Analysis of the androgen receptor CAG repeats length in Iranian patients with idiopathic non-obstructive azoospermia
Shohreh Zare-Karizi*, Mona Amin-Beidokhti, Mahnoosh Rahimi, Reza Mirfakhraie, *Department of Biology, Varamin Pishva Branch, Islamic Azad University, Varamin, IranDepartment of Medical Genetics, Shahid Beheshti University of Medical Sciences, Tehran, IranGenomic Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
ABSTRACT
Objective:To determine whether expansion of the CAG repeat in exon 1 of the AR gene is correlated with non-obstructive azoospermia (NOA) in Iranian infertile men. Methods:The CAG repeat length was investigated in 307 Iranian men, 104 infertile men with NOA and 203 fertile controls, using primers flanking the CAG repeat region in exon 1 of the AR gene. Results:The most common allele in the patients and controls was 23 (18.39%) and 21 (19.70%) CAG repeats with the mean number of (21.970±2.772) and (21.100±2.674), respectively (P=0.013). Conclusion:Although our results indicate a significant negative correlation between the length of CAG repeat and male infertility, however, other genetic modifiers may be required in order to cause male infertility.
ARTICLE INFO
Article history:
Received 22 February 2015
Received in revised form 7 October 2015
Accepted 10 November 2015
Available online 1 January 2016
?
Analysis of the androgen receptor CAG repeats length in Iranian patients with idiopathic non-obstructive azoospermia
Shohreh Zare-Karizi1*, Mona Amin-Beidokhti2, Mahnoosh Rahimi2, Reza Mirfakhraie2, 3*
1Department of Biology, Varamin Pishva Branch, Islamic Azad University, Varamin, Iran
2Department of Medical Genetics, Shahid Beheshti University of Medical Sciences, Tehran, Iran
3Genomic Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
ABSTRACT
Objective:To determine whether expansion of the CAG repeat in exon 1 of the AR gene is correlated with non-obstructive azoospermia (NOA) in Iranian infertile men. Methods:The CAG repeat length was investigated in 307 Iranian men, 104 infertile men with NOA and 203 fertile controls, using primers flanking the CAG repeat region in exon 1 of the AR gene. Results:The most common allele in the patients and controls was 23 (18.39%) and 21 (19.70%) CAG repeats with the mean number of (21.970±2.772) and (21.100±2.674), respectively (P=0.013). Conclusion:Although our results indicate a significant negative correlation between the length of CAG repeat and male infertility, however, other genetic modifiers may be required in order to cause male infertility.
ARTICLE INFO
Article history:
Received 22 February 2015
Received in revised form 7 October 2015
Accepted 10 November 2015
Available online 1 January 2016
Keywords:
Tel:+989125953602
E-mail:shohreh1357us@yahoo.com
Reza Mirfakhraie, PhD, Department of Medical Genetics, Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Koodakyar St.,Velenjak St., Shahid Chamran Highway, Tehran, Iran Zip:19395-4719.
Tel:+989121838075
E-mail:reza_mirfakhraie@yahoo.com
1. Introduction
Androgen receptor (AR; OMIM:313700), mediates the role of androgens including testosterone and dihydrotestosterone in regulating spermatogenesis and development of the male phenotype. Therefore, abnormalities in the AR that results in the defects in the androgen response pathway may be involved in the etiology of idiopathic azoospermia.
The AR gene is located at Xq11.12, composed of 8 exons[1]. The gene encodes 919 amino acids that form four domains in the protein structure, including the N-terminal transactivation domain encoded by exon 1, DNA-binding domain encoded by exons 2 and 3, hinge region encoded by part of exon 4, and C-terminal ligand-binding domain encoded by exons 4 to 8 domains[2].
There are two highly polymorphic CAG and GGN microsatellite repeats in the transactivation domain, which encodes for a polyglutamine and polyglycin stretches respectively. The number of CAG repeats varies from 9 to36 in healthy individuals with an average of 18-22 repeats[3]. The average number of repeat lengths varies significantly between different racial groups[1, 3]. It has been suggested that there is an inverse correlation between the CAG repeat number in exon 1 and male infertility; therefore, the longer polyglutamine tracts may be considered a risk factor for defective spermatogenesis due to reduced AR transcriptional activity both in vivo and in vitro[4, 5]. Conflicting results have been obtained from previous studies concerning the association between the lengths of the polyglutamine tract and developing idiopathic male infertility.
