Choriocarcinoma-associated pulmonary thromboembolism and pulmonary hypertension: a case report
Yan Zhu, Meining Yu, Luyao Ma, Hai Xu, Fanghong Rose Li,?
Departments of1Pathology;2Cardiothoracic Surgery;3Diagnostic Radiology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 210029, China.
Choriocarcinoma-associated pulmonary thromboembolism and pulmonary hypertension: a case report
Yan Zhu1, Meining Yu1, Luyao Ma2, Hai Xu3, Fanghong Rose Li1,?
Departments of1Pathology;2Cardiothoracic Surgery;3Diagnostic Radiology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 210029, China.
Cases of pulmonary embolism and pulmonary artery hypertension caused by choriocarcinoma represent a rare clinical emergency. We report a case of a 25-year-old woman who presented with pulmonary embolism and hypertension and died soon after complete pulmonary embolectomy. A related literature review revealed that almost all of these patients had previously experienced a spontaneous abortion (average, 6 months) and were not pregnant.
choriocarcinoma, pulmonary embolism, pulmonary artery hypertension, pulmonary thromboembolism, β-hCG
Introduction
Choriocarcinoma is a highly malignant gestational trophoblastic disease associated with high urine and serum β-hCG levels[1]. To date, few studies have reported pulmonary embolism (PE) and pulmonary hypertension caused by choriocarcinoma of the pulmonary artery[2–9]. Here, we report a young female patient with choriocarcinoma of the pulmonary artery who was clinically misdiagnosed with pulmonary thromboembolism (PTE) and died of acute cardiopulmonary failure very soon after embolectomy.
Case report
A 25 year-old female patient first experienced an episode of sudden dyspnea and frequent coughing after routine physical activity. She was initially diagnosed at a local hospital with acute pneumonia based on a chest X-ray, which showed several small, scattered radio-opaque shadows on both lungs. Antibiotic treatment was initiated, but her condition showed no improvement. The symptoms of exertional dyspnea continued.
Six months later, the patient was referred to our hospital because of severe orthopnea. Upon arrival, arterial blood gas analysis showed that the values of pH, PaCO2, and PaO2were 7.502, 26.3 mmHg, and 50 mmHg, respectively, under 2 L/minute oxygen inhalation. An emergent contrast-enhanced CT scan showed occlusion of the left pulmonary artery and right inferior pulmonary artery (Fig. 1A). The scan further revealed multiple patchy, cord-like, nodular fuzzy shadows in bilateral lungs (Fig. 1B). Meanwhile, a diagnosis of PE was made based on echocardiographs, which revealed severe pulmonary hypertension (pulmonary systolic pressure, 105 mmHg) and mild tricuspid regurgitation. Although her D-dimer test value was only 0.27 μg/mL, a pulmonary angiograph was still performed via the right femoral vein, which showed complete occlusion of the left pulmonary artery and rightinferior pulmonary artery. Bilateral ultrasound examination of her legs showed no evidence of deep-vein thrombosis.
