Effects of Feeding Time on the Growth Performance and Variation of RNA/DNA Ratio of the Chinese Soft-shelled Turtle, Pelodiscus sinensis
Yingguang JI, Haiyan LIU, Zhe SONGand Zhencai YANG*
1College of Life Science, Hebei Normal University, Shijiazhuang 050024, Hebei, China
2Hebei Chemical and Pharmaceutical College, Shijiazhang 050026, Hebei, China
Effects of Feeding Time on the Growth Performance and Variation of RNA/DNA Ratio of the Chinese Soft-shelled Turtle, Pelodiscus sinensis
Yingguang JI1,2, Haiyan LIU1, Zhe SONG1and Zhencai YANG1*
1College of Life Science, Hebei Normal University, Shijiazhuang 050024, Hebei, China
2Hebei Chemical and Pharmaceutical College, Shijiazhang 050026, Hebei, China
The present study was to investigate the effects of feeding time on growth of the Chinese soft-shelled turtle. The experiment was carried out with a photoperiod of 12L (light):12D (dark) with lights on at 06:00 and off at 18:00. Turtles were maintained at a temperature of 32 ± 0.2 °C in tanks throughout the length of the experiment. The turtles in group 1 to group 6 were fed respectively at 00:00, 04:00, 08:00, 12:00, 16:00 and 20:00 with 60 turtles each (Initial body weight 88.27 ± 0.09 g). Acrophases of postprandial RNA/DNA ratio in liver in each group was shown between 5h and 7h after feeding. A positive linear correlation could be seen between specific growth rate (SGR) and RNA/ DNA:SGR=1.1586RNA/DNA–0.7097 (r = 0.9328, P = 0.0066). The results indicated that the values at the acrophases of about 6h after feeding might be used as an instantaneous growth index in the Chinese soft-shelled turtle. The turtles in group 1 grew better than group 3, group 4, group 5, group 6, because they ate more, but they ate more and grew slower than group 2, whose feed conversion rate was also higher. Meanwhile, the SGR and feeding rate of turtles fed at 12:00 were the lowest from the six groups (P < 0.05) . Turtles fed in group 1, group 2 and group 6 developed more heavy fnal body weight (FBW), higher feeding rate and SGR than the other three groups. This probably suggested that turtles fed in scotophase grew better than that fed at photophase in total.
Feeding time, Growth performance, Pelodiscus sinensis, RNA/DNA ratio
1. Introduction
Studies have reported that different feeding times in fishes may affect their behavior and physiology (Vera et al., 2013; Boujard et al., 1995). Feeding time might also affect the feed conversion rate (FCR), protein retention, and food utilization (Boujard et al., 1992). Periodic feeding during daytime can affect the daily rhythms of the thyroid hormone and digestive enzymes (Montoya et al., 2010). A scheduled feeding time could help synchronize the expression of several clock genes (Feliciano et al., 2011). It was observed that rainbow trouts (Oncorhynchus mykiss) fed at dawn or in the early morning weighed more than those fed at any other time of the day (Gélineau et al., 1996). Essentially, it plays a very important role in affecting a large number of aquatic species. The lanternfish (Diaphus chrysorhynchus) was observed to prefer feeding at night (Tanaka et al., 2013). Similar results were observed in a study on juvenile Florida pompano (Trachinotus carolinus), in which the fish that fed in the evening were found to have longer body length and food conversion efficiency than the fish that fed in the morning (Heilman et al., 1999). The Chinese soft-shelled turtle (Pelodiscus sinensis), a poikilotherm, is a carnivorous commercial reptile that is widely cultured throughout Asia for its nutritional and medicinal properties. For this turtle, feeding time is an important factor that might impact its growth, a characteristic similar to that observed in rainbow trout (Boujard et al., 1995).