The aim of the present study was to determine the distribution of CAG repeat expantion in Iranian population and to investigate its association with non-obstructive azoospermia.
2. Material and methods
2.1. Patients
In a case-control study that was carried out between June 2012and July 2013 in Medical Genetics Department of Shahid Beheshti University of Medical Sciences, 104 non-obstructive azoospermic patients were studied to determine the effect of the polyglutamine tract length in male infertility. The patients were candidates for ICSI and referred from the whole country. Semen analysis was performed according to normal standard parameters using the World Health Organization (WHO) criteria[6]. Urological examination was performed in all of the patients for anatomical integrity of genital system. Hormone analysis including folloicle-stimulating hormone (FSH) and luteinizing hormone (LH) were also done. 203 age-matched fertile men who had at least one child with no history of requiring assisted reproduction technology were considered as control group. Informed written consent was obtained from each azoospermic and fertile control man.
2.2. Genotyping
DNA was extracted from the peripheral blood of each subject by a salting out method. A series of 11 sequence-tagged site (STS) markers located on Yq11 were selected for the detection of submicroscopic deletions in the azoospermia factor (AZF) region using multiplex polymerase chain reaction (M-PCR)[7]. The STS markers included sY84, sY86, sY176 and sY182 for AZFa; sY127 and sY134 for AZFb; and sY142, sY254, sY255, sY1191 and sY1291 for the AZFc region. The CAG repeats in exon 1 of the AR gene was amplified by using primers forward (5’- GGAAGTAGGTGGAAGATTCAGCCA -3’) and reverse (5’-AACGTGGATGGGGCAGCTGAGTCA -3’).
Genomic DNA (100 ng) was added to a mixture of 200 mmol/L Tris-HCl (pH 8.3), 100 mmol/L KCl, 3 mmol/L MgCl2, 5 mmol/L each dNTP, 10 per cent dimethyl sulfoxide (DMSO), 5 pmol/L of primer pairs, 2 U Taq DNA polymerase (Cynagen Co., Tehran, Iran), adjusted to a final volume of 25 μL. Amplifications were carried out on a Analytikjena thermocycler (Analytic jena Ltd., Germany) with the following program:Initial denaturation at 94 ℃ for 5 min and a subsequent series of 35 cycles of 94 ℃ for 30 sec (denaturation), 61 ℃ for 45 sec (annealing), and 72 ℃ for 1 min (extension). Final extension was carried out at 72 ℃ for 5 min. PCR products were sequenced on an ABI 3730XL automated DNA sequencer (Macrogen, Seoul, Korea).
2.3. Statistical analysis
Data were analyzed with SPSS 18 statistical software (SPSS Inc., Chicago, Illinois, USA). Differences in the mean of (CAG)n length between patients and controls was determined by the independent samples t-test. P values < 0.05 were considered statistically significant.
3. Results
Seventheen patients (16.35%) showed Y chromosome microdeletions and therefore were excluded from our study. The mean value for FSH concentration in patients with Y chromosome microdeletions was 44.90 mIU/mL compared with 35.50 mIU/mL in patients with no microdeletions (P<0.001). These values were 13.91 and 13.69 mIU/mL for LH concentration respectively (P=0.178). The mean serum FSH and LH levels were significantly higher in patients than that in the fertile controls (P<0.0001). The mean number of CAG repeats in exon 1 of the AR gene were 21.97±2.772 and 21.10±2.674 for infertile men and controls, respectively (P=0.013) (Table 1). The distribution of CAG repeat lengths in the studied groups is shown in Figure 1.