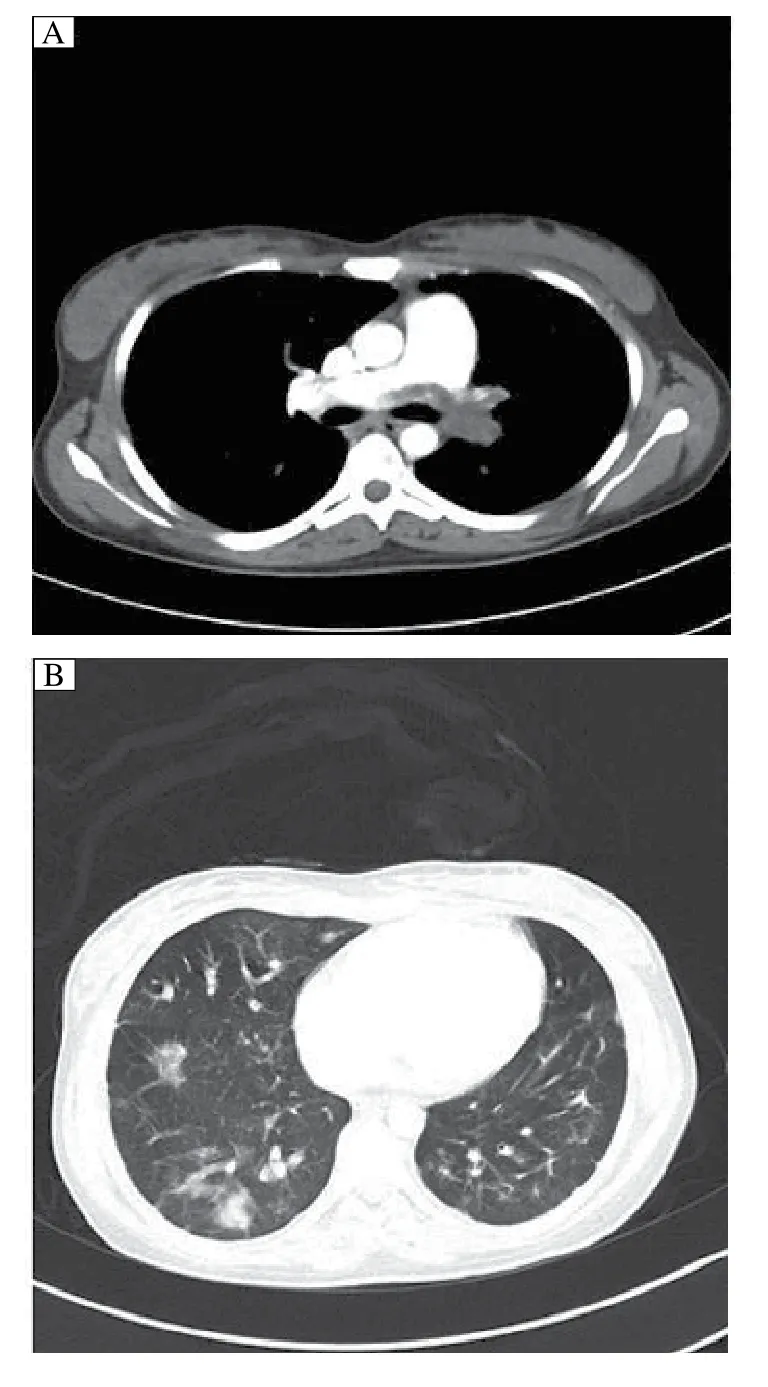
Fig. 1 Contrast-enhanced CT scan in a 25 year-old woman. A: CT scan demonstrates occlusion of the left pulmonary artery and right inferior pulmonary artery. B: CT scan shows multiple patchy, cordlike, nodular fuzzy shadows in bilateral lungs.
The patient claimed that she was a non-smoker, did not use oral contraception, and had experienced three previous spontaneous abortions (the most recent occurring one year previously). The patient had spent more than 10 hours playing card games every day during the past four years. An angiographic procedure for direct injection of 400,000 IU urokinase failed to dissolve the emboli. Since her PE was believed to have derived from deep venous thrombi (sedentary clinical history), a filter was also placed in the inferior vena cava.
The condition of the patient continued to deteriorate with progressive tachypnea, cyanosis, non- productive cough, and hemoptysis; therefore, a pulmonary embolectomy was carried out using a cardiopulmonary bypass procedure. Complete obstruction of the openings of the left pulmonary artery and right inferior pulmonary artery by the yellow-pink soft emboli was observed. The emboli were completely removed to re-establish blood flow from the bronchial artery. At the time of re-warming prior to decannulation, the pulmonary artery systolic pressure ranged from 90-110 mmHg and surpassed the aortic systolic pressure. The hemodynamic situation was so unstable that a 5 mm hole was made in the atrial septum to mitigate the condition. The patient was then transferred to the Cardio-thoracic Intensive Care Unit.
In spite of intensive efforts, the patient ultimately succumbed to cardiopulmonary failure and acute renal failure approximately 14 hours after embolectomy. Pathologic evaluation of pulmonary emboli showed macroscopic fragments of yellow-pink tissue measuring 4 cm at the greatest dimension (Fig. 2A). Postmortem histological evaluation showed that the viable tumor constituted only a thin peripheral rim with a central area of necrosis and focal hemorrhage (Fig. 2B). The tumor cells consisted of an intimate amalgamation of multinucleate syncytiotrophoblasts, mononucleate cytotrophoblasts, and intermediate trophoblasts. There was considerable cytological atypia in the trophoblasts with hyperchromatic nuclei and abnormal mitotic figures (Fig. 2B). Lack of villi and new blood vessel formation comprised the unique morphological features consistent with choriocarcinoma. Immunohistochemical staining demonstrated strong reactivity for hCG in syncytiotrophoblasts (Fig. 2C) and strong cytokeratin positivity was observed in all the tumor cells (Fig. 2D). Based on morphology and immunochemical analyses, the diagnosis of choriocarcinoma embolism was confirmed.