It has been reported that the RNA/DNA ratio is an effective tool to evaluate the nutritional status of released and wild Japanese flounder (Paralichthys olivaceus) juveniles and larval Pacific bluefin tuna (Thunnusorientails) (Gwak et al., 2003; Tanaka et al., 2008). Genetic information is considered to be transmitted as DNA→RNA→Protein; moreover, the concentration of DNA per unit mass is always stable. It has been observed that RNA/DNA ratios varied with the time of the day in Crassostrea angulata, and were higher at night (Chícharo et al., 2001). It was confirmed that RNA/DNA ratios exhibited a signifcantly positive correlation with recent growth rates in young-of-the-year tautog (Tautoga onitis) under field conditions (Mercaldo-Allen et al., 2006). Furthermore, the variation in RNA/DNA ratios in Calanus sinicus refected the radical changes in their growth (Ning et al., 2013). Moreover, signifcantly positive correlations between growth rate and RNA/DNA ratio were observed in Paracentrotus lividus (Frantzis et al., 1992). Therefore, RNA/DNA ratio is a reliable measure to evaluate the instantaneous growth in aquatic species. In Japanese founder, the variation in the RNA/DNA ratio exists while it might be species specifc in fed Crassostrea angulata (Chícharo et al., 2001). These variations could probably be attributed to postprandial rhythms, and could cause serious problems if left unchecked. A correlation between postprandial acrophase values of RNA/DNA ratio and growth might better explain this phenomenon. However, very few studies have reported the effects of feeding time on postprandial RNA/DNA ratio.
Most of the previous studies that evaluated the correlation between RNA/DNA ratio and growth in fshes were performed in a natural or semi-natural environment that was easily affected by several factors such as temperature, illumination etc., and hence, do not explain the exact effect of feeding time on instantaneous somatic growth. Since the results of these studies were conficting, the exact effects of feeding time on the growth of some specific animals remain unclear (Nunes et al., 2006). We used the Chinese soft-shelled turtle as the laboratory animal in the present study.
This study aimed to investigate the effects of feeding time on growth performance and to identify whether the acrophases of postprandial RNA/DNA ratio values exhibited a correlation with growth in the Chinese soft-shelled turtle under well-controlled experimental conditions.
2. Materials and Methods
2.1 Feeding and acclimation of turtlesPowdered feed was provided by Hebei Haitai Technology Corporation, Hebei, China. The feed was processed into small wet pellets (approximately 2 mm in diameter) and stored at ?20°C (21.4% moisture content) until use. Juvenile Chinese soft-shelled turtles (weighing approximately 10 g) were purchased from Shijiazhuang Kangtai Turtle Aquafarm (Luquan, Hebei, China). The experiment was carried out under a photoperiod of 12 h L (light):12 h D (dark), and illumination by fluorescent light (about 70 lux) was provided from 06:00 to 18:00. Air was supplied twice every day at 09:00 and 17:00, for 30 min each. The turtles were maintained at 32 ± 0.2°C in tanks flled with approximately 60 L of aerated water (pH 7.0–8.0; 20% renewal every day). The laboratory and the water in the tanks were sterilized once a week. The turtles were acclimated for 20 days; they were fed to satiation once at 08:00 every day, and the leftover feed was siphoned off.
2.2 Experimental procedureAfter acclimation, a total of 360 turtles with an initial body weight (IBW) of 88.27 ± 0.09 g were divided into 6 treatment groups; each group included 10 replicates, with 6 turtles per replicate. The turtles from treatment group 1 to treatment group 6 were fed at 00:00, 04:00, 08:00, 12:00, 16:00, and 20:00, respectively. The trial duration was 30 days. The experimental conditions were the same as those in the acclimation period. The feed pellets were prepared about 5 minutes before each feeding time, and were manually offered to each tank as a single meal every day. About 30 minutes later, the residual pellets in the tanks were collected, dried, and weighed every time, until the end of the trial. The dry weight of the feed consumed each day during the trial was recorded in 60 replicates. Based on these data, the cumulative consumption (CC) was calculated.
