Role of microRNA in liver regeneration
Peng-Sheng Yi,Ming Zhang and Ming-Qing XuChengdu,China
?
Role of microRNA in liver regeneration
Peng-Sheng Yi,Ming Zhang and Ming-Qing Xu
Chengdu,China
BACKGROUND:Liver regeneration is a complex process.microRNAs(miRNAs)are short,single-stranded RNAs that modify gene expression at the post-transcriptional level.Recent investigations have revealed that miRNAs are closely linked to liver regeneration.
DATA SOURCES:All included studies were obtained from PubMed,Embase,the ScienceDirect databases and Web of Science,with no limitation on publication year.Only studies published in English were considered.
RESULTS:We grouped studies that involved miRNA and liver regeneration into two groups:miRNAs as promoters and as inhibitors of liver regeneration.We summarized the relevant miRNAs separately from the related pathways.
CONCLUSIONS:Blocking or stimulating the pathways of miRNAs in liver regeneration may be novel therapeutic strategies in future regeneration-related liver managements.We may discover additional chemotherapy targets of miRNA.
(Hepatobiliary Pancreat Dis Int 2016;15:141-146)
KEY WORDS:microRNAs;
liver regeneration;
gene expression;
target;
pathway
Author Affiliations:Department of Liver and Vascular Surgery,West China Hospital,Sichuan University,Chengdu 610041,China(Yi PS,Zhang M and Xu MQ)
? 2016,Hepatobiliary Pancreat Dis Int.All rights reserved.
Published online December 30,2015.
Introduction
M icroRNAs(miRNAs)are a group of small regulatory RNAs,most of which are 22-nucleotides long.These miRNAs participate in a variety of pathological and physiological processes.Lee et al[1]identified the first miRNA(lin-4)in 1993.However,miRNAs were not recognized as a distinct class of biological regulators until the early 2000s when Pasquinelli and coworkers found the regulatory function of let-7 in invertebrates and vertebrates.[2]The biosynthesis of miRNA was firstly described by Bartel.[3]Generally,RNA polymerase II transcribes miRNA genes into hair-pin stem-loop structures,which is known as primary miRNA.Primary miRNA is transformed to precursor miRNAs within the nucleus by the Drosha-DGCR8,further processed by the Dicer1-TARBP2 complex and generated the mature miRNA duplexes.miRNAs function is via the inhibition of expression of protein-encoding genes and through binding to complementary sequences in the 3’-untranslated regions(UTRs)of their target mRNAs.Studies[4,5]demonstrated that miRNAs are correlated with cell proliferation,apoptosis,differentiation,and tumor progression.It was reported that differential expression of miRNAs might be involved in liver development or carcinogenesis.Commonly,in both cases,miRNAs modulate the expression of genes that function in proliferation and replication.[6]
The liver is a typical organ with a unique ability to regenerate after injury,which is the theoretical basis of liver transplantation.[7]The demand for high quality donor graft far outweighs the graft we can supply,one of the limitations is the gap between insufficient liver volume for the safety of donors and the adequate liver volume for survival of recipients.The capability of liver regeneration after partial hepatectomy or trauma is a critical prognostic factor for patients with living donor liver transplantation or acute liver injury.Liver regeneration is a complex process that consists of three main phases:initiation,proliferation,and metabolic adaption.In the past several decades,investigators have identified a large number of molecules that associated with liver regeneration,such as hepatocyte growth factor,cytokines,and matrix remodeling factors,etc.Additionally,the processes of initiation and termination of liver regeneration have already been partially clarified.However,the mechanism of liver regeneration is not completely understood.
