Novel monoclonal antibody against beta 1 integrin enhances cisplatin efficacy in human lung adenocarcinoma cells
Min-Young Kim, Woon-Dong Cho, Kwon Pyo Hong, Da Bin Choi, Jeong won Hong, Soseul Kim, Yoo Ri Moon, Seung-Myoung Son, Ok-Jun Lee,2, Ho-Chang Lee,2, and Hyung Geun Song,2,?
1Department of Pathology;2Research Institute, Chungbuk National University College of Medicine, Cheongju, 28644, Republic of Korea.
Novel monoclonal antibody against beta 1 integrin enhances cisplatin efficacy in human lung adenocarcinoma cells
Min-Young Kim1, Woon-Dong Cho1, Kwon Pyo Hong1, Da Bin Choi1, Jeong won Hong1, Soseul Kim1, Yoo Ri Moon1, Seung-Myoung Son1, Ok-Jun Lee1,2, Ho-Chang Lee1,2, and Hyung Geun Song1,2,?
1Department of Pathology;2Research Institute, Chungbuk National University College of Medicine, Cheongju, 28644, Republic of Korea.
The use of anti-beta 1 integrin monoclonal antibody in lung cancer treatment has proven beneficial. Here, we developed a novel monoclonal antibody (mAb), called P5, by immunizing mice with human peripheral blood mononuclear cells (PBMC). Its anti-tumor effect is now being tested, in a clinical phase Ⅲ trial, in combinatorial treatments with various chemical drugs. To confirm that P5 indeed binds to beta 1 integrin, cell lysates were immunoprecipitated with commercial anti-beta 1 integrin mAb (TS2/16) and immunoblotted against P5 to reveal a 140 kDa molecular weight band, as expected. Immunoprecipitation with P5 followed by LC/MS protein sequence analysis further verified P5 antigen to be beta 1 integrin. Cisplatin treatment upregulated cell surface expression of beta 1 integrin in A549 cells, while causing inhibition of cell growth. When cells were co-treated with different concentrations of P5 mAb, the cisplatin-mediated inhibitory effect was enhanced in a dose-dependent manner. Our findings show that a combinatorial treatment of P5 mAb and cisplatin in A549 cells resulted in a 30% increase in apoptosis, compared to baseline, and significantly more when compared to either the cisplatin or P5 alone group. The entire peptide sequences in CDR from variable region of Ig heavy and light chain gene for P5 mAb are also disclosed. Together, these results provide evidence of the beneficial effect of P5 mAb in combinatorial treatment of human lung adenocarcinoma.
CD29, beta 1 integrin, lung adenocarcinoma, monoclonal antibody, cisplatin
Introduction
Lung cancer is the most prevalent form of cancer worldwide and, it is the leading cause of cancerrelated death in men and the second leading cause in women[1,2]. Among lung cancers, non-small cell lung cancer (NSCLC) represents the most common type of cancer, accounting for 75-80% of all lung cancer occurrences[3].
The importance of beta 1 integrin in cell or tumor biology has been researched for decades. Beta 1 integrin transduces biochemical signals from extracellularenvironment, particularly those involved with growth, differentiation, invasiveness, and the metastatic potential of malignant cells[5]. Altered expression of integrins was implicated in tumor suppression and progression[6-8], where an increase in beta 1 integrins enhanced cancer cell viability by promoting survival and conferring resistance to chemotherapy in several tumor cell types[9-11].
The therapeutic approach using beta 1 integrin started from a report demonstrating that a monoclonal antibody against it improved the effectiveness of radiation therapy. Recently, beta 1 integrin mAb has been also actively used in a combinatorial treatment regime with anticancer drugs[12], demonstrating significantly improved outcome in progressive form of NSCLC where surgical treatment is difficult[13].
Cis-diaminodichloroplatinum (II) (CDDP; cisplatin) is one of the most effective and widely used DNA-damaging anti-cancer drugs for the treatment of various human cancers, including lung cancer[14]. However, cancer cells becoming resistant to cisplatin treatment remains a significant impediment to successful chemotherapy. To resolve this problem and to increase treatment efficacy, trials were performed using cisplatin and teniposide, or cisplatin and paclitaxel to compare the effectiveness of a combined treatment[15]. As result, the combinatorial treatment gave better efficiency for advanced NSCLC and has been recommended as one of the standard cares for advanced NSCLC patients. The new treatment regime takes a cancer-cell specific approach and promises less cytotoxicity than the older regime. As cancer cell-specific targets are only part of disease etiology, treatments combining a targeted approach and conventional drugs are yielding a synergistic effect.
In this study, we established a novel monoclonal antibody against beta 1 integrin from the hybridoma clone called P5 and evaluated its therapeutic efficacy when used in combination with cisplatin on A549 cells. Results are discussed to suggest the beneficial effect of this monoclonal antibody against lung adenocarcinoma.
