An unusual case of prolonged post-endoscopic retrograde cholangiopancreatography jaundice
Georgios Tziatzios,Paraskevas Gkolfakis,Ioannis S Papanikolaou,George Dimitriadis and Konstantinos TriantafyllouAthens,Greece
?
An unusual case of prolonged post-endoscopic retrograde cholangiopancreatography jaundice
Georgios Tziatzios,Paraskevas Gkolfakis,Ioannis S Papanikolaou,George Dimitriadis and Konstantinos Triantafyllou
Athens,Greece
ABSTRACT:Despite the effectiveness of endoscopic retrograde cholangiopancreatography(ERCP)for the treatment of choledocholithiasis,various complications have been described.We herein report the first case of prolonged post-ERCP jaundice due to toxicity of the contrast agent Iobitridol(?XENETIX,Guerbet,Roissy CdG Cedex,France)in a patient who underwent ERCP with sphincterectomy and common bile duct stone removal.While clinical improvement and normalization of aminotransferases and cholestatic enzymes after the procedure,an unexplained increase of direct bilirubin was noticed.A second ERCP was performed one week later,excluding possible remaining choledocholithiasis.Nevertheless,serum direct bilirubin increased further up to 15 mg/dL.Other potential causes of direct hyperbilirubinemia were ruled out and patient’s liver biopsy was compatible with drug-induced liver toxicity.Additionally,the cause-result time connection between the use of Iobitridol and bilirubin increase indicated the possibility of a toxic effect related to the repeated use of the particular contrast agent.Iobitridol,a contrast agent,can induce prolonged direct hyperbilirubinemia.
(Hepatobiliary Pancreat Dis Int 2016;15:220-222)
KEY WORDS:endoscopic retrograde cholangiopancreatography;
jaundice;
adverse effect;
Iobitridol
Author Affiliations:Hepatogastroenterology Unit,2nd Department of Internal Medicine and Research Institute,“Attikon” University General Hospital,Medical School,University of Athens,Athens,Greece(Tziatzios G,Gkolfakis P,Papanikolaou IS,Dimitriadis G and Triantafyllou K)
? 2016,Hepatobiliary Pancreat Dis Int.All rights reserved.
Published online July 17,2015.
Introduction
E ndoscopic retrograde cholangiopancreatography(ERCP)with sphincterectomy and stone removal remains the gold standard intervention for choledocholithiasis.[1]The major complications related to ERCP involve acute pancreatitis,bleeding,sepsis and perforation.Multiple studies have defined an incidence rate of about 7%.[2,3]Prolonged post-ERCP jaundice has been described as a rare post-ERCP complication.[4]We report one case of post-ERCP prolonged direct hyperbilirubinemia due to toxicity of a new generation contrast agent used for the procedure.
Case report
A 56-year-old Caucasian man presented with jaundice and right upper quadrant abdominal pain lasting for 3 days.The patient underwent laparoscopic cholecystectomy for acute cholecystitis two months before his admission.He was not taking any medication;he had no history of jaundice or anemia,hepatitis exposure,alcohol abuse,blood transfusion or travelling abroad.No signs of chronic hepatopathy were noticed.On admission,laboratory examinations revealed normal blood count,while liver function tests showed serum total bilirubin 9.6 mg/dL(normal <1.2 mg/dL),direct bilirubin 6.3 IU/L(normal <1.0 IU/L),alanine aminotransferase 440 IU/L(normal <41 IU/L),aspartate aminotransferase 213 IU/L(normal <38 IU/L),alkaline phosphatase 166 IU/L(normal <120 IU/L),and gamma-glutamyl transpeptidase(γ-GT)490 IU/L(normal <70 IU/L).Laboratory results indicating acute inflammation were absent.
