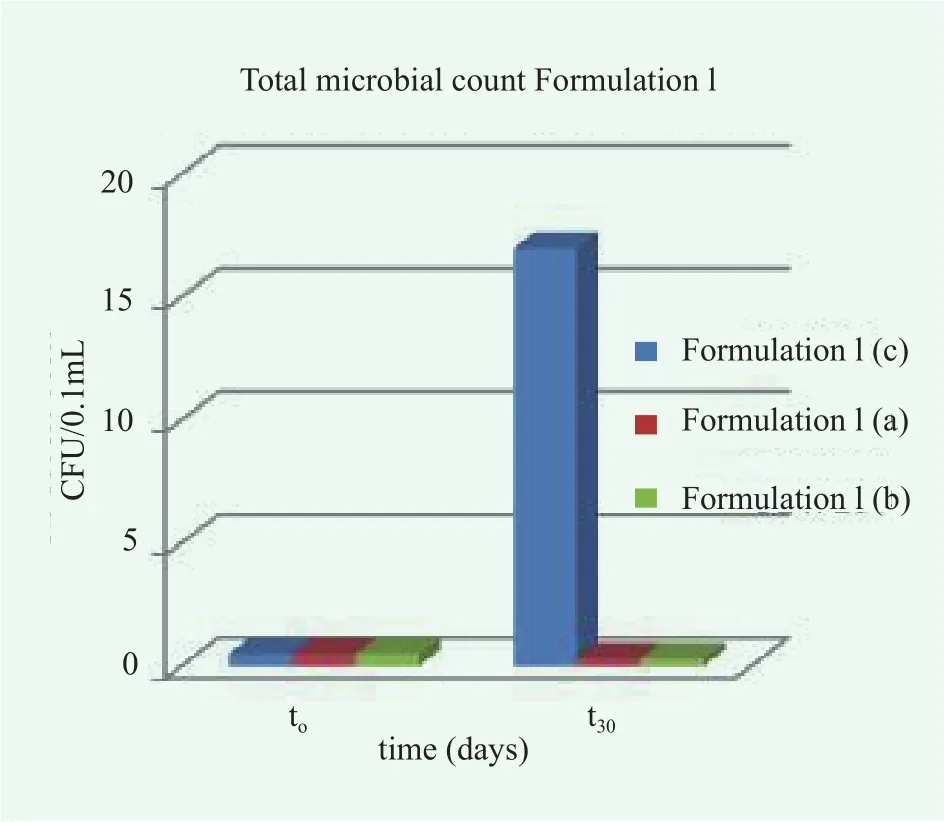
Figure 5. The total microbial count in Formulation I without (c)and with preservatives, (a) and (b)
It is to be noted that since Formulation I was prepared from commercial raw materials and following common industrial protocols, it is not sterile. A total microbial count was carried out at time zero (t0) and after 30 days (t30) both on formulations without preservatives and with methylchloroisothiazolinone and methylisothiazolinone (0.1% w/w) or colloidal silver (0.5 mg L-1).As shown in Figure 5, in the absence of preservatives, microbial growth in Formulation I(c) is observed. In the presence of preservatives, no microbial initial contamination or growth was observed.
Moreover, the Challenge Test was performed not only on these formulations, but also on those preserved with colloidal silver at concentration 0.1 and 0.05 mg L-1with respect to the weight of the cosmetic product. After an appropriate contamination with microorganisms listed in Table 2, the capacity of defending against microbial attack of cosmetic formulations was verified; the reduction of the total viable count was monitored, for each tested microbial strain, within a given time span, according to the criteria of acceptability emanated by ISO 11930:2012.
In order to satisfy the criteria of evaluation of preserving system provided by European Pharmacopoeia 7thedition,[9,10]the microbial count for each tested microbial strain was performed also after 2 days from inoculation, in addition to 7,14 and 28 days. Furthermore, for all conditioner formulations analyzed, the log reduction values were calculated and compared to the minimum values required for evaluation criterion A or B, reported in Table 2.
Regarding to Formulation I samples, the results of Challenge Test do not always satisfy the acceptance criteria.
The trends of microbial concentration during the incubation time in conditioner Formulation I contaminated with S.aureus without preservatives (c), with colloidal silver (b)and with methylchloroisothiazolinone/methylisothiazolinone(a) are shown in Figure 6.