Materials and methods
Cells and reagents
Human cancer cell lines K562(ATCC, #CCL-243), TF-1 (ATCC, #CRL-2003), IM9 (ATCC, #CCL-159), Ramos (ATCC, #CRL-1596), Raji (ATCC, #CCL-86), Jurkat (ATCC, #TIB-152), CCRF-CEM (ATCC, #CCL-119), Molt-4 (ATCC, #CRL-1582), HL-60 (ATCC, #CCL-240), KATOⅢ (ATCC, #HTB-103), A549 (ATCC, #CCL-185), SW620 (ATCC, #CCL-227) and J82 (ATCC, #HTB-1) were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS). Other cell lines, HeLa (ATCC, #CCL-2), Caki-1 (ATCC, #HTB-46) and DU145 (ATCC, #HTB-81) were maintained in DMEM supplemented with 10% FBS.
P5 mAbs were purified from ascites using DEAE-anion exchange chromatography. Cisplain [cisdiaminodichloroplatinum (CDDP)] was purchased from Choong-Wae Pharmaceutical (Seoul, Korea). Fluorescein isothiocyanate (FITC)-conjugated goat antimouse secondary antibody and horseradish peroxidase (HRP)-conjugated goat anti-mouse secondary antibody were supplied from DINONA (Seoul, Korea). Sulfo-NHS-LC-biotin was purchased from Thermo Scientific (Rockford, IL, USA). HRP-conjugated streptoavidin was purchased from DAKO (Kyoto, Japan). Anti-beta 1 integrin mAb (TS2/16) and ELC-western blotting system were purchased from Santa Cruz Biotech (Santa Cruz, CA, USA) and GE Amersham (Braunschweig, Germany), respectively. EZ-Cytox Cell Viability Assay Kit was purchased from Daeil Lab (Seoul, Korea). Annexin V-FITC and propidium iodide (PI) was purchased from BD Biosciences (San Jose, CA, USA).
Generation of monoclonal antibody
Six weeks-old Balb/c mice were immunized 3 times with human peripheral blood mononuclear cell (PBMC) at 4-2-2 week intervals. Three days prior to spleen harvest for fusion, mice were boosted with anti-CD40 mAb. Splenocytes from immunized mice were fused with SP2/0 mouse myeloma cell line (ATCC, #CRL-1581). Finally, P5 mAb was established through repeating screenings and limiting dilutions 3 times[16].
TF-1 cell preparation
TF-1 cells (1 × 108) of erythroleukemia origin were harvested and washed with cold phosphate-buffered saline (PBS). Cells were resuspended with 1 mL of buffer following preparation of biotinylation buffer (1% Sulfo-NHS-LC-biotin in 20 mmol/L sodium bicarbonate, and 150 mmol/L sodium chloride). Cell suspensions were incubated at 4°C for 2 hours and washed twice with PBS. Biotinylated cells were lysed in lysis buffer (1% Nonidet P-40; NP-40 in 50 mmol/L Tris-HCl pH7.4, 50 mmol/L EDTA and 1 mmol/L phenyl-methylsulfonyl- fluoride). For immunoprecipitation, protein G agarose beads were preincubated with P5 mAb in PBS overnight at 4°C with rotation. Biotinylated cell lysates were incubated with antibody-bead complex overnight at 4°C with rotation. Protein-antibody bead complex was washed with buffers 1, 2, 3 in numerical order (Buffer 1;1% NP-40 in 1 mol/L NaCl, Buffer 2; 0.1% NP-40 in 0.5 mol/L NaCl, Buffer 3; 0.1% NP-40 in PBS). Final samples were resuspended in reducingloading buffer and separated by electrophoresis in SDS on 10% polyacrylamide gels. Proteins were transferred on PVDF membrane (Millipore, Billerica, MA, USA), and the membrane was incubated with HRP-conjugated streptavidin and visualized using the ELC-western blotting system.
Immunoprecipitation and immunoblotting
For immunoprecipitation (IP), protein G agarose beads were preincubated with anti-beta 1 integrin mAb (TS2/16) in PBS overnight at 4°C with rotation. Subsequently, antibody-bead complex was incubated with TF-1 cell lysates overnight at 4°C with rotation. Resulting protein-antibody bead complex was immunoblotted with P5 mAb and HRP-conjugated goat antimouse secondary antibody. The blots were developed using ELC-western blotting system.
Protein analysis
The protein-antibody bead complex resulting from immunoprecipitation described above was separated by electrophoresis in SDS on 10% polyacrylamide gels. Protein bands stained with Coomassie blue R-250 (Bio-Rad Labs, Ramsey, MN, USA) were excised from the gel and sent to ProteomeTech (Seoul, Korea) for protein sequence analysis.
Flow cytometric analysis of beta 1 integrin expression
A549 cells (2 × 105) were seeded onto 6-well culture plates, incubated with cisplatin (1 μg/mL), and detached with 0.05% trypsin/0.02% EDTA at defined time points (12, 24, 48 hours) for analysis. Non-treated controls were prepared in parallel for each time point. Cells were incubated with either saturating amounts of purified P5 mAb or a control immunoglobulin for 30 minutes at 4°C. Thereafter, cells were washed twice with PBS, incubated with FITC-conjugated goat anti-mouse secondary antibody for 20 minutes at 4°C, and washed twice again with PBS. The resulting cells were fixed with 1% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) prior to be analyzed using FACS caliber (BD Biosciences, San Jose, USA) equipped with a CELLQuest software.