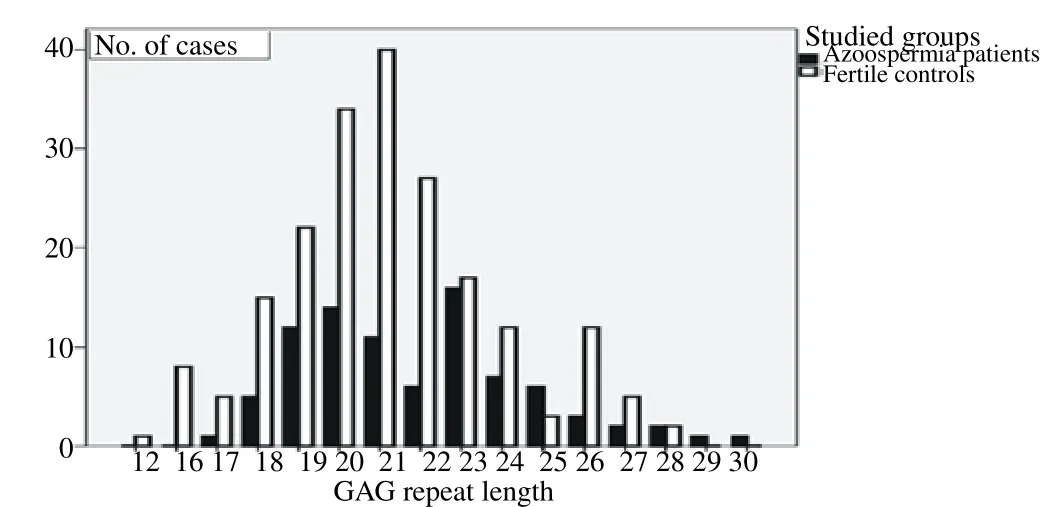
Figure 1. Distribution of the CAG repeat lengths in the studied groups.
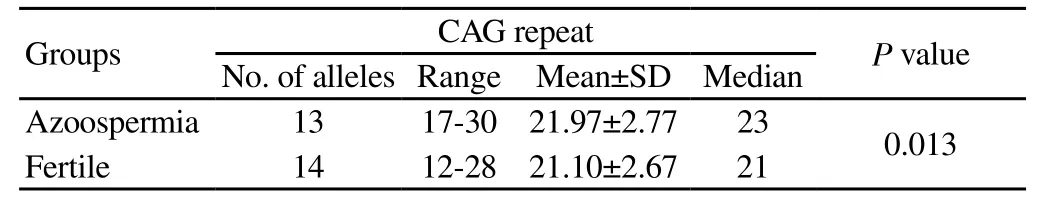
Table 1 Comparison of CAG repeats length between non-obstructive azoospermia patients and fertile controls.
4. Discussion
The CAG repeat lengths in the exon 1 of the AR gene were analyzed in several studies worldwide. The first report on association between CAG tract length variation and male infertility was published by Tut et al. in 1997. They showed that a CAG repeat of 28 or more results in fourfold increase risk for impaired spermatogenesis[4]. This observation was confirmed by Mifsud et al. who showed that having >26 (CAG)n repeats is associated with sevenfold increase of being infertile[8, 9]. However, the association between the length of CAG repeat and male infertility has been questionable. Some investigators have reported a statistical significant longer CAG tracts in infertile men[1, 2, 10-12], while, others showed no association[3, 5, 12-16]. Decrease in CAG repeats in the androgen receptor gene has been also reported in men withdefective sperm production[12, 17]. The results of the present study indicate a significant difference in the mean length of the CAG repeats in azoospermia patients (21.97±2.772) compared with fertile controls (21.10±2.674) (P=0.013).
Several factors including ethnicity, sample size and the inclusion criteria may explain the observed discrepancies among previous studies concerning the association between CAG repeat number and male infertility. The first and most important factor is differences in the ethnicity and the genetic backgrounds of the studied patients. It has been shown that the size of CAG repeats varies in a race specific manner. Short CAG repeats are more prevalent in black Americans than Asians[2]. Interestingly, conflicting results have also obtained from studies on the same population with mixed ethnic origin. In two previous studies conducted by Barden et al. and Mosaad et al, the association between CAG repeat length and male infertility was investigated in Egyptian population and contradictory results were obtained. The main reason was that the genetic background of the studied people in these regions is quite different[2, 3]. This issue should be concerned in similar studies on other heterogeneous populations such as Iranian population due to mixed ethnicity.