Recently,emerging evidence has shown a close association between miRNAs and liver regeneration.Kren et al[8]revealed the role of miRNA in the regulation of c-Mycand p53,which caused an expression alteration in liver regeneration.Afterward,a variety of miRNAs including micro-21 and micro-34 were found to participate in liver regeneration.[9,10]Generally,the study protocols on miRNAs in liver regeneration are similar.In general,liver samples were processed for RNA extraction with the TRIZOL reagent.Briefly,the frozen tissue was placed in TRIZOL reagent and immediately homogenized by using a prechilled mortar and pestle.The samples were then processed for isolation of total RNA.miRNA was isolated from frozen liver specimens by different techniques following the manufacturer’s recommendations.Finally,miRNA was detected by qRT-PCR miRNA assays,which is the standardized equipment for this detection.However,few reviews have summarized the function of specific miRNAs in liver regeneration.The latest review by Finch et al[4]presented an overview of miRNAs in liver development,regeneration,and disease.The present review is to summarize our knowledge of miRNAs in the modulation of liver regeneration.
miRNAs promote liver regeneration and related pathway
A variety of miRNAs have been reported to promote liver regeneration.Castro et al[11]harvested RNA at 3-72 hours after partial hepatectomy in rats and found that the feeding of ursodeoxycholic acid induced a sustained increase of proliferative miRNAs at early phase after partial hepatectomy.In all,26 miRNAs were found to be differentially expressed by 1.5-fold or more following partial hepatectomy,especially micro-21.They elucidated that micro-19a,micro-21,and micro-214 target the phosphatase and tensin homolog deleted on chromosome ten(PTEN),which is a negative regulator of the PI3K/Akt survival pathway.Additional studies demonstrated that PI3K/Akt is key mediator in the modulation of regeneration by IL-6.Chou et al[12]described the anti-apoptotic role of Mcl-1L during liver regeneration and found that Mcl-1L was stimulated by IL-6 through the JAK/PI3K/ Akt/CREB signaling pathway.Therefore,PI3K/Akt might be a common pathway in liver regeneration.
Several other miRNAs,such as micro-106a,micro-20a,micro-20b,and micro-93,are modulators of vascular endothelial growth factor(VEGF).[11]VEGF has been widely accepted as a key mediator in carcinogenesis due to its angiogenic effects.Scartozzi and colleagues[13]recommended VEGF and VEGFR genotyping as the predictor factors of patients with hepatocellular carcinoma who receiving treatment with sorafenib.Liver regeneration is a process that consists of proliferation and angiogenesis,and VEGF is a potent angiogenic factor.It has been demonstrated that tissue resection increases this cytokine which plays an important role during liver regeneration.[14]In addition,VEGF acts through the mediation of liver endothelial cells to communicate with neighboring parenchymal cells.This communication promotes the expression of VEGF and its receptors,and induces the proliferation of endothelial cells.
Raschzok et al[15]investigated the expression of 323 miRNAs after hepatectomy,and the expression level of 29 miRNAs was significantly altered.Among the 29 miRNAs,7 miRNAs(micro-33,micro-153,micro-298,micro-301b,micro-489,micro-743b and micro-883)were up-regulated.Interestingly,none of these changes reached statistical significance in the early phase of regeneration,but rather,the significant upregulation peaked at 24 hours after hepatectomy,which is consistent with DNA replication.This result revealed that these 7 miRNAs play key roles in the early phase of liver regeneration,primarily during G1/S.Putative targets of these miRNAs are CDK6,RAP2A,TNF,CCND1,and MAP3K1.[15]Salehi et al[16]reported that the expression of micro-126,micro-130a,micro-20a and micro-520e was significantly upregulated after liver transplantation.They also discovered that angiogenesis was induced by micro-126 through the VEGF signaling pathway,which is similar to the pathway through which micro-106a,micro-20a,micro-20b,and micro-93 modulate regeneration.Increasing expression of micro-520e attenuated the expression of the membrane-bound complement regulator CD46 and thereby increased the expression of the complement components C4b and C3b,which are mediators of early liver regeneration.As well-known,the liver is the primary source of complement proteins,and emerging evidences indicate that the complement cascade(especially C3 and C5)might regulate liver regeneration.Rutkowski et al[17]found that C3 and C5 double knockout mice showed significant hepatocyte apoptosis following partial hepatectomy,in addition,reconstitution of C3 or C5 attenuated this injury,reconstitution of both almost completely prevented apoptotic injury.