At the end of the experiment, the first sample from each group was collected at its corresponding feeding time. Subsequently, one replicate per group (6 turtles) was collected every hour (Table 1). The possibility of any error in weight measurement was minimized by wiping the water on the surface of the turtle’s body with gauze. The final body weight (FBW) of each turtle was recorded. Based on these data, the weight gain (WG), specific growth rate (SGR), and FCR of each replicate were calculated. The turtles were then euthanized immediately. Their liver samples were preserved in liquid nitrogen immediately after collection and transferred to a laboratory freezer at ?70°C until the measurement of RNA/DNA ratio. Nucleic acids in the liver samples of 3 turtles in each replicate (6 turtles) were randomly selected. DNA in the liver was determined using the Cell and Tissue Genomic DNA Extraction kit (Columntype; Shanghai Generay Biotech Co., Ltd., Shanghai, China), while RNA was determined using the TotalRNA Extraction kit (Trizol-column; Shanghai Generay Biotech). The postprandial RNA/DNA ratios of the replicates in each group after every hour and 9 h after the last feed were determined after cultivation.

Table 1 The order and its corresponding time of the replicatecollection in the turtles of the present research.
2.3 CalculationsData used for analysis were the averages of each individual replicate of the 6 turtles.
(1) Weight gain (WG) (g): FBW (g) ? IBW (g)
(2) Specifc growth rate (% d?1): SGR = 100 × (LN FBW? LN IBW)/t, where t is the duration of the trial
(3) Feeding rate (FR) (%): FR = CC/([IBW + FBW]/2) × 100
(4) Feed conversion ratio: FCR = CC/WG
2.4 Statistical analysisOne-way analysis of variance (ANOVA) was used to analyze the effects of feeding time on WG, SGR, FR, and FCR of growth performance in the Chinese soft-shelled turtle. Duncan’s multiple comparison was used if the differences in the effects between groups were significant. The correlation of SGR with RNA/ DNA ratio was analyzed by linear regression. Data were analyzed using STATISTICA 6.0 (StatSoft). P < 0.05 was considered statistically signifcant.
3. Results
3.1 Effect of feeding time on growth performanceTotal survival rate was 98.9%. Turtles from group 2 gained approximately 52% more weight than those from group 4 (Table 2). There were significant differences in WG (F1,59= 2.582, P < 0.05) and SGR (F1,59= 2.521, P< 0.05) between turtles from group 4 and those from the other 5 groups. The WG and SGR in scotophase were observed to greater than those in photophase. Feeding time had a significant effect on FR in the trial among the groups (F5,54= 4.16, P < 0.05). Turtles fed at 00:00 h exhibited significant differences compared with those fed during the photophase (F5,54= 2.58, P < 0.05). The FR in scotophase was greater than that in photophase. There were no signifcant differences in the FCR among the 6 groups of the Chinese soft-shelled turtle (P > 0.05). The FCR was minimum at dawn (04:00 h; 0.98 ± 0.03 [mean ± S.E]) and maximum at noon (12:00 h; 1.25 ± 0.07 [mean ± S.E.]) (Table 2). The feeding time did affect the FCR in all the turtles; however, the effect did not signifcantly differ between the groups.
In this study, the FR, WG, and SGR in the present research in group 1, group 2, and group 6 were more than those in the other 3 groups, indicating that turtles that fed in scotophase consumed greater feed and exhibited greater growth than turtles that fed in photophase.
3.2 Effect of feeding time on postprandial variations of RNA/DNA ratioThe relationship of linear regression between RNA/DNA ratios and feeding time (hours after feeding) was analyzed. The regression equation was: RNA/DNA = 0.0344T + 2.0596 (F2,160= 6.395, P = 0.0124, T = hours after feeding), from which a positive correlation between RNA/DNA ratio and feeding timewas revealed. Most acrophases were observed about 6 h (from 5 h to 7 h) after feeding (Figure 1).