Another factor that may explain the observed discrepancies is different diagnostic criteria that may affect the results. In some studies karyotype analysis, detection of Y chromosome microdeletions, and screening for the CFTR and AR gene mutations were performed in the patients. However, not all of these excluding criteria were considered in all studies. In addition, in the majority of studies a heterogenous group of infertile men including nonobstructive azoospermia, oligozoospermia, severe aligozoospermia and oligoasthenoteratozoospermia were investigated. The CAG expansion situation may be different in each type of male infertility. In addition separate statistical analysis in each infertile group may result in the reduction in the sample size that is not preferred when a genetic variant is incompletely penetrant. Finally, the inclusion criteria for the control group were different in the previous studies. It should be noted that not all individuals with normal spermiogram are fertile and proven fertility does not always mean to have normal sperm parameters.
It was mentioned that longer CAG repeat lengths result in reduced AR transcriptional activity. However, the molecular mechanisms beyond this situation are not clearly understood. Some of the hypotheses postulate that the increase in the length of the CAG tract might impair the interaction between androgen receptor and coactivator molecules such as p160 that result in the alteration of the activation function 1 (AF1) located in the N-terminal region of the AR[18]. Others have also suggested that longer than normal CAG repeats results in a reduced amount of AR messenger RNA and protein[19]. There are also some clinical evidences for this inverse correlation between the length of CAG repeats in the AR gene and its transcriptional activity. In patients with Kennedy disease, characterized by spinal and bulbar muscular atrophy associated with reduced virilisation and defective spermatogenesis, the CAG repeat lengths are more than 40 repeats[2, 5]. Moreover, Zinn et al. have suggested that the polyglutamine length might influence the phenotype of klinefelter syndrome[20].
In conclusion, the present study showed a significant correlation between CAG repeat length and risk of non-obstructive azoospermia in Iranian population. The variability of the reported results might be mainly due to different ethnicity and genetic modifiers in the studied populations.
Declare of interest statement
We declare that we have no conflict of interst.
Acknowledgment
The authors are grateful to Yazd Clinical Centre for Infertility, Day Hospital IVF section, and Kowsar Infertility Treatment Center for their kind collaboration.
References
[1] Mostafa T, El-Shahid LH, El Azeem AA, Shaker O, Gomaa H, Abd El Hamid HM. Androgen receptor-CAG repeats in infertile Egyptian men. Andrologia 2012; 44(3):147-151.
[2] Mosaad YM, Shahin D, Elkholy AA, Mosbah A, Badawy W. CAG repeat length in androgen receptor gene and male infertility in Egyptian patients. Andrologia 2012; 44(1):26-33.
[3] Badran WA, Fahmy I, Abdel-Megid WM, Elder K, Mansour R, Kent-First M. Length of androgen receptor-CAG repeats in fertile and infertile Egyptian men. J Androl 2009; 30(4):416-425.
[4] Tut TG, Ghadessy FJ, Trifiro MA, Pinsky L, Yong EL. Long polyglutamine tracts in the androgen receptor are associated with reduced trans-activation, impaired sperm production, and male infertility. J Clin Endocrinol Metab 1997; 82(11):3777-82.
[5] Ferlin A, Bartoloni L, Rizzo G, Roverato A, Garolla A, Foresta C. Androgen receptor gene CAG and GGC repeat lengths in idiopathic maleinfertility. Mol Hum Reprod 2004; 10(6):417-421.
[6] World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva, Switzerland:WHO; 2012.
[7] Krausz C, Hoefsloot L, Simoni M, Tuttelmann F, European Academy of A, European molecular genetics quality N. EAA/EMQN best practice guidelines for molecular diagnosis of Y-chromosomal microdeletions:state-of-the-art 2013. Andrology 2014; 2(1):5-19.
[8] Mifsud A, Sim CK, Boettger-Tong H, Moreira S, Lamb DJ, Lipshultz LI, et al. Trinucleotide (CAG) repeat polymorphisms in the androgen receptor gene:molecular markers of risk for male infertility. Fertil Steril 2001; 75(2):275-281.