Discussion
Choriocarcinoma of the pulmonary artery has been described as a rare cause of PE and pulmonary artery hypertension (PAH). Such cases represent a potentially fatal emergency due to cardiopulmonary failure[4–6]. However, the diagnosis is difficult to establish because of the tendency of these tumors to present characteristics of more common diseases such as PTE. Almost all choriocarcinomas of the pulmonary artery present with PE, and PAH occurs after nonmolar abortions (mostly spontaneous abortions) and rarely after normal pregnancies. The low incidence of choriocarcinoma of the pulmonary artery and the long latency after a live birth or nonmolar abortion make early diagnosis of this condition very challenging[2–7]. As such, curative chemotherapy may therefore be delayed or withheld[2,5].
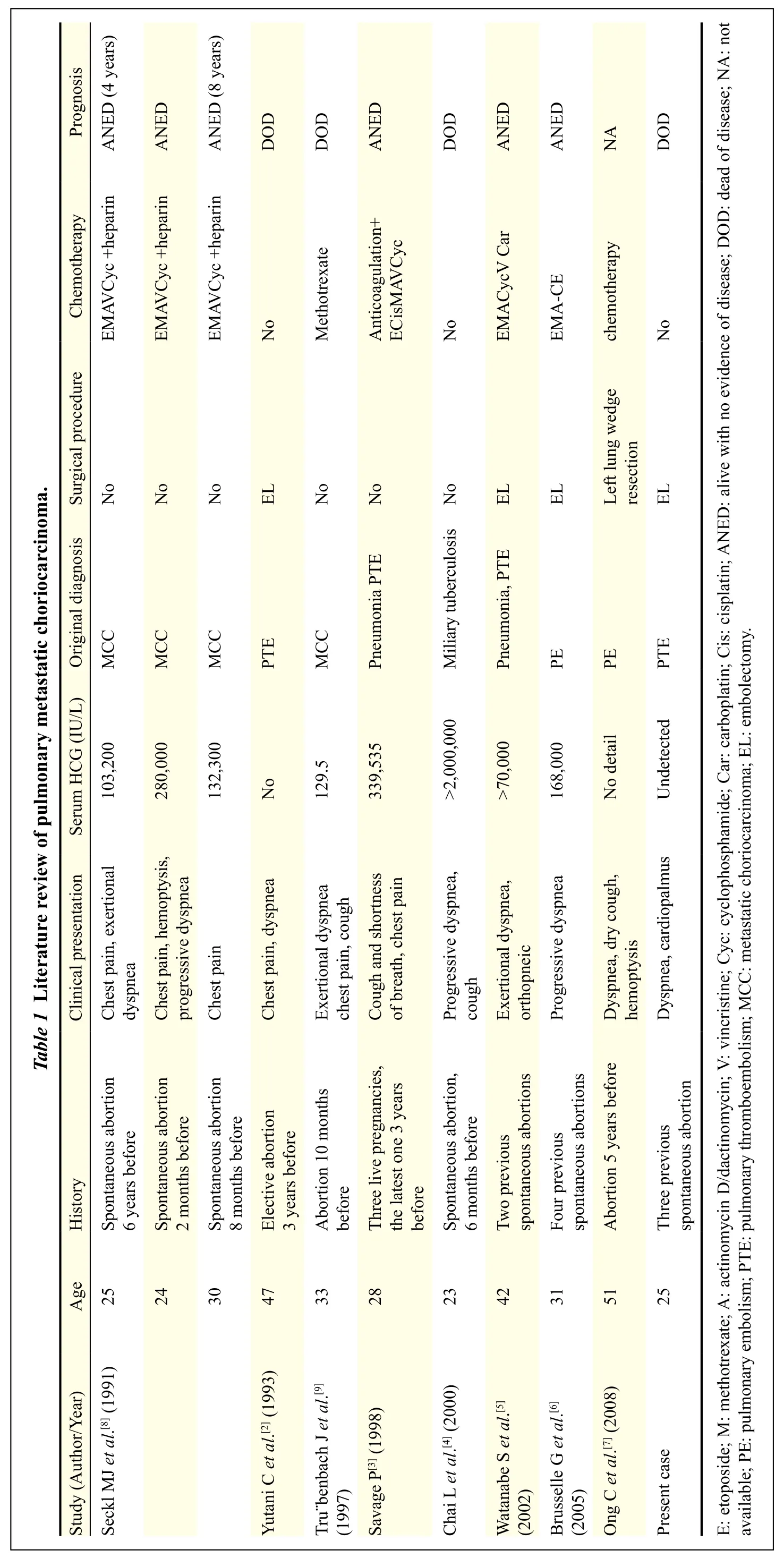
Bagshawe first reported choriocarcinoma of the pulmonary arteries, identified at necropsy in patients who died of PAH in 1959. He suggested that choriocarcinoma emboli could be the result of a progressive neoplasticobstruction of pulmonary arteries leading to PAH and respiratory distress. In addition to the case reported here, we reviewed 10 cases of pulmonary choriocarcinoma presenting with PE and PAH reported between 1991 and 2008; the findings of these 11 cases are summarized in Table 1[2–9]. Almost all of the patients (10/11) had a history of abortion with latency periods ranging from two months to six years and no primary uterine tumor identified. Among these cases, seven (7/10) were spontaneous abortion and, only one patient (1/11) a normal delivery three years previously. The most important risk factor, a history of hydatidiform mole, was absent from the cases included in our review; almost all cases occur after a spontaneous abortion and rarely after normal full term pregnancy. Owing to regular check-ups for potential malignant sequelae after hydatidiform molar pregnancies, few of these patients present with advanced disease, such as pulmonary hypertension. Therefore, almost all trophoblastic tumors that occur after a normal pregnancy are choriocarcinomas.
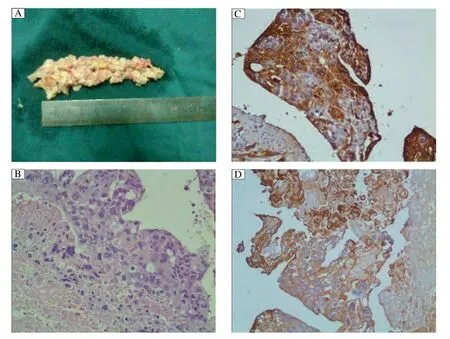
Fig. 2 Pathologic evaluation of pulmonary embolism. A: Macroscopic examination of the yellow-pink soft embolus obtained during pulmonary embolectomy. B: Tumor characterized by atypical trophoblasts with polymorphic and hyperchromatic nuclei as well as abnormal mitotic figures. C: Syncytiotrophoblasts were strongly positive for