Cytotoxicity assay
A549 cells (4 × 103cells suspended in 100 μL of RPMI 1640 supplemented with 10% FBS) were seeded onto a 96-well plate (SPL Lifesciences, Pocheon, Korea) and incubated overnight at 37°C with 5% CO2to allow the cells to adhere to the plate. Next day, the culture supernatant was removed, and the antibody (dissolved in serum-free RPMI at a concentration of 10 μg/mL), either alone or in combination with 1 μg/mL of cisplatin, was added to the cells. Cells in fresh medium without antibody or cisplatin were used as control. Plates were incubated at 37°C for a given incubation period, after which 10 μL of the solution from EZ-Cytox Cell Viability Assay kit was added to each well and incubated at 37°C with 5% CO2for 2 hours. The extent of reduction to formazan within cells was quantified by measuring absorbance at 480 nm using SpectraMax M5 (Molecular Devices, Sunnyvale, USA).
Apoptosis assay
A549 cells (1 × 105) in 100 μL of RPMI 1640 supplemented with 10% FBS were seeded onto a 6 well plate (SPL Lifesciences, Pocheon, Korea). Plates were incubated overnight at 37°C with 5% CO2to allow cells to adhere to the plate. The culture supernatant was removed, and the antibody (dissolved in serum-free RPMI at a concentration of 10 μg/mL), either alone or in combination with 1 μg/mL of cisplatin, was added to cells. Cells in fresh medium without antibody or cisplatin were used as control. Apoptotic cell death was assessed 48 hours later by determining the percentage of cells positive for FITC-labeled Annexin V and PI. The proportion of Annexin V/PI positive cells were analyzed using FACS caliber equipped with CELLQuest software.
Statistical analysis
Data were expressed as mean ± SD. Comparisons between experimental groups were made by one-way ANOVA. The differences in mean values were considered significant at P < 0.05.
Sequence analysis
RNA was extracted from P5 hybridoma clone (RNeasy Plus Mini Kit, QIAGEN Inc., Valencia, CA, USA) to analyze the gene sequence for immunoglobulin variable regions. PCR (Biorad, Hercules, CA) was performed using Mouse Ig-Primer Set (Novagen, Wisconsin, USA) to yield P5 mAb variable region of DNA. Sequencing analysis on the PCR product was performed at Comogenetech (Daejeon, Korea), and the CDRs were confirmed using IMGT/V-QUEST (V-QUEry and STandardization) software, an integrated software program that analyzes immunoglobulin (IG) and T cell receptor (TR) rearranged nucleotide sequences[17-18].
Results
Establishment and characterization of anti-beta integrin mAb (P5)
Mice were alternatively immunized with human PBMC to produce P5 mAb. Identification and confirmation ofresulting P5 mAB as a novel antibody against beta 1 integrin was made possible by the following methods. First, cell lysates were immunoprecipitated with commercial anti-beta 1 integrin mAb (TS2/16) and immunoblotted with P5 mAb, which revealed a 140 kDa molecular weight band corresponding to the expected molecular weight of beta 1 integrin antigen (Fig. 1). Second, the confirmation of protein beta 1 integrin was performed using LC/MS protein sequence analysis (Fig. 2). The results demonstrated that P5 antigen is expressed on several cancer cell lines (Table 1).
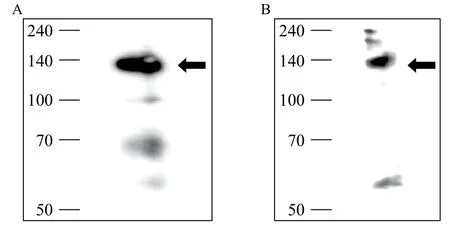
Fig. 1 Sequential immunoprecipitation (A) and immunoblotting (B) for beta 1 integrin from TF-1 cell lysates. A: Molecular weight of target antigen of P5 monoclonal antibody (mAb) was confirmed to be ~140 kDa via immunoprecipitation of TF-1 cell lysates. B: Immunoprecipitation-immunoblotting was performed using anti-beta 1 integrin mAb (TS2/16) and P5 mAb from TF-1 cell lysates to ensure that P5 mAb recognized beta 1 integrin.
Cisplatin treatment increases beta 1 integrin expression on A549 cells
We tested if cisplatin treatment would affect the cellular expression of beta 1 integrin in A549 cells. WhenA549 cells were incubated with cisplatin (1 μg/mL), subsequently detached and analyzed by flow cytometry, their beta 1 integrin expression was increased at 24 hours (125%) and 48 hours (184%) compared to untreated cells (Fig. 3). This result reveals that cisplatin treatment increases beta 1 integrin expression on A549 cells.
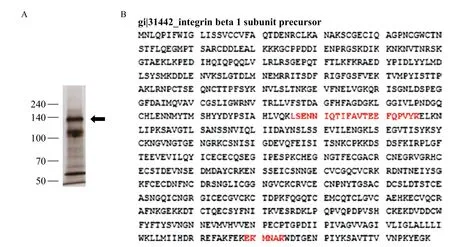
Fig. 2 LC-MS/MS protein sequence analysis of P5 antigen. A: Silver staining of immunoprecipitated proteins with P5 mAb from TF-1 cell lysate. The immunoprecipitated band (arrowhead) was digested in SDS-PAGE gel and then subjected to LC-MS/MS analysis. B: LC mass spectrometry reveals that the target molecule of P5 mAb is beta 1 integrin (matched peptides shown in bold red).