Abdominal ultrasound showed a dilated(9 mm)common bile duct and indicated the presence of a stone in the region of the ampulla of Vater.Two days later the patient underwent ERCP with sphincterectomy and stone extraction,under conscious sedation with 2 mg of midazolam.We used 10 mL of the contrast agentIobitridol(?XENETIX,Guerbet,Roissy CdG Cedex,France)to opacify the bile duct.The drainage of the contrast from the common bile duct was complete after stone extraction.We observed clinical improvement as well as a progressive decrease of aminotransferases and cholestatic enzymes during the next days,while a marked increase was noted in both total(up to 15 mg/dL)and direct bilirubin(up to 12 mg/dL)(Fig.).One week later,a second abdominal ultrasonography was performed.Amorphous echogenic material detected in the common bile duct suggested a high suspicion of remaining choledocholithiasis,which led to the decision to perform a second ERCP.Midazolam 2 mg once again was used during ERCP for conscious sedation.Normal cholangiography using 10 mL of the same contrast agent ruled out choledocholithiasis.The following days,despite the normalization of aminotransferases and cholestatic enzymes,the levels of both total(up to 18 mg/dL)and direct bilirubin(up to 15 mg/dL)were increased further(Fig.).
Infections including hepatitis A,B,C and other hepatotropic viruses(cytamegalovirus,herpes simplex virus,and Epstein-Barr virus)were ruled out.Immunological studies on ANA,SMA,AMA,anti-M2 and anti-LKM showed negative results with the exception of a 3-fold increase in IgG-4 values(377 mg/dL).Ceruloplasmin and ferritin levels were normal.The patient underwent MRCP that was negative for intra- or extrahepatic bile duct pathology.The review of patient’s history provided no new information.Liver biopsy revealed mild inflammatory and lymphohistocytic infiltrations without bile duct loss or damage.Moderate intrahepatic cholestasis with occasional focal necrotic areas was also noticed,and findings were compatible with drug-induced liver toxicity.Without any treatment,the bilirubin level decreased slowly,and the patient was discharged 35 days after admission.Two months later,tests of liver function showed nothing abnormal(Fig.).
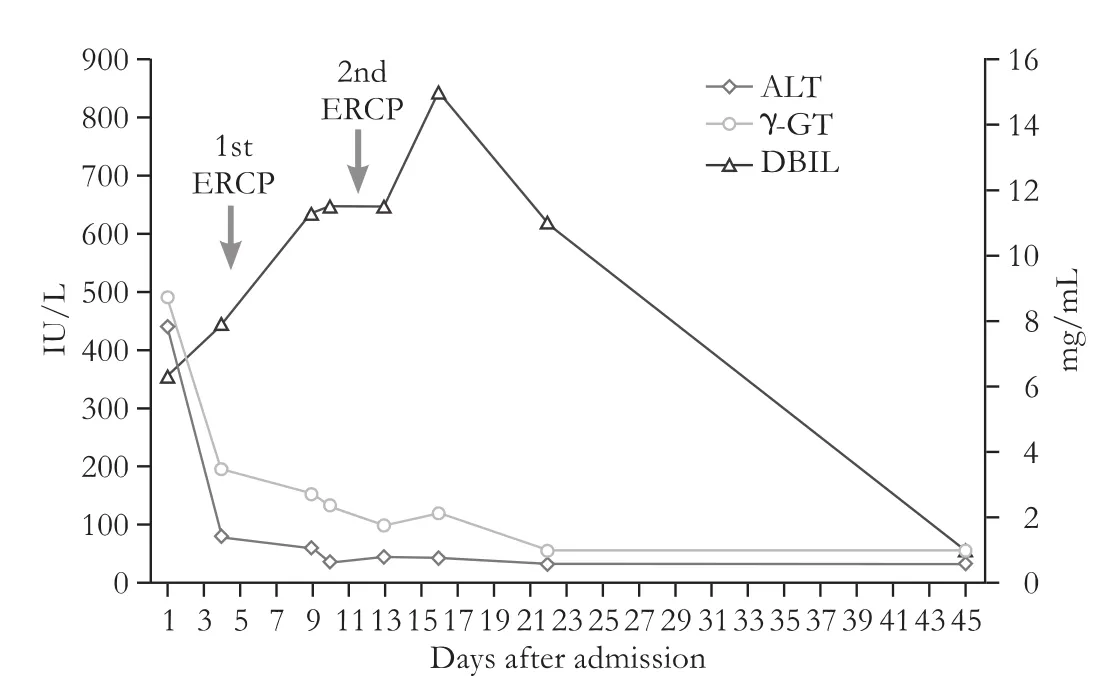
Fig.Liver enzymes course from admission until normalization.ALT:alanine aminotransferase;γ-GT:gamma-glutamyl transpeptidase;DBIL:direct bilirubin.