A study[18]on the role of specific miRNAs in liver regeneration showed that micro-21 promotes liver regeneration directly via the inhibition of Btg2.Btg2 is a cell cycle inhibitor that prevents the activation of forkhead box M1,which is essential for DNA synthesis in hepatocytes after 2/3 partial hepatectomy.The PI3K/Akt survival pathway is known to be related to micro-21 in liver regeneration,[11]and it is likely that micro-21 may participate in the regulation of liver regeneration by other pathways.On the basis of the above findings,Ng et al[19]knocked out micro-21 in rodent hepatocytes during liver regeneration after 2/3 partial hepatectomy.Theirresults revealed that the knockout of micro-21 prevented the progression of hepatocytes into S phase of the cell cycle,which occurred mainly through a decrease in the levels of cyclin D1 protein,but not through a decrease in CCND1 mRNA.Afterward,they found that the upregulation of micro-21 facilitated cyclin D1 translation in the early phase of liver regeneration via the releasing of Akt1/mTOR.They concluded that micro-21 promoted rapid hepatocyte proliferation during liver regeneration by the acceleration of cyclin D1 translation.Bai et al[20]recently confirmed the above findings.We concluded that micro-21 exerts an effect during the early phase of liver regeneration via the activation of molecular cascade signals such as the cyclin proteins and PTEN.micro-34a is another molecular cascade involved in liver regeneration.Unlike the other miRNAs,micro-34a is significantly upregulated during the termination of liver regeneration and therefore,regarded as a representative of miRNAs participating in termination of liver regeneration.[21]Moreover,micro-34a expression is associated with cell cycle arrest and cell death of malignant tumors;micro-34a also influences the response of human cholangiocarcinoma cell lines to chemotherapy.[22,23]The major miRNAs that enhance liver regeneration are presented in Table 1.
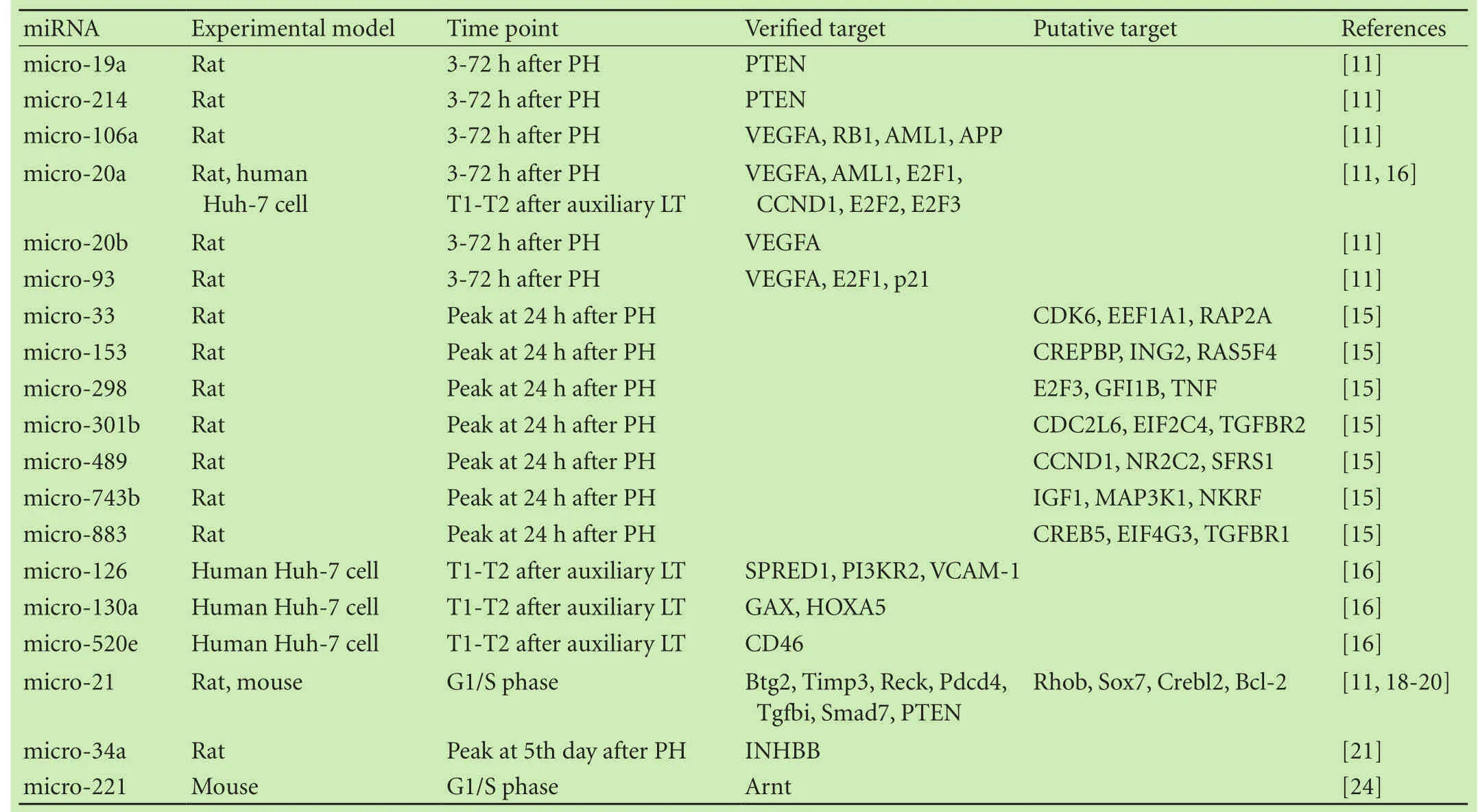
Table 1.miRNAs promoting liver regeneration
miRNAs repress liver regeneration and related pathway