[9] Tahmasbpour E, Balasubramanian D, Agarwal A. A multi-faceted approach to understanding male infertility:gene mutations, molecular defects and assisted reproductive techniques (ART). J Assist Reprod Genet 2014; 31(9):1115-1137.
[10] Castro-Nallar E, Bacallao K, Parada-Bustamante A, Lardone MC, Lopez PV, Madariaga M, et al. Androgen receptor gene CAG and GGN repeat polymorphisms in Chilean men with primary severe spermatogenic failure. J Androl 2010; 31(6):552-559.
[11] Giagulli VA, Carbone MD, De Pergola G, Guastamacchia E, Resta F, Licchelli B, et al. Could androgen receptor gene CAG tract polymorphism affect spermatogenesis in men with idiopathic infertility? J Assist Reprod Genet 2014; 31(6):689-697.
[12] Ryan CP, Crespi BJ. Androgen receptor polyglutamine repeat number:models of selection and disease susceptibility. Evol Appl 2013; 6(2):180-196.
[13] Akinloye O, Gromoll J, Nieschlag E, Simoni M. Androgen receptor gene CAG and GGN polymorphisms in infertile Nigerian men. J Endocrinol Invest 2009; 32(10):797-804.
[14] Hadjkacem-Loukil L, Hadj-Kacem H, Hadj Salem I, Bahloul A, Fakhfakh F, Ayadi H. Genotyping of Tunisian azoospermic men with Sertoli cell-only and maturation arrest. Andrologia 2011. doi:10.1111/ j.1439-0272.2010.01088.x.
[15] Peterlin B, Zorn B, Teran N, Kunej T. Analysis of the CAG repeat number in exon 1 of the androgen receptor gene in Slovene men with idiopathic azoospermia and oligoasthenoteratozoospermia. Asian J Androl 2007; 9(2):280-282.
[16] Martinez-Garza SG, Gallegos-Rivas MC, Vargas-Maciel M, Rubio-Rubio JM, de Los Monteros-Rodriguez ME, Gonzalez-Ortega C, et al. Genetic screening in infertile Mexican men:chromosomal abnormalities, Y chromosome deletions, and androgen receptor CAG repeat length. J Androl 2008; 29(6):654-660.
[17] Nenonen HA, Giwercman A, Hallengren E, Giwercman YL. Non-linear association between androgen receptor CAG repeat length and risk of male subfertility--a meta-analysis. Int J Androl 2011; 34(4):327-332.
[18] Gottlieb B, Lombroso R, Beitel LK, Trifiro MA. Molecular pathology of the androgen receptor in male (in)fertility. Reprod Biomed Online 2005; 10(1):42-8.
[19] Dietzsch E, Laubscher R, Parker MI. Esophageal cancer risk in relation to GGC and CAG trinucleotide repeat lengths in the androgen receptor gene. Int J Cancer 2003; 107(1):38-45.
[20] Zinn AR, Ramos P, Elder FF, Kowal K, Samango-Sprouse C, Ross JL. Androgen receptor CAGn repeat length influences phenotype of 47,XXY (Klinefelter) syndrome. J Clin Endocrinol Metab 2005; 90(9):5041-5046.
Androgen receptor
CAG repeat
Non-obstructive azoospermia
doi:Document heading 10.1016/j.apjr.2015.12.013
*Corresponding author:Sohreh Zare-Karizi, PhD, Department of Biology, Varamin Pishva Branch, Islamic Azad University, Naghsh e Jahan, Pishva,Varamin, Iran Zip:33817-74895.
 Asian Pacific Journal of Reproduction2016年1期
Asian Pacific Journal of Reproduction2016年1期
- Asian Pacific Journal of Reproduction的其它文章
- Diagnostic and decision-making difficulties:Placenta accreta at nine weeks’gestation
- Male masturbation device for the treatment of premature ejaculation
- Risk factors and adverse perinatal outcomes associated with low birth weight in Northern Tanzania:A registry-based retrospective cohort study
- Returning of cyclicity in infertile Corriedale Sheep with natural progesterone and GnRH based strategies
- Effect of cooling to different sub-zero temperatures on boar sperm cryosurvival
- Milk supplements in a glycerol free trehalose freezing extender enhanced cryosurvival of boar spermatozoa