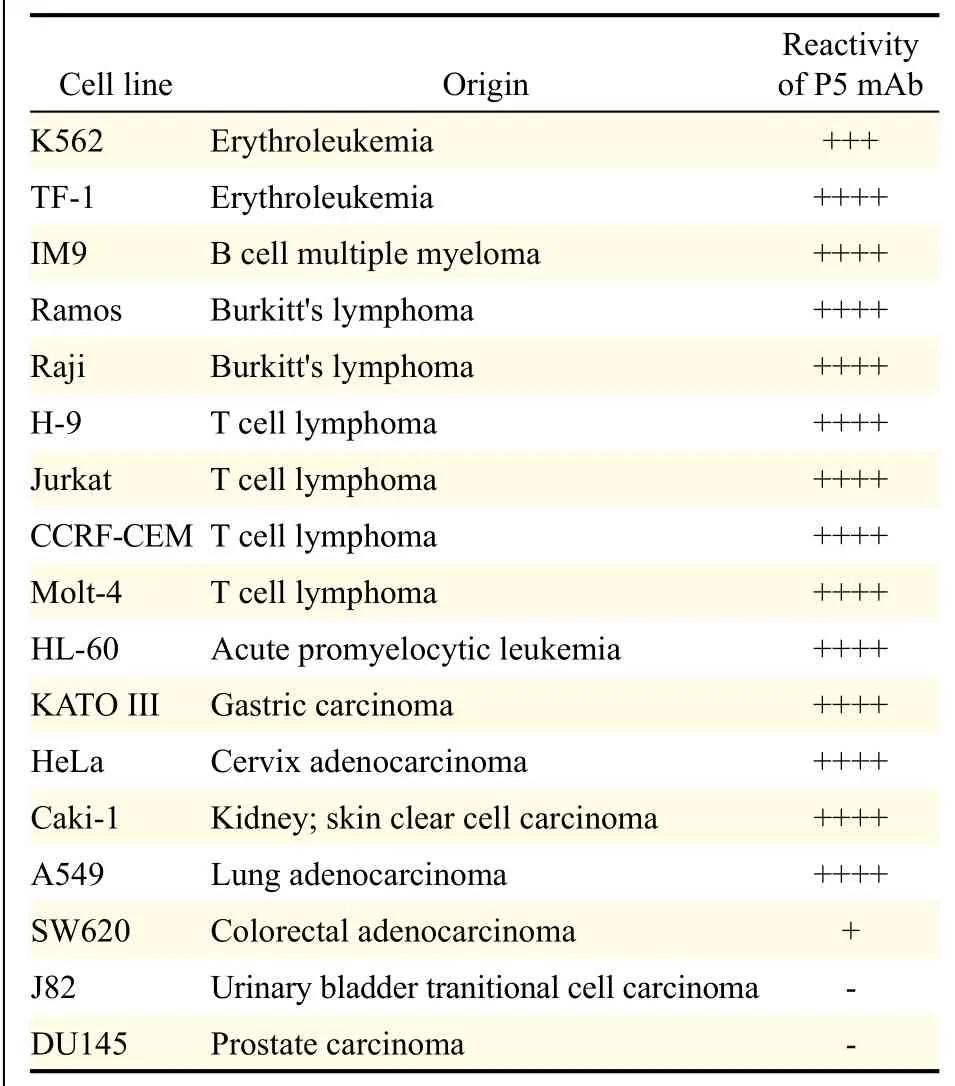
Table 1 Immunoreactivity of P5 mAb on various human cancer cell lines.

Fig. 3 Cisplatin treatment up-regulates beta 1 integrin expression in A549 cells. A549 cells were seeded onto 6 well culture plates and incubated with cisplatin (1 μg/mL), detached with trypsin/EDTA at defined time points (12, 24, 48 hours), and prepared for measurement. Non-treated controls were prepared in parallel at each time point. When determined by flow cytometry, beta 1 integrin expression was increased by 25% and 84% at 24 and 48 hours following cisplatin treatment, respectively.
Inhibition of cell growth for cisplatin-treated A549 cells by P5 mAb
To determine if a combined treatment of P5 mAb and cisplatin exerts synergistic effect on cell growth, A549 cells were plated onto 96 well plate and P5 mAb and cisplatin were added to the culture medium. The viability of A549 cells was assessed at defined time points (12, 24, and 48 hours) using EZ-Cytox Cell Viability Assay Kit. When treated individually, cell growths were inhibited at 48 hours by 13% and 12% by P5 mAb and cisplatin, respectively (Fig. 4A). When used in combination, P5 mAb and cisplatin synergistically inhibited the growth of A549 cells by 21% at 48 hours (Fig. 4A). Increasing concentration of P5 mAb (0.1, 0.5, 1, 5, 10, and 20 μg/mL, Fig. 4B) or cisplatin (1, 5, and 10 μg/mL, Fig. 4C) in the culture medium while maintaining constant concentration of either cisplatin (1 μg/mL, Fig. 4B) or P5 mAb (10 μg/mL) inhibited the growth of A549 cells in a dose dependent manner.