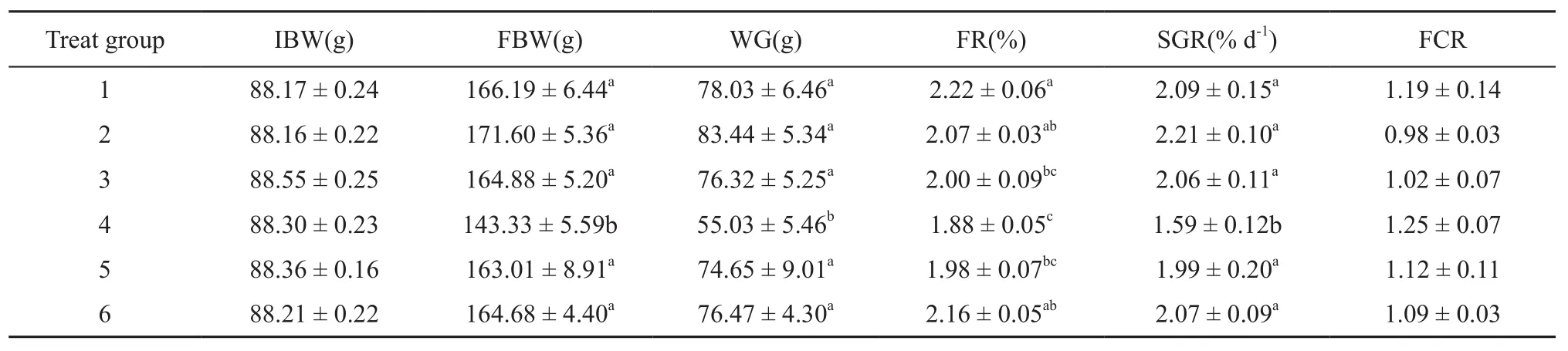
Table 2 Growth performance of the Chinese soft-shelled turtles reared under different feeding times.
3.3 Correlation between SGR and RNA/DNA ratioThe peaks of the RNA/DNA ratios at 6 h after feeding in groups were selected to assess regression with growth. A positive linear regression was observed, with the following regression equation: SGR = 1.1586 RNA/ DNA ? 0.7097 (r = 0.9328, P = 0.0066) (Figure 2). On the other hand, the mean RNA/DNA ratios of each group were selected to assess regression with growth. A negative correlation was observed (P > 0.05). A similar result was observed when the correlation between SGR and the maximum RNA/DNA ratio was evaluated (P > 0.05).
4. Discussion
4.1 Growth performance at different feeding timesIt has been demonstrated that feeding time affects growth in a large number of fshes (Vera et al., 2013; Sveier and Lied, 1998). In the present study, the SGR of the turtles in the 3 groups that fed in the dark was considerably higher than that of the turtles in the other groups that fed during daytime. This result was in agreement with that observed in a study on the European sea bass, Dicentrarchus labrax, L., in which the lowest SGR was shown by the group that fed at 12:00h (Azzaydi et al., 2000). Same reports could be seen in sea bass (Madrid et al., 1997). However, conflicting results have also been reported in silver carp (Hypophthalmichthys molitrix) (Wang et al., 1989). The overall growth performance could be affected by various elements and factors, such as the feeding strategy and the photoperiod and it is also the result of a combined action of genes, the environment, and nutrition (Taylor et al., 2006). Compared to the previous studies, however, the turtles fed in dark grew better than those fed during daylight, probably because of the growth hormone mRNA expression activities and the active metabolism activities (Ayson et al., 2007). The turtles in group 1 grew better than those in group 3, group 4, group 5, and group 6, because they consumed greater feed; however, they grew slower and exhibited a higher FCR than the turtles in group 2, probably because of decreased digestibility coefficient and nutrient uptake efficiency owing to too much food intake. Nevertheless, turtles that fed at night exhibited the highest growth.