miRNAs seem to play a bigger role in the suppression rather than promotion of liver regeneration.Generally,the downregulation of miRNAs after partial hepatectomy has been defined as an inhibitor of regeneration.Raschzok et al[15]defined statistically significant alterations as differences in expression alteration of more than 20%.A microarray analysis showed that 22 miRNAs were significantly downregulated,and out of these 22 miRNAs,13 showed a differential expression of more than 25%.Seven members of the let-7 family were also commonly expressed,and these are known to be closely involved in cell cycle regulation.CDC34,CDC25,MAP4K4,FOXG1,and IL-10,among others,were considered to be putative targets.[25]IL-10 is a potent anti-inflammatory cytokine,and in a previous study by Yin et al[26]it was validated that IL-10 modulated the production of proinflammatory cytokines and subsequently suppressed STAT3 activation in the liver.Therefore,IL-10 seems to be a novel target binding to the let-7 family of proteins in the regulation of regeneration.Shu et al[27]investigated whether a negative feedback loop exists between miRNAs and target genes.They revealed that the overexpression of several miRNAs,including let-7a,micro-17-92 cluster,micro-29,micro-30,and micro-424 in rat hepatocytes caused a decrease in the number of hepatocytes,which is in accordance with the process of DNA synthesis.Taken together,they hypothesized that miRNAs play a role in liver regeneration in part by binding to the 3’UTRs of the miRNA-processing pathway genes.In addition,they suggested that the level of alterations in miRNAs was regulated by negative feedback loops between miRNAs and their target genes.Salehi et al[16]showed a significant downregulation of micro-503,micro-23a,micro-150,micro-663 and micro-654 after transplantation and subsequent sequential biopsies.micro-150 is known to promote the expression of TNF-α,which is a key initiator of liver regeneration.Previously,TNF-α was considered to be a critical mediator of liver damage,and recently,investigators observed that liver regeneration was impaired in TNF-α receptor knockout mice,which supports the concept that TNF-α participates in the onset of regeneration.[14]Reduced expression of micro-503 caused an increase in the expression of pivotal cell cycle genes including cyclins D1,E1,E2,and F as well as Wee1,CDC25A and CHK1.All of the above pathways are involved in the coordination of liver regeneration through the promotion of cell proliferation.[16]