P5 mAb treatment with cisplatin increases apoptosis in A549 cells
To determine the effect of P5 mAb and cisplatin treatment on the induction of apoptosis in A549 cells, cells were plated onto 6 well plate and incubated with P5 mAb (10 μg/mL) and cisplatin (1 μg/mL) for 48 hours. At the end of incubation period, cells were detached, and cell death was assessed by determiningthe proportion of PI-positive cells labeled with Annexin V. Incubation with P5 mAb or cisplatin increased the apoptosis in A549 cells by 12.99% and 12.6%, respectively. Combination of P5 mAb and cisplatin synergistically increased the apoptosis by 27.2% (Fig. 5).
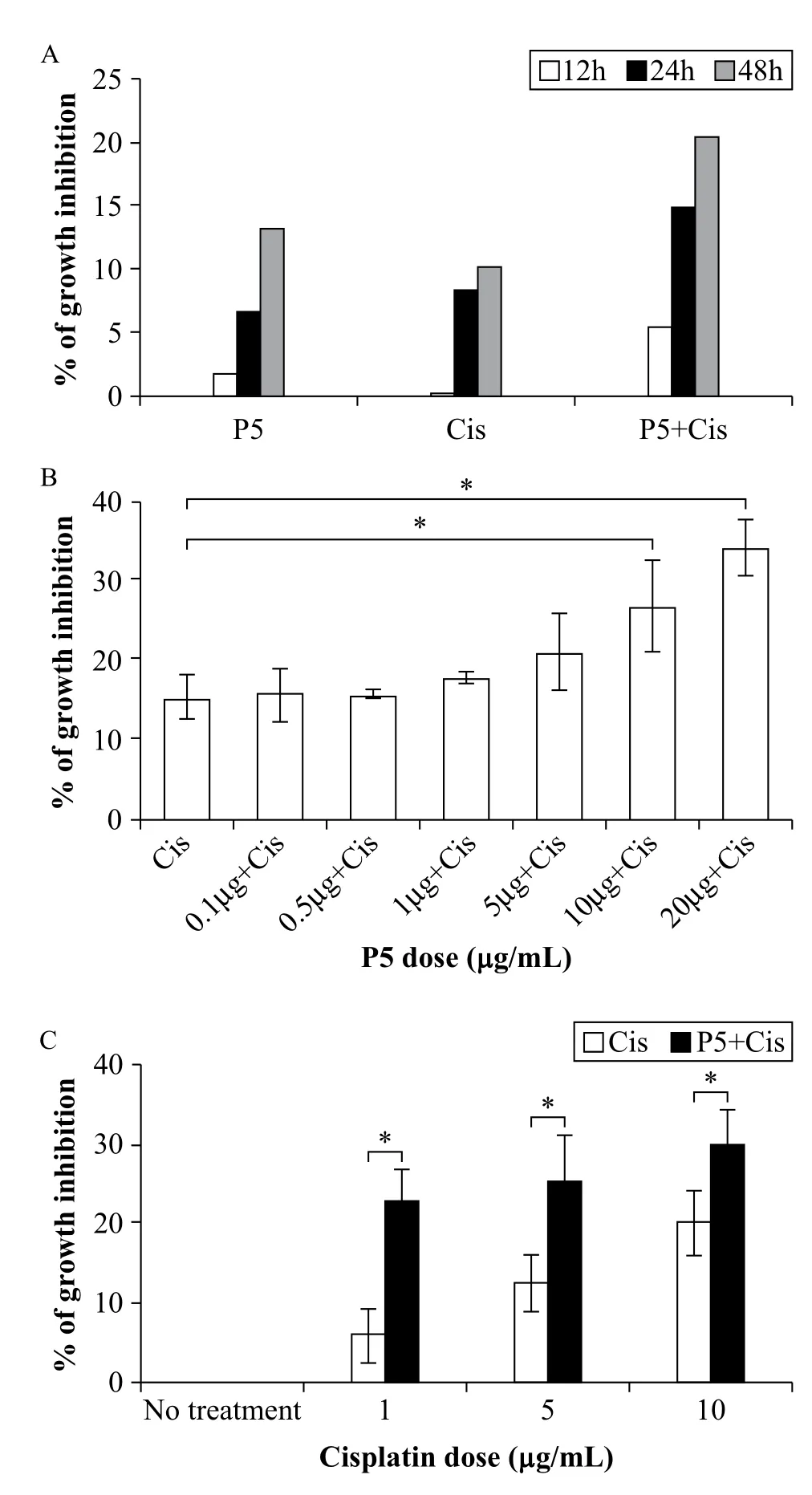
Fig. 4 Inhibitory effect of P5 mAb, cisplatin, or both on A549 cell growth. A: Cells were plated at 4 × 103/well and treated with P5 mAb (10 μg/mL), cisplatin (1 μg/mL), or P5 mAb (10 μg/mL) + Cisplatin (1 μg/mL) at defined time points (12, 24, and 48 hours). Combined treatment resulted in more significant growth inhibition (almost double compared to that of single treatment) at 48 hours. B and C: Dose-dependent inhibitory effect of P5 mAb and cisplatin when used with fixed concentration of either cisplatin (B, 1 μg/mL) or P5 mAb (C, 10 μg/mL) on A549 lung cancer cells. P5 mAb concentrations used were from 0.1 to 20 μg/mL (B), whereas cisplatin concentrations were from 0 to 10 μg/mL (C). Cell growth inhibition was determined by EZ-cytox reagent. Bars, means ± SD of a representative experiment done in triplicate from three independently performed experiments. *P < 0.05.