There is controversy concerning whether choriocarcinoma of the pulmonary artery is primary or metastatic. Almost all results of pelvic ultrasound and gynecologic examination of the uteri and ovaries were negative, even by autopsy. Rare cases of PE due to choriocarcinoma in women occurring many years after hysterectomy have been reported[7]. Moreover, Tanimura and colleagues revealed trophoblasts in the pulmonary arteries in autopsies of nine of ten patients who died after delivery or abortion. Therefore, it has been speculated that primary choriocarcinoma of the pulmonary arteries in women may be due to malignant transformation of normal villous trophoblasts which entered the pulmonary artery at the time of abortion or delivery; however, the others consider that pulmonary choriocarcinoma derives metastatically from a regressed tumor previously present in the uterus.
Diagnosis of pulmonary choriocarcinoma embolism is very difficult in the early stages. Similar to our case, patients commonly present with non-specific symptoms such as chest pain, cough and dyspnea, as well as abnormal findings of chest X-rays (diffuse radiopaque shadows in the bilateral lungs). These manifestations are commonly misdiagnosed as pneumonia due to the absence of molar pregnancy history and gynecologic symptoms. As the quantity and volume of the pulmonary artery embolus increases, symptoms of pulmonary hypertension are present. Chest CT imaging may show intraluminal contrast filling defects (emboli) in the pulmonary artery down to the segmental and subsegmental levels, as well as multiple peripheral opacities in both lungs. At this stage, thoracic surgeons frequently misdiagnosed the condition as pulmonary thromboembolism and as a result, the duration from onset to treatment ranged from one to 12 months (average 6 months).
Typically, serum β-hCG levels in patients with choriocarcinoma of the pulmonary artery are high, ranging from 1,000 mIU/mL to over 1,000,000 mIU/mL, and urine pregnancy tests are always positive. In most developed countries, urine and/or serum β-hCG evaluation is a routine screening test in female patients regardless of the disorders that bring them to clinics. However, there are still many hospitals around the world (especially in developing countries) in which urine and/or serum β-hCG is still not on the list of routine tests. Similarly, the clinician responsible for the case described here noted the long sedentary history of the patient but disregarded the three spontaneous abortions; therefore, urine and/or serum β-hCG tests were consistently omitted. Thus, urine and/or serum β-hCG test should always be a frontline test ordered when female patients present with the symptoms of PE and PAH to rule out the possibility of choriocarcinoma.