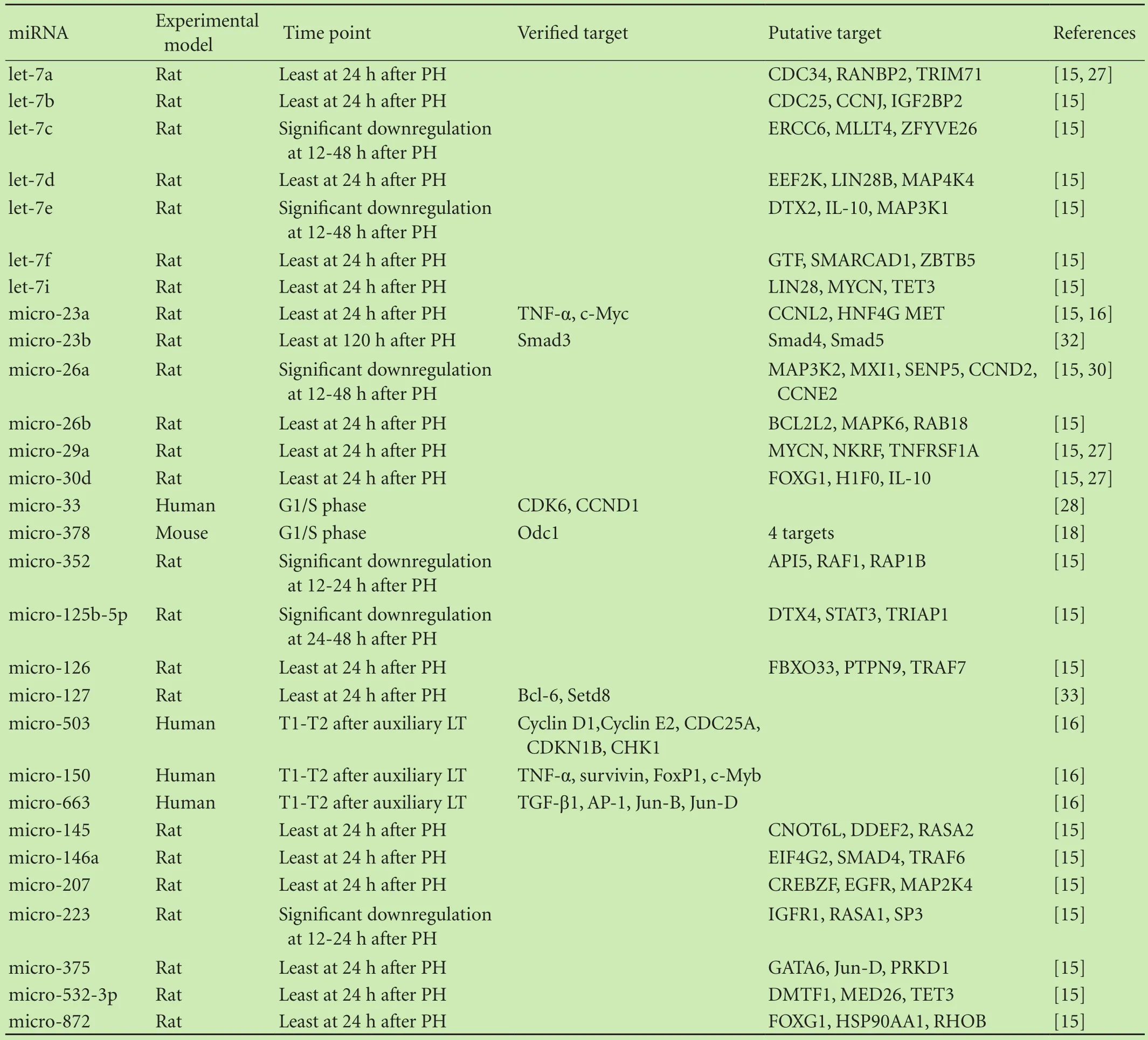
Table 2.miRNAs suppressing liver regeneration
To gain insight into particular miRNAs that inhibit liver regeneration,investigators have conducted a large number of studies in recent years.Cirera-Salinas et al[28]demonstrated that micro-33 inhibited cell proliferation through the suppression of CDK6 and CCND1.The overexpression of micro-33 induced a significant G1 phase arrest in Huh7 and A549 cell lines.In addition,a previous study[29]showed that micro-33 directly targets the 3’UTR of CDK6 and CCND1 separately from the known target ABCA1.Zhou et al[30]revealed that antimicro-26a enhanced proliferation during liver regeneration,and they presumed CCND2 and CCNE2 to be potential target genes of micro-26a.In summary,a variety of miRNAs play a role in the inhibition of regeneration through cyclins,which belong to a large family of regulatory proteins that function as accessory subunits in a variety of cyclin-dependent kinase complexes.They generally act as enzyme activators that drive the cell cycle through transitions between phases.Cyclin D is a cyclin subtype that specifically works with CDK4 and CDK6.Unlike most cyclins,cyclin D expression is not cyclical,but rather,is expressed in response to proliferative signals.Cyclin D primarily plays a role in cellular responses to mitogenic signals.Herein,we may block or stimulate pathways that involve cyclins to modulate liver regeneration.Recently,Yu et al[31]found that an inhibitor of micro-150 significantly elevated the expression levels of VEGFA mRNA 48 hours after transfection.Thus,they hypothesized that VEGFA might be a downstream target of micro-150 during liver regeneration.Similar to micro-34a,micro-23b was elucidated to play a role in termination of liver regeneration.Investigators[32]found that downregulation of micro-23b contributed to activation of the TGF-β1/Smad3 signal pathway during the termination of liver regeneration;however,the miRNAs associated with the termination of liver regeneration are not well studied.The major miRNAs that inhibit liver regeneration are listed in Table 2.
Conclusions