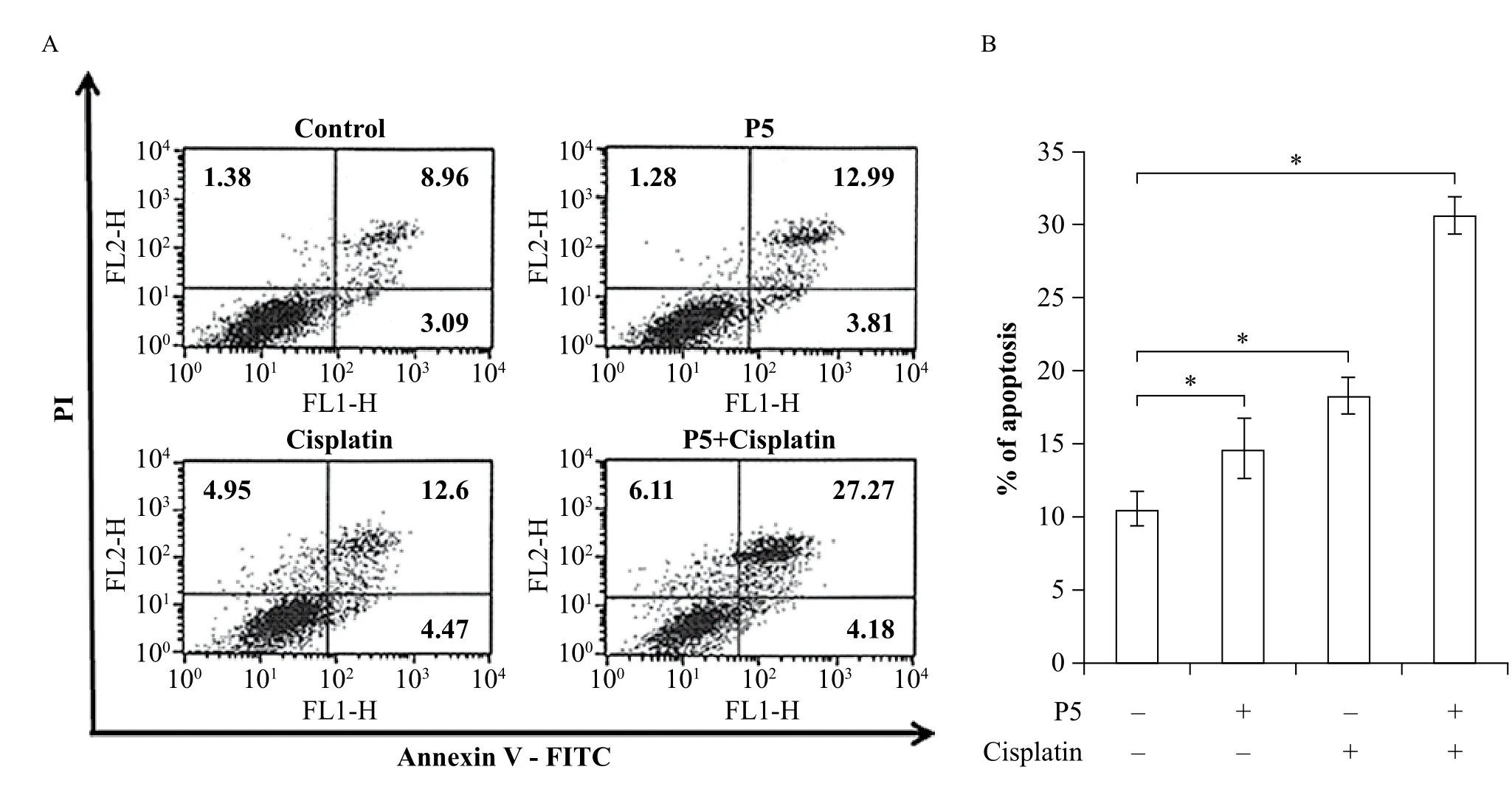
Fig. 5 Combined treatment of P5 mAb with cisplatin increases apoptosis in A549 cells. A & B: Apoptotic cell death, as evidenced by percent Annexin V/PI double positive cells (upper right quadrant from each analysis (A), was significantly increased by 15% in the combined treatment group compared to the single treatment group (B). Cells were plated at 1 × 105/well and placed in 10% FBS. P5 mAb (10 μg/mL) and cisplatin (1 μg/mL) were added simultaneously and cells were incubated for 48 hours. Cell death was assessed 48 hours later and the proportion of Annexin V/PI positive cells was assessed by flow cytometry (see methods). Bars, means ± SD of a representative experiment done in triplicate from three independently performed experiments. *P < 0.05.
Sequencing analysis of the variable region of P5 mAb
RNA from P5 hybridoma cells was subjected to PCR, and the gene sequences were analyzed to obtain sequence information for the variable regions of P5 mAb. Sequencing results yielded CDR1 (GYTFSNYW), CDR2 (ILPGSVNT), and CDR3 (ALATPYYALDS) in the heavy chain variable region whereas CDR1(ESLLHSNGNTY), CDR2 (RMS), and CDR3 (MQHLEYPFT) in the light chain region (Fig. 6).