Seckl and coworkers reported three cases of young women with complete remission following chemotherapy of choriocarcinoma embolism. The diagnosis was based mainly on high β-hCG levels in non-pregnant women[8], indicating that a definitive histological diagnosis is not essential before initiation of chemotherapy, particularly since surgery is associated with considerable morbidity[3,8]. Recently, FDG-PET/CT has been utilized to assist diagnosis for increased metabolism of the embolus.[9]Multiagent chemotherapy includes etoposide, methotrexate, actinomycin D, cyclophosphamide, cisplatin, vincristine, and dactinomycin, leading to a cure rate in excess of 89%. Thus, chemotherapy coupled with biochemical and radiological monitoring constitutes an attractive management approach for pulmonary choriocarcinoma embolism therapy.
In conclusion, pulmonary choriocarcinoma embolism is now a curable disease which is commonly associated with spontaneous abortion, a long latency and empty uteri. In order to avoid unnecessary deaths, it is very important for the clinician to recognize the non-specific clinical characteristics in the early stages and to use urine and/or serum β-hCG as the frontline test in the fertile female patients who are clinically suspected of PE; the earlier the diagnosis and initiation of chemotherapy, the better the prognosis.
References
[1] Kurman RJ, Lora HE, Ronnett BM. Blaustein’s pathology of the female genital tract[M]. 6th ed. London: Springer New York Dodrecht Heidelberg London, 2011,1101-1108.
[2] Yutani C, Imakita M, Ishibashi-Ueda H, et al. Pulmonary hypertension due to tumor emboli: a report of three autopsy cases with morphological correlations to radiological findings[J]. Acta Pathol Jpn, 1993,43(3):135-141.
[3] Savage P, Roddie M, Seckl MJ. A 28-year-old woman with a pulmonary embolus[J]. Lancet, 1998,352(9121):330.
[4] Chai L, Ong KC, Ng SB. A case of pulmonary tumor embolism mimicking miliary tuberculosis[J]. Res pirology, 2000,5(3):297-299.
[5] Watanabe S, Shimokawa S, Sakasegawa K, et al. Choriocarcinoma in the pulmonary artery treated with emergency pulmonary embolectomy[J]. Chest, 2002, 121(2):654-656.
[6] Brusselle G, Van Nooten G, Delrue L, et al. Cor pulmonale and respiratory failure in a young woman[J]. Respiration, 2005,72(5):549-551.
[7] Ong C, Low SY, Thirugnanam A, et al. Metastatic trophoblastic disease masquerading as pulmonary embolism[J]. Thorax, 2008, 63(11):1030.
[8] Seckl MJ, Rustin GJS, Newlands ES, et al. Pulmonary embolism, pulmonary hypertension, and choriocarcinoma[J]. Lancet, 1991,338(8778):1313-1315.
[9] Tr?benbach J, Pereira PL, Huppert PE, et al. Primary choriocarecinoma of the pulmonary artery mimicking pulmonary embolism[J]. Br J Radiol, 1997,70(836):843-845.
? Fanghong Rose Li, MD, Department of Pathology, The First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, Jiangsu 210029, China. Tel/Fax: 025-86862086/025-86862086, E-mail: fli@njmu.edu.cn.
17 April 2014, Accepted 28 August 2014, Epub 16 March 2015
R737.33, Document code: B
The authors reported no conflict of interests.
 THE JOURNAL OF BIOMEDICAL RESEARCH2016年3期
THE JOURNAL OF BIOMEDICAL RESEARCH2016年3期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Safety evaluation of a polyherbal formulation containing hydroalcoholic extracts of Hippophae salicifolia, Nyctanthes arbor-tristis, Ocimum tenuiflorum, and Reinwardtia indica in rodents
- Effects of microalgal polyunsaturated fatty acid oil on body weight and lipid accumulation in the liver of C57BL/6 mice fed a high fat diet
- Caspase-1 inhibition attenuates activation of BV2 microglia induced by LPS-treated RAW264.7 macrophages
- Novel monoclonal antibody against beta 1 integrin enhances cisplatin efficacy in human lung adenocarcinoma cells
- Human lgG Fc promotes expression, secretion and immunogenicity of enterovirus 71 VP1 protein
- Effect of vitamin D3 on production of progesterone in porcine granulosa cells by regulation of steroidogenic enzymes