miRNAs play a critical role in the regulation of liver regeneration.Based on their promotion or suppression of regeneration,they are typically grouped into two categories:promoters and inhibitors.Among the miRNAs that enhance regeneration,micro-21 is most intensively investigated.micro-21 plays an important role in the early phase of liver regeneration,and promotes the cells from G1 to S phase transition,and thus,leads the progression of the cell cycle.Small-for-size syndrome is a recognizable clinical syndrome,which presents as a reduction in the volume of the liver to maintain normal liver function.The clinical manifestations include prolonged cholestasis and coagulopathy,portal hypertension,and ascites.[34]Living donor liver transplantation was widely performed in the last decade,which made small-for-size syndrome a challenge for clinicians.Perioperative promotion of liver regeneration will undoubtedly improve the short and long-term outcome of recipients,and agonists of miRNAs that promote liver regeneration may be at the frontier of future clinical practice.Different miRNAs play different role in liver regeneration via the common pathways that also involved in carcinogenesis and tumor progression.Therefore,either blockade of the promoters or enhancement of the inhibitors in tumor progression may exert anti-tumor effects.Prospective investigations may focus on more targets of miRNAs and common pathways in the regulation of liver regeneration to provide clues on how to block or stimulate this process.
Contributors:YPS wrote the main body of the manuscript under the supervision of XMQ.ZM and XMQ revised the draft.XMQ is the guarantor.
Funding:None.
Ethical approval:Not needed.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
1 Lee RC,Feinbaum RL,Ambros V.The C.elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14.Cell 1993;75:843-854.
2 Pasquinelli AE,Reinhart BJ,Slack F,Martindale MQ,Kuroda MI,Maller B,et al.Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA.Nature 2000;408:86-89.
3 Bartel DP.MicroRNAs:genomics,biogenesis,mechanism,and function.Cell 2004;116:281-297.
4 Finch ML,Marquardt JU,Yeoh GC,Callus BA.Regulation of microRNAs and their role in liver development,regeneration and disease.Int J Biochem Cell Biol 2014;54:288-303.