Discussion
Intrahepatic cholestasis clinically characterized by jaundice may be attributed to many different causes.In our patient,infection with hepatotropic viruses was excluded by serological testing.Our patient did not use any medication and the only agent used during pro-,peri- or post-ERCPs was midazolam(2 mg),needed for conscious sedation.A study[5]showed that midazolam is not related to cases of clinically apparent liver injury,although other benzodiazepines occasionally contribute to prolonged cholestasis(2-6 months).The quick normalization of cholestatic liver enzymes and the previous exposure to midazolam during cholecystectomy without increase of bilirubin level unlikely make midazolam and elevated bilirubin level related.However,this possibility cannot be excluded.In our patient,there was no evidence of an underlying autoimmune or metabolic disease since ceruloplasmin and ferritin levels were normal.An isolated elevation of IgG4 titers was insufficient to develop a possible autoimmune pancreatitis according to the Mayo Clinic HISORt criteria.[6]During hospitalization,there was no evidence of co-existing sepsis as the underlying factor that triggered laboratory manifestations in our patient.The patient remained afebrile,and his white cell count and other inflammation markers(e.g.CRP)were constantly normal.
Pathological report suggested a possible liver toxic effect caused by the contrast agent used in ERCP.[7]A “withdrawal test” was also relevant to a transient drug-induced liver injury since the liver test was normal after the removal of the potential toxicity(Fig.).A “re-challenge test”could confirm the diagnosis but it was thought to expose the patient to unnecessary danger and therefore was considered unethical.However,the repeated ERCP in differential diagnosis functioned as an unintentional re-challenge test.This allowed us to establish a clear time correlation between the administration of the contrast agent and the subsequent elevation of bilirubin level,thus strengthening our hypothesis of an underlying association.
Iobitridol is an iodinated contrast agent used for whole-body computed tomography,venography,intraarterial angiography and ERCP.[8]It is well tolerated[9,10]and its gastrointestinal side effects include nausea,vomiting and abdominal pain.[8]The agent is not metabolized,not taken up by hepatocytes and does not interfere with liver metabolism.Nevertheless,local effects,immune toxicological effects,and cellular effects can never be excluded.To our knowledge,this is the first case of prolonged jaundice induced by Iobitridol.
There were 2 cases of prolonged cholestasis characterized by jaundice due to a similar iodinated contrast agent Meglumine Amidotrizoate(ANGIOGRAFIN?,Schering AG,Germany).[4]The authors proposed the disruption of the plasma canalicular membranes caused by the high-pressure contrast agent infusion during ERCP as the possible mechanism of liver injury.This mechanism is difficult to explain hyperbilirubinemia in our patient since neither an alkaline phosphatase elevation nor an acinarization of the hepatic parenchyma during ERCP was observed.There is evidence that systemic distribution of the contrast agent from the bile duct might be responsible for direct toxic liver injury[11-13]since the contrast agent fills in the bile duct and might spread extracellularly into the nearby tissues.The exact mechanism however remains unknown.In contrast to these reported cases,in whom corticosteroids were used,[4]our patient presented complete normalization of biochemical results without any special treatment.This allows us to speculate a potential idiosyncratic reaction as the cause of the syndrome.
In conclusion,prolonged jaundice due to iodinated contrast agents could be considered as a rare post-ERCP complication,and physicians should be aware of it.
Contributors:TK proposed the study.TG,GP and PIS collected the literature.TG and GP wrote the first draft.All authors contributed to the design and interpretation of the study and to further drafts.TK is the guarantor.