Turtles that fed at different times showed different WG, CC, and FR. Food availability was one of the main factors affecting the growth of Alboran Sea sardine larvae (Sardina pilchardus), and played an important role in controlling the growth of Calanus sinicus in the fields (Ramírez et al., 2004; Ning et al., 2013). The results of the present study showed that turtles that fed at 04:00 h exhibited the least FCR without the least CC, which could be attributed to a high enzymatic activity, similar to that observed in tilapia (Oreochromis niloticus) (Luo et al., 2014). Turtles that fed in scotophase exhibited better growth, probably because the feeding times synchronized with their growth rhythm, which was different from the fish that grew better by feeding at daytime, and improved their nutrition deposition rate, which consequently led to maximum somatic growth rate.
4.2 Postprandial variations of RNA/DNA ratios
Relationships between RNA/DNA ratio and growth have been reported in several fishes (Tanaka et al., 2008; Gwak et al., 2003). Positive correlation between the RNA/DNA ratios and growth rates were recorded in larval cod (Gadus morhua) reared under controlled conditions (Buckley, 1979). Studies in winter flounder (Pseudopleuronectes americanus) indicated that RNA/ DNA ratio was a valid indicator of growth and nutritional condition (Mercaldo-Allen et al., 2008). We used linear regression to assess the relationship between RNA/ DNA ratio and time (hours after feeding), and observed asignifcant positive correlation. Visual inspection in Fig. 1 found a rising tendency of association in each group, and was consistent with the results of linear regression, indicating that the RNA/DNA ratio increased after feeding in the Chinese soft-shelled turtles.
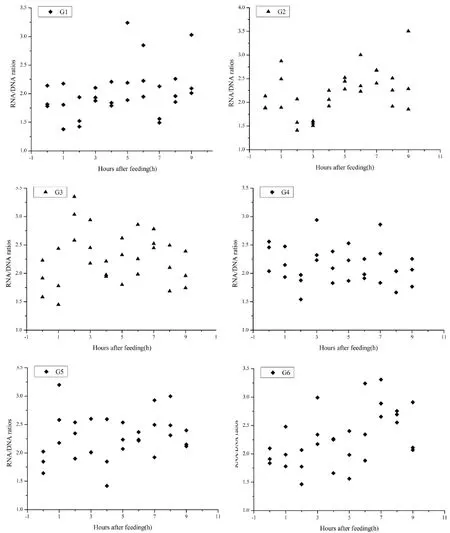
Figure 1 Postprandial variations of RNA/DNA ratios in liver of juvenile turtles fed at different times. G1?G6 represented group1 to group 6. The DNA in liver were determined using the Cell and Tissue DNA Extraction kit (Column-type). The RNA in liver were determined using the Total RNA Extraction kit (Trizol-column). Both kits were got from Shanghai Generay Biotech Co., Ltd.
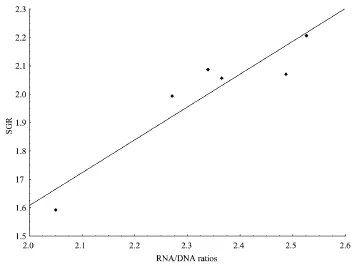
Figure 2 The regression of SGR with postprandial RNA/DNA ratios in the Chinese soft-shelled turtle. Data of SGR were shown as total means of each group. The data of RNA/DNA ratios were values 6 h after feeding times.
Most studies on fish concentrated on the variations in the RNA/DNA ratio over a long experimental period, which were calculated in days or months. However, the current study aimed to evaluate the postprandial variation in RNA/DNA ratio within 9 h after feeding. We found that the acrophases of the turtles that fed at 00:00, 04:00, and 16:00 were 5–6 h, 6 h, and 6–8 h, respectively, while that of the turtles in the other groups was approximately 6–7 h (Figure 2). Overall, the acrophases of RNA/DNA ratio scatterplots appeared approximately 6 h after feeding. We obtained a linear equation using the acrophase values (6 h after feeding) from each group and assessed their regression with total SGR. However, the postprandial RNA/DNA ratio at 1 or 2 h after feeding in some groups exhibited a high standard error. Further studies to minimize this error are warranted.