5 Lakner AM,Bonkovsky HL,Schrum LW.microRNAs:fad or future of liver disease.World J Gastroenterol 2011;17:2536-2542.
6 Mott JL.MicroRNAs involved in tumor suppressor and oncogene pathways:implications for hepatobiliary neoplasia.Hepatology 2009;50:630-637.
7 Jeng KS,Huang CC,Chu SH,Lin CK,Lin CC,Chen KH.Factors affecting the regeneration of liver graft after living related liver transplantation:a preliminary study.Transplant Proc 2013;45:1354-1359.
8 Kren BT,Wong PY,Shiota A,Zhang X,Zeng Y,Steer CJ.Polysome trafficking of transcripts and microRNAs in regenerating liver after partial hepatectomy.Am J Physiol Gastrointest Liver Physiol 2009;297:G1181-1192.
9 Li WQ,Chen C,Xu MD,Guo J,Li YM,Xia QM,et al.The rno-miR-34 family is upregulated and targets ACSL1 in dimethylnitrosamine-induced hepatic fibrosis in rats.FEBS J2011;278:1522-1532.
10 Bala S,Marcos M,Szabo G.Emerging role of microRNAs in liver diseases.World J Gastroenterol 2009;15:5633-5640.
11 Castro RE,Ferreira DM,Zhang X,Borralho PM,Sarver AL,Zeng Y,et al.Identification of microRNAs during rat liver regeneration after partial hepatectomy and modulation by ursodeoxycholic acid.Am J Physiol Gastrointest Liver Physiol 2010;299:G887-897.
12 Chou CH,Lai SL,Chen CN,Lee PH,Peng FC,Kuo ML,et al.IL-6 regulates Mcl-1L expression through the JAK/PI3K/Akt/ CREB signaling pathway in hepatocytes:implication of an anti-apoptotic role during liver regeneration.PLoS One 2013;8:e66268.
13 Scartozzi M,Faloppi L,Svegliati Baroni G,Loretelli C,Piscaglia F,Iavarone M,et al.VEGF and VEGFR genotyping in the prediction of clinical outcome for HCC patients receiving sorafenib:the ALICE-1 study.Int J Cancer 2014;135:1247-1256.14 Tilg H.Cytokines and liver diseases.Can J Gastroenterol 2001;15:661-668.
15 Raschzok N,Werner W,Sallmon H,Billecke N,Dame C,Neuhaus P,et al.Temporal expression profiles indicate a primary function for microRNA during the peak of DNA replication after rat partial hepatectomy.Am J Physiol Regul Integr Comp Physiol 2011;300:R1363-1372.
16 Salehi S,Brereton HC,Arno MJ,Darling D,Quaglia A,O’Grady J,et al.Human liver regeneration is characterized by the coordinated expression of distinct microRNA governing cell cycle fate.Am J Transplant 2013;13:1282-1295.
17 Rutkowski MJ,Sughrue ME,Kane AJ,Ahn BJ,Fang S,Parsa AT.The complement cascade as a mediator of tissue growth and regeneration.Inflamm Res 2010;59:897-905.
18 Song G,Sharma AD,Roll GR,Ng R,Lee AY,Blelloch RH,et al.MicroRNAs control hepatocyte proliferation during liver regeneration.Hepatology 2010;51:1735-1743.
19 Ng R,Song G,Roll GR,Frandsen NM,Willenbring H.A microRNA-21 surge facilitates rapid cyclin D1 translation and cell cycle progression in mouse liver regeneration.J Clin Invest 2012;122:1097-1108.
20 Bai YN,Yu ZY,Luo LX,Yi J,Xia QJ,Zang Y.MicroRNA-21 accelerates hepatocyte proliferation in vitro via PI3K/Akt signaling by targeting PTEN.Biochem Biophys Res Commun 2014;443:802-807.
21 Chen H,Sun Y,Dong R,Yang S,Pan C,Xiang D,et al.Mir-34a is upregulated during liver regeneration in rats and is associated with the suppression of hepatocyte proliferation.PLoS One 2011;6:e20238.
22 Meng F,Henson R,Lang M,Wehbe H,Maheshwari S,Mendell JT,et al.Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines.Gastroenterology 2006;130:2113-2129.