Funding:None.
Ethical approval:Not needed.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
1 Adler DG,Baron TH,Davila RE,Egan J,Hirota WK,Leighton JA,et al.ASGE guideline:the role of ERCP in diseases of the biliary tract and the pancreas.Gastrointest Endosc 2005;62:1-8.
2 Andriulli A,Loperfido S,Napolitano G,Niro G,Valvano MR,Spirito F,et al.Incidence rates of post-ERCP complications:a systematic survey of prospective studies.Am J Gastroenterol 2007;102:1781-1788.
3 Wang P,Li ZS,Liu F,Ren X,Lu NH,Fan ZN,et al.Risk factors for ERCP-related complications:a prospective multicenter study.Am J Gastroenterol 2009;104:31-40.
4 Dourakis SP,Mayroyannis C,Alexopoulou A,Hadziyannis SJ.Prolonged cholestatic jaundice after endoscopic retrograde cholangiography.Hepatogastroenterology 1997;44:677-680.
5 http://livertox.nih.gov/Midazolam.htm;Accessed on November 06,2014.
6 Chari ST,Smyrk TC,Levy MJ,Topazian MD,Takahashi N,Zhang L,et al.Diagnosis of autoimmune pancreatitis:the Mayo Clinic experience.Clin Gastroenterol Hepatol 2006;4:1010-1016.
7 Barritt AS 4th,Lee J,Hayashi PH.Detective work in druginduced liver injury:sometimes it is all about interviewing the right witness.Clin Gastroenterol Hepatol 2010;8:635-637.
8 MHRA.Summary of Product Characteristics.http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1362976132452.pdf.Accessed on July 20,2014.
9 Maurer M,Heine O,Wolf M,Freyhardt P,Schnapauff D,Hamm B.Safety and tolerability of iobitridol in general and in patients with risk factors:results in more than 160,000 patients.Eur J Radiol 2011;80:357-362.
10 McCormack PL.Iobitridol:a review of its use as a contrast medium in diagnostic imaging.Clin Drug Investig 2013;33:155-166.
11 Chavalitdhamrong D,Donepudi S,Pu L,Draganov PV.Uncommon and rarely reported adverse events of endoscopic retrograde cholangiopancreatography.Dig Endosc 2014;26:15-22.
12 Draganov P,Cotton PB.Iodinated contrast sensitivity in ERCP.Am J Gastroenterol 2000;95:1398-1401.
13 Draganov PV,Forsmark CE.Prospective evaluation of adverse reactions to iodine-containing contrast media after ERCP.Gastrointest Endosc 2008;68:1098-1101.
Received October 9,2014
Accepted after revision March 11,2015
Announcements for this section should be submitted in the correct format at least 3 months before the required date of publication.This list is provided as a service to readers;inclusion does not imply endorsement by the Hepatobiliary &Pancreatic Diseases International.
Section editor
Shui-Ying Lei
Email:hbpdint@126.com
April,2016
AACR annual meeting 2016
April 16-20,2016;Ernest N.Morial Convention Center,New Orleans,Louisiana,USA
The AACR annual meeting highlights the best cancer science and medicine from institutions all over the world.Attendees are invited to stretch their boundaries,form collaborations,attend sessions outside their own areas of expertise,and learn how to apply exciting new concepts,tools,and techniques to their own research.Program committee chairperson is Scott A.Armstrong,Memorial Sloan Kettering Cancer Center,New York,New York,USA.For more information,please visit:http://www.aacr.org/Meetings/Pages/MeetingDetail.aspx?EventItemID=63#.VfFHZ_nvPGg.