AcknowledgementsThis research was financially supported by the National Natural Science Foundation of China (No. 31172085). Thanks Shijiazhuang Kangtai Turtle Aquafarm (Luquan, Hebei, China) for supplying the juvenile Chinese soft-shelled turtles.
Ayson F. G., Jesus-Ayson E. G. T., Takemura A. 2007. mRNA expression patterns for GH, PRL, SL, IGF-I and IGF-II during altered feeding status in rabbitfsh, Siganus guttatus. Gen Comp Endocr, 150: 196–204
Azzaydi M., Martínez F. J., Zamora S., Sánchez-Vázquez F. J., Madrid J. A. 2000. The influence of nocturnal vs.diurnal feeding under winter conditions on growth and feed conversion of European sea bass (Dicentrarchus labrax, L.). Aquaculture, 182: 329–338
Boujard T., Leatherland J. F. 1992. Circadian rhythms and feeding time in fshes. Environ Biol Fish, 35: 109–131
Boujard T., Gélineau A. Corraze G. 1995. Time of a single daily meal influences growth performance in rainbow trout, ncorhynchus mykiss (Walbaum). Aquac Res, 26: 341–349
Buckley L. J. 1979. Relationships Between RNA-DNA Ratio, Prey Density, and Growth Rate in Atlantic Cod (Gadus morhua) Larvae. J Fish Res Board Can, 36: 1497–1502
Chícharo L. M. Z., Chícharo M. A., Alves F., Amaral A., Pereira A., Regala J. 2001. Diel variation of the RNA/DNA ratios in Crassostrea angulata (Lamarck) and Ruditapes decussatus (Linnaeus 1758) (Mollusca: Bivalvia). J Exp Mar Biol Ecol, 259: 121–129
Feliciano A., Vivas Y., De Pedro N., Delgado M. J., Velarde E., Isorna E. 2011. Feeding time synchronizes clock gene rhythmic expression in brain and liver of goldfsh (Carassius auratus). J Biol Rhythm, 26: 24–33
Frantzis A., Grémare A., Vétion G. 1992. Growth rates and RNA:DNA ratios in Paracentrotus lividus (Echinodermata: Echinoidea) fed on benthic macrophytes. J Exp Mar Biol Ecol, 156: 125–138
Gélineau A., Mambrini M., Leatherland J. F., Boujard T. 1996. Effect of Feeding Time on Hepatic Nucleic Acid, Plasma T3, T4, and GH Concentrations in Rainbow Trout. Physiol Behav, 59: 1061–1067
Gwak W. S., Tanaka Y., Tominaga O., Tsusaki T., Tanaka M. 2003. Field evaluation by RNA/DNA ratios on post-release nutritional status of released and wild Japanese flounder Paralichthys olivaceus juveniles. J Exp Mar Biol Ecol, 293: 107–124
Heilman M. J., Spieler R. E. 1999. The daily feeding rhythm to demand feeders and the effects of timed meal-feeding on the growth of juvenile Florida pompano, Trachinotus carolinus. Aquaculture, 180: 53–64
Luo G. Z., Gao Q., Wang C. H., Liu W. C., Sun D. C., Li L., Tan H. X. 2014. Growth, digestive activity, welfare, and partial cost-effectiveness of genetically improved farmed tilapia (Oreochromis niloticus) cultured in a recirculating aquaculture system and an indoor biofoc system. Aquaculture, 422: 1–7
Madrid J. A., Azzaydi M., Zamora S., Sánchez-Vázquez F. J. 1997. Continuous Recording of Uneaten Food Pellets and Demand-Feeding Activity: A New Approach to Studying Feeding Rhythms in Fish. Physiol Behav, 62: 689–695
Mercaldo-Allen R., Kuropat C., Caldarone E. M. 2006. A model to estimate growth in young-of-the-year tautog, Tautoga onitis, based on RNA/DNA ratio and seawater temperature. J Exp Mar Biol Ecol, 329: 187–195
Mercaldo-Allen R., Kuropat C., Caldarone E. M. 2008. An RNA:DNA-based growth model for young-of-the-year winter flounder Pseudopleuronectes americanus (Walbaum). J Fish Biol, 72: 1321–1331