Figure 6. Trends of microbial concentration vs. time in conditioner Formulation I contaminated with S.aureus without preservatives, (c), with colloidal silver at different concentration (b) and with methylchloroisothiazolinone/methylisothiazolinone (a)
In this case, the reduction of microbe population over time is satisfactory in all cases. Different results were obtained for the samples of conditioner contaminated with P.aeruginosa, E.coli, A.brasiliensis and C.albicans, as shown in Figures 7~10.
Overall, the conditioner without preservatives, Formulation I(a), fails the Challenge Test since in many cases the acceptance criteria are not satisfied. Particularly, for P.aeruginosa, E.coli and A.brasiliensis, neither Criterion A nor Criterion B of the method ISO 11930:2012 are fulfilled. In the case, C.albicans,even though the limits for acceptability are reached, an evident microbe contamination persist over time. A preservative,either methylchloroisothiazolinone methylisothiazolinone or colloidal silver, is required to avoid microbe proliferation and growth. In the case of colloidal silver, the effect of the preservative concentration was also investigated. In some cases the lowest considered concentration was not sufficient to completely suppress in few days the microbe proliferation, pointing to the need of a correct dosage of preservatives in the formulation.
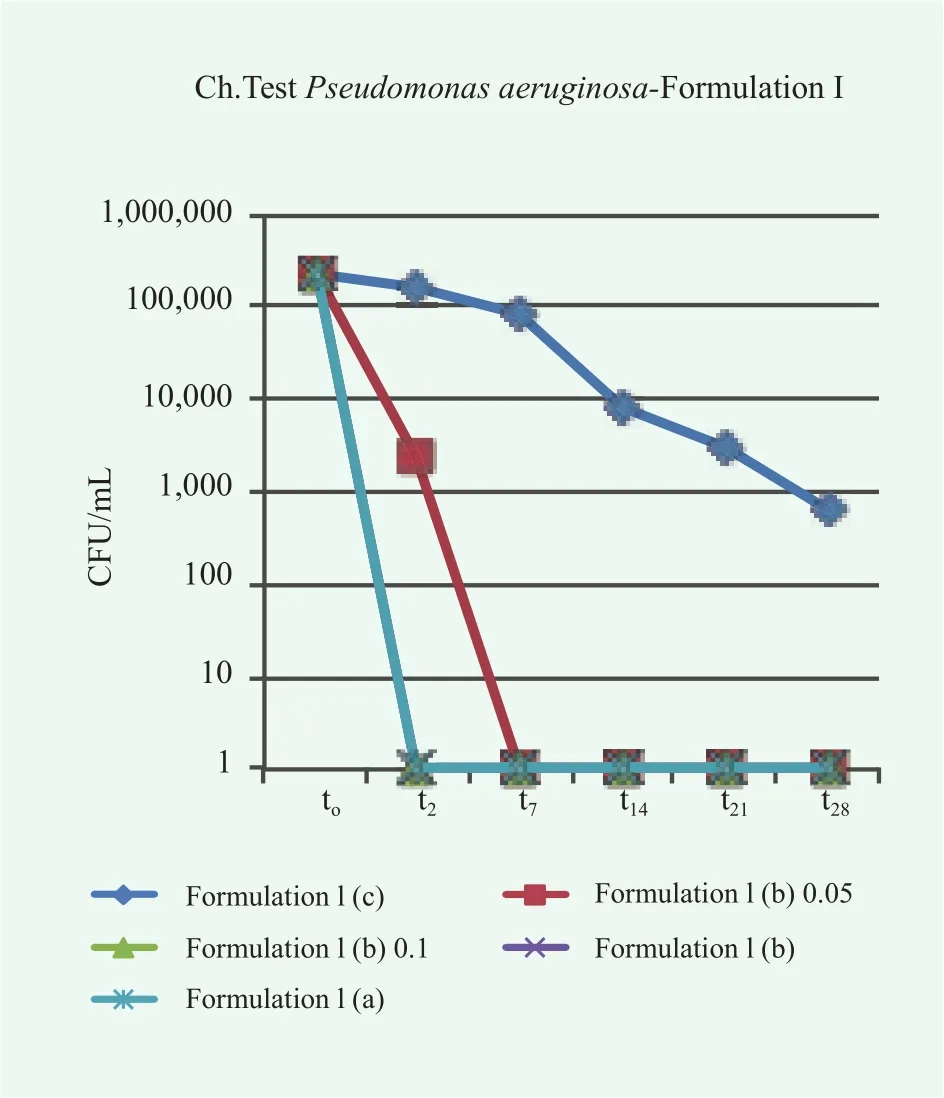
Figure 7. Trends of microbial concentration vs. time in conditioner Formulation I contaminated with P. aeruginosa without preservatives, (c), with colloidal silver at different concentration (b) and with methylchloroisothiazolinone/methylisothiazolinone (a)