May
McMaster international review course in internal medicine(MIRCIM)
May 6-7,2016;Krakow,Poland
This course is organized by McMaster University Department of Medicine,Jagiellonian University Medical College,and the Polish Society of Internal Medicine.Co-chairs of the Organizing Committees are Akbar Panju,MB ChB FRCPC FRCP(Edin),FRCP(Glasg)FACP,McMaster University,Canada;Paul O’Byrne,MB FRCP(C)FRSC,Chair,Department of Medicine,Mc-Master University,Canada;and Piotr Gajewski,MD PhD FACP,Polish Society of Internal Medicine,Medycyna Praktyczna.The aim of MIRCIM 2016 is an accessible presentation of the most practical,up-to-date,evidence-based knowledge useful in everyday practice.The presentations will provide a unique opportunity to discuss the most relevant statements presented in the latest guidelines,with take-home messages for immediate implementation in your patients’care.The broad array of issues analysed during the lectures and panel discussions will undoubtedly benefit a wide audience of general internists,subspecialists,hospitalists,family physicians,residents and fellows in training specialising in internal medicine.The sheer diversity of topics makes the McMaster Course a uniquely rich educational experience,providing the basis for a holistic approach to challenges faced today by medical professionals around the world.For more information,please visit:http:// www.globaleventslist.elsevier.com/events/2016/05/mcmaster-international-review-course-in-internal-medicine-mircim/.
NASH:Beyond the acronym:certainties and clinical dilemmas
May 12-14,2016;Riga,Latvia
Scientific organizing committees are Jean-Francois Dufour,Bern,Switzerland;Manuel Romero-Gomez,Sevilla,Spain;and Vlad Ratziu,Paris,France.Topics covered include NASH,epidemiology,disease history and prognostic,diagnosis progress,and disease management.The conference will be an excellent place to discuss epidemiology and pros and cons for population screening together with the analysis of individual risk factors from genes to environment.NAFLD as a systemic disease is associated with several pathological conditions from cardiovascular to kidneys and lung diseases.Diagnostic methods would be addressed,including liver histology as well as non-invasive methods.Defining safe and accurate noninvasive diagnostic methods are an unmet need that is mandatory to resolve for the improvement of knowledge and management of this entity.Mechanism of disease progression could allow us to look for new therapeutic targets.Lastly,patient selection for therapeutic interventions starting with approaches to promote weight loss using diet and physical exercise interventions to bariatric surgery will be reviewed.In non-responders patients,emerging pharmacologic options would fill the gap to increase success rate in NASH resolution.For further information,please visit:https://events.easl.eu/Event-Portal/Information/MR/HOME.aspx.
ILTS workshop:liver transplantation in HCV positive recipients
May 19-20,2016;San Francisco,CA,USA
The ILTS workshop and consensus conference will take place the 2 days prior to digestive diseases week(DDW)being held in San Diego,CA,so any delegates would be able to attend this workshop and conference as well as DDW.For further information,please contact:Norah Terrault,MD,MPH,Consensus Co-Chair,Professor of Medicine and Surgery,Director,Viral Hepatitis Center,Division of Gastroenterology,University of CaliforniaSan Francisco,San Francisco,California,Norah.Email:Terrault@ucsf.edu;Or Lisa D.Pedicone,PhD,Consensus Committee Consultant,Executive Vice President,Clinical Affairs,Focus Medical Communications,Parsippany,New Jersey 07054,USA.Tel:+973-520-1822;Email:lpedicone@focusmeded.com.
Digestive eisease week?(DDW)
May 21-24,2016;San Diego,USA
DDW is considered the largest and most prestigious meeting in the world for the GI professional.Every year it attracts more than 15 000 physicians,researchers and academics from around the world who desire to stay up-to-date in the field.The meeting is the year’s best opportunity to learn about the latest advances in gastroenterology,hepatology,endoscopy and gastrointestinal surgery,prevention,diagnosis and treatment of digestive disorders,and cutting-edge technological advances.For more information,please visit:www.ddw.org.
Singapore hepatitis conference(SHC)2016
May 27-28,2016;Singapore,Singapore Organized by Singapore Hepatitis Conference Pte Ltd.,the 3rd Singapore hepatitis conference(SHC 2016)will be held on May 27-28,2016 at Suntec Singapore.SHC 2016 is a dynamic platform poised to create awareness,provide practical tips and new updates,and share exciting developments in the clinical management of HBV and HCV.SHC is pleased to announce its second year of collaboration with the European Association for the Study of the Liver(EASL).EASL will showcase yet another highly anticipated “Best of EASL” sessions over the 2-day Conference.The scientific program provided a multi-disciplinary approach to the assessment,diagnosis and treatment strategies to eradicate HBV and HCV.For more information,please visit:http://singaporehepatitisconference.com/.