Montoya A., López-Olmeda J. F., Yúfera M., Sánchez-Muros M. J., Sánchez-Vázquez F. J. 2010. Feeding time synchronises daily rhythms of behaviour and digestive physiology in Gilthead seabream (Sparus aurata). Aquaculture, 306: 315–321
Ning J., Li C. L., Yang G., Wan A. Y., Sun S. 2013. Use of RNA:DNA ratios to evaluate the condition and growth of the copepod Calanus sinicus in the Southern Yellow Sea. Deep-SeaReserch II, 97: 109–116
Nunes A. J. P., Sá M. V. C., Carvalho E. A., Neto H. S. 2006. Growth performance of the white shrimp Litopenaeus vannamei reared under time- and rate-restriction feeding regimes in a controlled culture system. Aquaculture, 253: 646–652
Ramírez T., Cortés D., García A., Carpena A. 2004. Seasonal variations of RNA/DNA ratios and growth rates of the Alboran Sea sardine larvae (Sardina pilchardus). Fish Res, 68: 57–65
Sveier H., Lied E. 1998. The effect of feeding regime on growth, feed utilisation and weight dispersion in large Atlantic salmon (Salmo salar) reared in seawater. Aquaculture, 165: 333–345
Tanaka H., Sassa C., Ohshimo S., Aoki I. 2013. Feeding ecology of two lanternfishes Diaphus garmani and Diaphus chrysorhynchus. J Fish Biol, 82: 1011–1031
Tanaka Y., Satoh K., Yamada H., Takebe T., Nikaido H., Shiozawa S. 2008. Assessment of the nutritional status of feldcaught larval Pacifc bluefn tuna by RNA/DNA ratio based on a starvation experiment of hatchery-reared fsh. J Exp Mar Biol Ecol, 354: 56–64
Taylor J. F., North B. P., Porter M. J. R., Bromage N. R., Migaud H. 2006. Photoperiod can be used to enhance growth and improve feeding efficiency in farmed rainbow trout, Oncorhynchus mykiss. Aquaculture, 256: 216–234
Vera L. M., Montoya A., Sánchez-Vázquez F. J. 2013. Effectiveness of the anaesthetic MS-222 in gilthead seabream, Sparus aurata: Effect of feeding time and day-night variations in plasma MS-222 concentration and GST activity. Physiol Behav, 110: 51–57
Wang J. Q., Flickinger S. A., Be K., Liu Y., Xu H. W. 1989. Daily food consumption and feeding rhythm of silver carp (Hypophthalmichthys molitrix) during fry to fingerling period. Aquaculture, 83: 73–79
*Corresponding author: Prof. Zhencai YANG, from Hebei Normal University, China, with his research focusing on aquatic biology.
E-mail: zcyang@mail.hebtu.edu.cn
Received: 19 March 2015 Accepted: 8 December 2015
 Asian Herpetological Research2016年4期
Asian Herpetological Research2016年4期
- Asian Herpetological Research的其它文章
- Isolation and Characterization of 15 Microsatellite DNA Loci for the Alpine Stream Frog Scutiger boulengeri (Anura: Megophryidae)
- A Field Observation and Signifcant Range Extension of Manouria impressa in Myanmar
- Major Factors Affecting the Distribution of Anuran Communities in the Urban, Suburban and Rural Areas of Shanghai, China
- Effects of Dietary Vitamins A, B2, and B6Supplementation on Growth and Feed Utilization of Juvenile Chinese Soft-shelled Turtle Pelodiscus sinensis according to an Orthogonal Array Experiment
- Plasticity in Metamorphic Traits of Rice Field Frog (Rana limnocharis) Tadpoles: The Interactive Effects of Rearing Temperature and Food Level
- Pathological Changes in Andrias davidianus Infected with Chinese Giant Salamander Ranavirus