Fig. 6 P5 mAb variable region amino acids sequence (deduced). (A) Heavy chain variable region sequence. (B) Light chain variable region sequence. CDRs are in bold and underlined.
Discussion
In this study, we provide evidence that the novel monoclonal antibody against beta 1 integrin (CD29) enhances cisplatin efficacy in human adenocarcinomacell line A549. In addition, we prove that combinatorial treatment of P5 mAb with cisplatin shows better efficacy than chemotherapy alone.
P5 mAb was produced by immunizing BALB/c mice with human PBMC. Its binding was further characterized by performing cell-binding profiling using various human origin cell lines, measured with flow cytometry. Based on Western blotting results, channel catfish beta 1 molecule has a molecular mass of approximately 130 kDa (19). When TF-1 cell lysates overexpressing P5 antigen were used for immunoprecipitation to determine the molecular weight of P5 antigen, a discrete band was observed at approximately 140 kDa under reducing condition. We also confirmed that P5 mAb recognized the same target molecule as commercial anti-beta 1 integrin mAb by LC/MC protein sequence analysis.
Less is known on the relevance of beta 1 integrin in lung tumors, despite the fact that it has aberrant expression and has been correlated with patient survival reduction[20,21]. The results presented in this study prove for the first time that the use of novel P5 mAb against beta 1 integrin can overcome the limitation of cisplatin.
A previous report showed that beta 1 integrin and EGFR functionally co-operate in lung cancer cells, giving rise to a crucial signaling platform required for primary tumor formation and the invasive behavior of tumor cells[21]. They also demonstrated that the proliferation of A549 is affected both in vitro and in vivo via silencing beta 1 integrin[21]. Based on this finding, we hypothesized that our P5 mAb could act as functional blocker of beta 1 integrin and exert an antitumor effect on lung adenocarcinoma cells either alone or as combinatorial therapy with other drugs such as cisplatin.
Over-expression of beta 1-integrin protected hepatocarcinoma cell (HCC) lines from apoptosis induced by chemotherapeutic reagents[22]. Thus, we sought to determine the change in surface beta 1 integrin expression following cisplatin treatment in A549 cells using flow cytometry. As result, we confirmed that the surface expression of beta 1 integrin on A549 following 48 hours of cisplatin treatment doubled when compared to no treatment control (Fig. 3).
If over-expression of beta 1 integrin in A549 could protect cells from apoptosis by cisplatin treatment, functional block of beta-1 integrin could enhance the antitumor efficacy by cisplatin treatment. Indeed, our results indicated that P5 mAb and cisplatin combination therapy significantly inhibited A549 cell proliferation in vitro, and this effect was the highest at 48 hours of treatment (Fig. 4A). We also confirmed that this growth inhibition occurred in a dose dependent manner, with inhibitory effect augmented as P5 mAb concentration increased (Fig. 4B). On the other hand, only a marginal increase in growth inhibition activity was observed when cells were treated with a fixed concentration of P5 mAb in combination with an increasing concentration of cisplatin. In this case, the highest significant inhibitory effect was observed at 1 μg/mL cisplatin. To further prove that this growth inhibition activity occurred via apoptosis, we investigated Annexin V and PI positive cells. The result revealed~20% more AnnexinV positive cells in the combination therapy group than the negative control group (Fig. 5).
Currently, volociximab is in clinical trial as monoclonal therapy that targets alpha 5 beta 1 integrin. This monoclonal therapy causes tumor cells to undergo apoptotic cell death by inhibiting neoangiogenesis via blocking the interaction between alpha 5 beta 1 integrin and fibronectin[10,23]. Through our current work, we confirmed that our P5 mAb similarly induces apoptotic cell death in tumor cells and the effect is enhanced by combinatorial treatment with cisplatin. We disclosed the full CDR sequences of the variable region of heavy and light chain immunoglobulin gene (Fig. 6). The antibody is now in the process of being developed as a chimeric monoclonal antibody for anti-cancer therapy.
In conclusion, our results demonstrate that P5, the novel anti-beta 1 integrin mAb, has a potential to be utilized as a novel therapeutic antibody for the treatment of human lung adenocarcinoma. P5 mAb also exhibits a synergistic antitumor effect when used in combination with cisplatin. P5 mAb is a novel antibody that has different sequence than volociximab, but a similar effect. We believe that P5 mAb has a potential to be developed as an effective antibody therapy targeting beta 1 integrin.
Acknowledgements
This work was supported in 2015 by a research grant from Chungbuk National University.
References
[1] Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006[J]. CA Cancer J Clin, 2006;56(2):106-130.
[2] Parkin DM. Global cancer statistics in the year 2000[J]. Lancet Oncol, 2001;2(9):533-543.
[3] Brognard J, Clark AS, Ni Y, et al. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation[J]. Cancer Res, 2001;61(10):3986-3997.
[4] Blau HM, Baltimore D. Differentiation requires continuous regulation[J]. J Cell Biol, 1991;112(5):781-783.
[5] Juliano RL. The role of beta 1 integrins in tumors[J]. Semin Cancer Biol, 1993;4(5):277-283.
[6] Dedhar S, Hannigan GE. Integrin cytoplasmic interactions and bidirectional transmembrane signalling[J]. Curr Opin Cell Biol, 1996;8(5):657-669.