June
Liver fibrosis:the next goal of targeted therapy?
June 17-18,2016;Porto,Portugal
Scientific organizing committees are Sophie Lotersztajn,Paris,France;Massimo Pinzani,London,UK;and Christian Trautwein,Aachen,Germany.The conference is dedicated to all the current key areas of research in liver fibrogenesis and will focus on a number of open issues requiring further scientific efforts.The role of genetic/epigenetic factors and of the immune system,and the reversibility of fibrosis and methodologies for the identification of more reliable targets for drug development will represent some of the hot topics to be covered.Young scientists are particularly encouraged to participate and will have the opportunity to present their original results during a dedicated session.The format is intended to generate active interactions and discussion between basic scientists and clinicians,and to foster future collaborative efforts to better understand the pathogenesis of tissue fibrosis in chronic liver disease.Ultimately,to improve patient management.For further information,please visit:https://events.easl.eu/Event-Portal/Information/EventInformation.aspx?EventInfor mationPageCode=HOME&EventCode=MONP.
Cost-effectiveness in liver disorders:from prevention to transplantation
June 23-25,2016;Budapest,Hungary
At this Budapest meeting,cost-effectiveness of different liver diseases will be analyzed by medical and health-economy experts to help physicians,insurance companies and decision makers in rational allocation of resources.Main topics include health benefit/costeffectiveness of primary prevention(vaccination,diet,environmental factors);screening and surveillance(viral hepatitis,NAFLD,HCC);diagnostic algorithms(enzyme elevation,nodules,rare liver diseases);medical therapy(HCV,HBV,HCC,NFLD,NASH,cirrhosis etc.);surgical/invasive therapy(TIPS,RFA,transplantation);burden of liver diseases from scientific;and payers’perspectives.For further information,please visit:http://www.celdbudapest.eu/.
August
World congress of internal medicine(WCIM)2016,Bali
August 22-25,2016;Bali Nusa Dua Convention Center(BNDCC),Bali,Indonesia
The congress is organized by Indonesian Society of Internal Medicine.The 33rd world congress of internal medicine(WCIM)is designed to promote scientific knowledge,medical advancement,and the delivery of effective healthcare in the field of internal medicine.The scientific papers will be mostly presented and moderated by leaders in the field and attendees will benefit from the lively discussions on thought-provoking and timely topics in internal medicine.For further information,please visit:http://www.wcimbali2016.org/.
doi:10.1016/S1499-3872(15)60402-7 10.1016/S1499-3872(16)60083-8)
Corresponding Author:Konstantinos Triantafyllou,MD,Hepatogastroenterology Unit,2nd Department of Internal Medicine and Research Institute,“Attikon” University General Hospital,Medical School,University of Athens,1 Rimini Street,124 62 Athens,Greece(Tel:+30-210-5832089;Fax:+30-210-5326422;Email:ktriant@med.uoa.gr)
 Hepatobiliary & Pancreatic Diseases International2016年2期
Hepatobiliary & Pancreatic Diseases International2016年2期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- OIder age at first birth is a risk factor for pancreatic cancer:a meta-anaIysis
- Pathologic response to preoperative transarterial chemoembolization for resectable hepatocellular carcinoma may not predict recurrence after liver resection
- Role of microRNA in liver regeneration
- Surgical treatment of synchronous colorectal liver and lung metastases:the usefulness of thoracophrenolaparotomy for single stage resection
- Monocyte chemoattractant protein-1,transforming growth factor-β1,nerve growth factor,resistin and hyaluronic acid as serum markers:comparison between recurrent acute and chronic pancreatitis
- Improvement of gastric emptying by enhanced recovery after pancreaticoduodenectomy