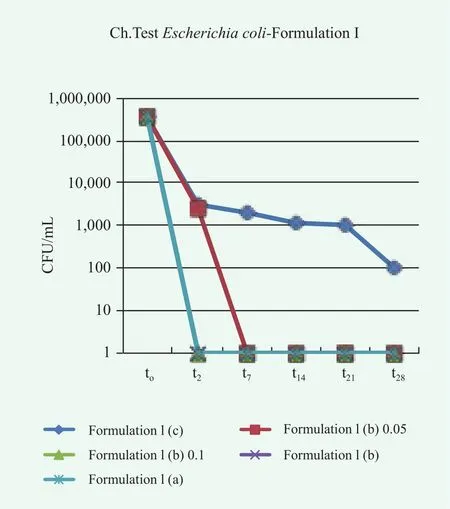
Figure 8. Trends of microbial concentration vs. time in conditioner Formulation I contaminated with E.coli without preservatives, (c), with colloidal silver at different concentration (b) and with methylchloroisothiazolinone/methylisothiazolinone (a)
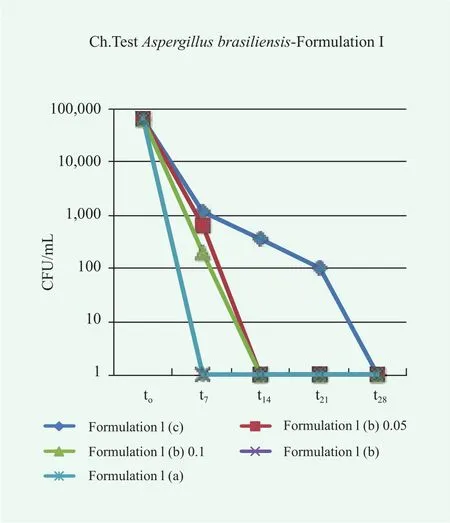
Figure 9. Trends of microbial concentration vs. time in conditioner Formulation I contaminated with A.brasiliensis without preservatives, (c), with colloidal silver at different concentration (b) and with methylchloroisothiazolinone/methylisothiazolinone (a)
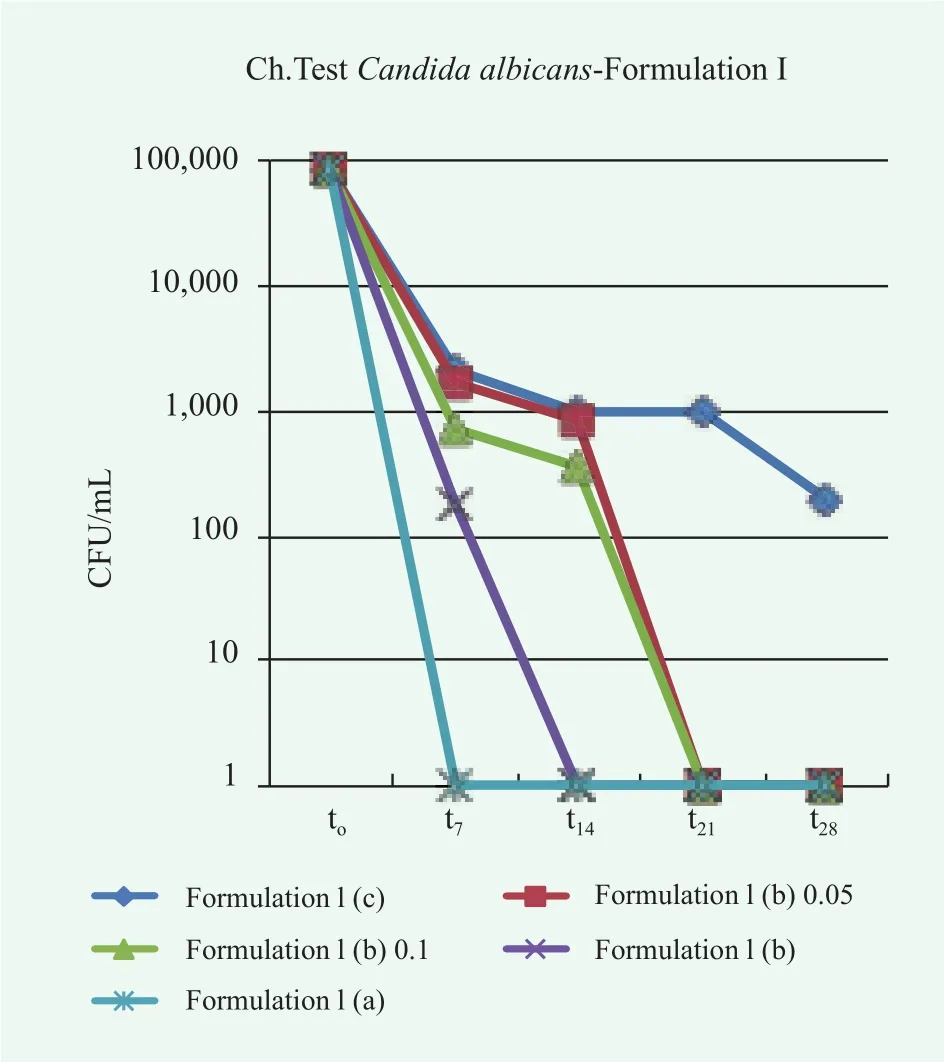
Figure 10. Trends of microbial concentration vs. time in conditioner Formulation I contaminated with C. albicans without preservatives, (c), with colloidal silver at different concentration (b) and with methylchloroisothiazolinone/methylisothiazolinone (a)
Conclusions
The EPR results show that the preservatives do not change the microstructural properties of conditioner formulations. The surfactant mixtures form small micellar aggregates surrounded by a relatively unaffected aqueous medium.
Besides, the microbiological analyses, and in particular,the Challenge Test highlights that the presence of preservatives is fundamental in the conditioner formulations. This result,probably due to the high content of water, points to the need of a careful health safety assessment for formulations based on eco- and bio-compatible components.
[1] Campana R.; Scesa C.; Patrone V.; Vittoria E.; Baffone W.Microbiological study of cosmetic products during their use by consumers: health risk and efficacy of preservative systems. letters in applied microbiology 2006, 43, 301—306.