23 Ghawanmeh T,Thunberg U,Castro J,Murray F,Laytragoon-Lewin N.miR-34a expression,cell cycle arrest and cell death of malignant mesothelioma cells upon treatment with radiation,docetaxel or combination treatment.Oncology 2011;81:330-335.
24 Yuan Q,Loya K,Rani B,M?bus S,Balakrishnan A,Lamle J,et al.MicroRNA-221 overexpression accelerates hepatocyte proliferation during liver regeneration.Hepatology 2013;57:299-310.
25 Zhu XM,Wu LJ,Xu J,Yang R,Wu FS.Let-7c microRNA expression and clinical significance in hepatocellular carcinoma.J Int Med Res 2011;39:2323-2329.
26 Yin S,Wang H,Park O,Wei W,Shen J,Gao B.Enhanced liver regeneration in IL-10-deficient mice after partial hepatectomy via stimulating inflammatory response and activating hepatocyte STAT3.Am J Pathol 2011;178:1614-1621.
27 Shu J,Kren BT,Xia Z,Wong PY,Li L,Hanse EA,et al.Genomewide microRNA down-regulation as a negative feedback mechanism in the early phases of liver regeneration.Hepatology 2011;54:609-619.
28 Cirera-Salinas D,Pauta M,Allen RM,Salerno AG,Ramírez CM,Chamorro-Jorganes A,et al.Mir-33 regulates cell proliferation and cell cycle progression.Cell Cycle 2012;11:922-933.
29 Marquart TJ,Allen RM,Ory DS,Baldán A.miR-33 links SREBP-2 induction to repression of sterol transporters.Proc Natl Acad Sci U S A 2010;107:12228-12232.
30 Zhou J,Ju W,Wang D,Wu L,Zhu X,Guo Z,et al.Downregulation of microRNA-26a promotes mouse hepatocyte proliferation during liver regeneration.PLoS One 2012;7:e33577.
31 Yu ZY,Bai YN,Luo LX,Wu H,Zeng Y.Expression of microRNA-150 targeting vascular endothelial growth factor-A is downregulated under hypoxia during liver regeneration.Mol Med Rep 2013;8:287-293.
32 Yuan B,Dong R,Shi D,Zhou Y,Zhao Y,Miao M,et al.Downregulation of miR-23b may contribute to activation of the TGF-β1/Smad3 signalling pathway during the termination stage of liver regeneration.FEBS Lett 2011;585:927-934.
33 Pan C,Chen H,Wang L,Yang S,Fu H,Zheng Y,et al.Downregulation of MiR-127 facilitates hepatocyte proliferation during rat liver regeneration.PLoS One 2012;7:e39151.
34 Tucker ON,Heaton N.The ‘small for size’liver syndrome.Curr Opin Crit Care 2005;11:150-155.
Received November 17,2014
Accepted after revision July 15,2015
doi:10.1016/S1499-3872(15)60036-4
Corresponding Author:Ming-Qing Xu,MD,PhD,Department of Liver and Vascular Surgery,West China Hospital,Sichuan University,Chengdu 610041,China(Tel/Fax:+86-28-85422870;Email:xumingqing0018@163.com)
 Hepatobiliary & Pancreatic Diseases International2016年2期
Hepatobiliary & Pancreatic Diseases International2016年2期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- OIder age at first birth is a risk factor for pancreatic cancer:a meta-anaIysis
- Pathologic response to preoperative transarterial chemoembolization for resectable hepatocellular carcinoma may not predict recurrence after liver resection
- An unusual case of prolonged post-endoscopic retrograde cholangiopancreatography jaundice
- Surgical treatment of synchronous colorectal liver and lung metastases:the usefulness of thoracophrenolaparotomy for single stage resection
- Monocyte chemoattractant protein-1,transforming growth factor-β1,nerve growth factor,resistin and hyaluronic acid as serum markers:comparison between recurrent acute and chronic pancreatitis
- Improvement of gastric emptying by enhanced recovery after pancreaticoduodenectomy