[7] Hemler ME, Mannion BA, Berditchevski F. Association of TM4SF proteins with integrins: relevance to cancer[J]. Biochim Biophys Acta, 1996;1287(2-3):67-71.
[8] Plath T, Detjen K, Welzel M, et al. A novel function for the tumor suppressor p16(INK4a): induction of anoikis via upregulation of the alpha(5)beta(1) fibronectin receptor[J]. J Cell Biol, 2000;150(6):1467-1478.
[9] Hodkinson PS, Elliott T, Wong WS, et al. ECM overrides DNA damage- induced cell cycle arrest and apoptosis in small-cell lung cancer cells through beta1 integrin-dependent activation of PI3-kinase[J]. Cell Death Differ, 2006;13(10):1776-1788.
[10] Aoudjit F, Vuori K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells[J]. Oncogene, 2001;20(36):4995-5004.
[11] Morozevich GE, Kozlova NI, Preobrazhenskaya ME, et al. The role of beta1 integrin subfamily in anchorage-dependent apoptosis of breast carcinoma cells differing in multidrug resistance[J]. Biochemistry (Mosc), 2006;71(5):489-495.
[12] Cordes N, Park CC. Beta1 integrin as a molecular therapeutic target[J]. Int J Radiat Biol, 2007;83(11-12):753-760.
[13] Besse B, Tsao LC, Chao DT, et al. Phase Ib safety and pharmacokinetic study of volociximab, an anti-alpha5beta1 integrin antibody, in combination with carboplatin and paclitaxel in advanced non-small-cell lung cancer[J]. Ann Oncol, 2013;24(1):90-96.
[14] Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance[J]. Oncogene, 2003;22(47):7265-7279.
[15] Giaccone G, Splinter TA, Debruyne C, et al. Randomized study of paclitaxel-cisplatin versus cisplatin-teniposide in patients with advanced non-small-cell lung cancer. The European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group[J]. J Clin Oncol, 1998;16(6):2133-2141.
[16] Hong KP, Shin MH, Yoon S, et al. Therapeutic effect of anti CEACAM6 monoclonal antibody against lung adenocarcinoma by enhancing anoikis sensitivity[J]. Biomaterials, 2015;67:32-41.
[17] Hyung-Geun S, Kwon Pyo H, Yoo Ri M, et al. Generation of 1D4 Single Chain Fv-Fc[J]. Journal of Biomedical Research, 2011;12(3):157-164.
[18] Giudicelli V, Chaume D, Lefranc MP. IMGT/V-QUEST, an integrated software program for immunoglobulin and T cell receptor V-J and V-D-J rearrangement analysis[J]. Nucleic Acids Res, 2004;32(Web Server issue):W435-440.
[19] Qian Y, Noya M, Ainsworth AJ. Molecular characterization and leukocyte distribution of a teleost beta1 integrin molecule[J]. Vet Immunol Immunopathol, 2000(1-2);76:61-74.
[20] Okamura M, Yamaji S, Nagashima Y, et al. Prognostic value of integrin beta1-ILK-pAkt signaling pathway in non-small cell lung cancer[J]. Hum Pathol, 2007;38(7):1081-1091.
[21] Morello V, Cabodi S, Sigismund S, et al. Beta1 integrin controls EGFR signaling and tumorigenic properties of lung cancer cells[J]. Oncogene, 2011; 30(39):4087-4096.
[22] Zhang H, Ozaki I, Mizuta T, et al. Beta 1-integrin protects hepatoma cells from chemotherapy induced apoptosis via a mitogen-activated protein kinase dependent pathway[J]. Cancer, 2002;95(4):896-906.
[23] Almokadem S, Belani CP. Volociximab in cancer[J]. Expert Opin Biol Ther, 2012;12(2):251-257.
? Hyung Geun Song, Department of Pathology, Chungbuk National University College of Medicine, 1 Chungdae-ro, Seowon-gu, Cheongju 28644, Korea. Tel: 82-43-261-2853, Fax:82-43-269–6269, E-mail: hgsong@chungbuk.ac.kr.
01 Feb 2016, Revised 24 Feb 2016, Accepted 25 Feb 2016, Epub 20 April 2016
R730.53, Document code: A.
The authors reported no conflict of interest.
 THE JOURNAL OF BIOMEDICAL RESEARCH2016年3期
THE JOURNAL OF BIOMEDICAL RESEARCH2016年3期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Safety evaluation of a polyherbal formulation containing hydroalcoholic extracts of Hippophae salicifolia, Nyctanthes arbor-tristis, Ocimum tenuiflorum, and Reinwardtia indica in rodents
- Choriocarcinoma-associated pulmonary thromboembolism and pulmonary hypertension: a case report
- Effects of microalgal polyunsaturated fatty acid oil on body weight and lipid accumulation in the liver of C57BL/6 mice fed a high fat diet
- Caspase-1 inhibition attenuates activation of BV2 microglia induced by LPS-treated RAW264.7 macrophages
- Human lgG Fc promotes expression, secretion and immunogenicity of enterovirus 71 VP1 protein
- Effect of vitamin D3 on production of progesterone in porcine granulosa cells by regulation of steroidogenic enzymes