[2] Ying G G. Fate, behavior and effects of surfactants and their degradation products in the environment. Environment International 2006, 32, 417—431.
[3] Yamane M.; Toyo T.; Inoue K.; et al. Aquatic toxicity and biodegradability of advanced cationic surfactant APA—22 compatible with the aquatic environment. Journal of Oleo Science 2008, 57, 529—538.
[4] ISO 22716: Cosmetics—Good Manufacturing Practices (GMP)—Guidelines on Good Manufacturing Practices, 2007.
[5] Zago C.; Galiano F. Preservative cosmetic composition comprising electrolytic colloidal silver. Eur. Pat. Appl., EP 2018839 A1 20090128,2009.
[6] ISO 11930: Cosmetics—Microbiology—Evaluation of the Antimicrobial Protection of A Cosmetic Product, 2012.
[7] Regulation (EC) N° 1223/2009: The cosmetic products regulation.[8] ISO 29621: Cosmetics—Microbiology—Guidelines for the Risk Assessment and Identification of Microbiologically Low—Risk Products, 2010.
[9] Siegert W. ISO 11930—A comparison to other methods to evaluate the efficacy of antimicrobial preservation. sofw journal 2012, 138,44—53.
[10] European Pharmacopoeia 7thedition, 5.1.3. Efficacy of Antimicrobial Preservation, 2010.
 China Detergent & Cosmetics2017年1期
China Detergent & Cosmetics2017年1期
- China Detergent & Cosmetics的其它文章
- Are you ready to join in the ITP 2017 ?
- Regulatory Status of Wet Wipes Used on Human Body in Europe,United States, Canada, Australia and China
- Introduction to Technical Safety Standards for Cosmetics
- China National Standard—Test Method for Biodegradability of Surfactants (GB/T I5818—2006)
- For Beauty — JALA Group are Here to You
- Development of Chinese Kids Toothpaste Market
