Percutaneous resection of upper tract urothelial cell carcinoma:When,how, and is it safe?
Willem E.Strijbos,Bart van der Heij
Department of Urology,Zuyderland Medical Centre,Heerlen,The Netherlands
ORIGINAL ARTICLE
Percutaneous resection of upper tract urothelial cell carcinoma:When,how, and is it safe?
Willem E.Strijbos*,Bart van der Heij
Department of Urology,Zuyderland Medical Centre,Heerlen,The Netherlands
Percutaneous tumour
resection;
PCTR;
UTUC;
Endoscopic
management;
Upper tract urothelial
cell carcinoma;
Tumour surface area
Introduction:In the management of upper tract urothelial cell carcinoma(UTUC) endoscopic,nephron sparing procedures like ureterorenoscopy(URS)or percutaneous tumour resection(PCTR)still play a very limited role.This could lead to possible unnecessary radical nephroureterectomies(RNU),still being the gold standard treatment.The risk of chronic kidney disease(CKD)later in life is important.In this study we present the results of 24-year experience with PCTR in a single institution.
Methods:We identified 44 patients who underwent PCTR between 1992 and 2015.Radical resection was achieved in 40 patients who were included in this study.Demographic and clinical data,including tumour recurrence,progression to RNU,tumour grade and overall survival(OS)were retrospectively acquired.An outcome analysis was conducted.
Results:Median age at diagnosis was 68 years(range 42-94 years).Low grade tumours were found in 37 patients(92.5%)and high grade tumours in three patients(7.5%).Median followup was 53 months during which 20 patients developed upper tract recurrences(50.0%).The longest time to recurrence was 97 months.At follow-up 11 patients(27.5%)underwent an RNU and two patients died from UTUC.RNU could be avoided in 29 patients(72.5%).In this study we found that multifocality is a significant risk factor for recurrence,but not for stage progression to RNU.
Conclusion:PCTR is a surgically and oncologically safe procedure.Renal preservation in patients with UTUC who are eligible for percutaneous resection can be achieved in the majority of patients.Selection criteria for PCTR should be further refined,leading to a wider application of PCTR in the future.Follow-up needs invasive procedures and should be long term. ?2016 Editorial Office of Asian Journal of Urology.Production and hosting by Elsevier B.V.Ltd. This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/ licenses/by-nc-nd/4.0/).
1.Introduction
Endoscopic treatment of upper tract urothelial cell carcinoma(UTUC)arose in 1985,when Huffman et al.[1]reported on a ureteroscopic tumour resection.In 1986 Streem and Pontes[2]reported on the first percutaneous approach for the treatment of UTUC[2].Experience with endoscopic treatment of UTUC has historically been limited to a few centres worldwide,treating sub-groups of patients with imperative indications for non-removal of renal units, either ureteroscopically with laser ablation techniques or percutaneously with electro-resection.With increasing experience endoscopic management has been extended to selected patients with elective indications in a few centres.
UTUC is a very rare disease[3,4].It occurs in 2%-5%of all urothelial cell tumours.An increase can be seen in the Netherlands since 2005 of 1-1.5 per 100,000 inhabitants per year.
Radical nephroureterectomies(RNU)is considered the“gold standard”treatment for UTUC because of its often advanced stage and aggressive nature at presentation[5]. Conservative management for UTUC has been used for patients with solitary kidneys,end stage chronic kidney disease(CKD),bilateral UTUC or patients in poor surgical condition.But the question arises whether conservative treatment is an option in the presence of a normal contra lateral kidney.Is it possible to use treatment algorithms akin to the conservative treatment options for non invasive bladder cancer?In renal cell carcinoma(RCC)nephron sparing surgery is widely used for better overall survival (OS)due to preservation of kidney function and reduced risk for CKD[6-8].A loss of kidney function after RNU is obvious.Nephron sparing surgery for UTUC will most likely have a better OS as well.But which patient is suitable for conservative treatment?Several studies have addressed this question[9,10].The recently published EAU guideline on urothelial cell carcinoma of the upper urinary tract indicates that conservative treatment can be discussed in low-risk patients with a functional contra lateral kidney avoiding the morbidity associated with open or laparoscopic radical surgery and not compromising oncological outcomes and kidney function[11].Sixty percent of patients with UTUC present with invasive disease,as compared with only 15%-20%of patients with bladder cancer[12].
This underlines the need for proper patient selection.In 2015 Motamedinia et al.[10]presented a large study in which they conclude that nephron-sparing endoscopic resection continues to be an increasingly relevant choice for the management of patients with UTUC.
With this study we intend to support this view whole heartedly and contribute to determining selection criteria for patients who could or even should undergo percutaneous tumour resection(PCTR)for the treatment of UTUC.
2.Patients and methods
2.1.Patients
This study received local Institutional Review Board approval.A retrospective analysis was performed of all patients treated with PCTR of urothelial carcinoma of the kidney and/or proximal ureter between 1992 and 2015.In 1995 we reported on the first 10 patients treated with PCTR in ourcentre[13].The files offourofthese patients were not retrievable.These patients are not included in this study.
A total of 190 patients with an initial diagnosis of UTUC were identified of whom 44 patients underwent PCTR. Imperative indications for endoscopic treatment were a solitary kidney,risk of developing of CKD,bilateral disease and a poor physical condition due to co-morbidity and/or age.
The chance of developing CKD was dependent on a multitude of factors such as serum creatinine,body mass index,diabetes mellitus and cardiovascular status.An exact cut-off level of creatinine was not used in our study.
2.2.Pathology and cytology
Tumours were classified as low or high grade according to the WHO grading criteria[10].Earlier cohorts of patients who were classified using the WHO 1973 grading criteria were reclassified as low grade in case of grade 1 or 2 and as high grade in case of grade 3.Urine cytology results were determined.
2.3.Patient selection and technique
Patient selection for PCTR was based on imaging of the renal collecting system.The continuity and integrity of the collecting system wall at the tumour location has always been the determining factor for eligibility for PCTR.With the availability of flexible ureteroscopes a diagnostic ureterorenoscopy(URS)was performed for con firmation of the findings on imaging.Small tumours that could be coagulated were treated in the same session.
All patients were treated in a percutaneous nephrolithotomy(PCNL)setup in prone position.In case of hydronephrosis access to the collecting system was reached by puncture using ultrasonic guidance without the need for a contrast medium.If the kidney was not dilated a ureteral catheter was placed ipsilaterally and a diluted contrast medium was used to induce a mild dilatation.After the ultrasonically guided puncture a percutaneous tract was then established by dilatation using the Alken dilators,thus having exact control over the extent of dilation into the lumen of the collecting system.The nephrostomy tract was dilated up to 28 Fr outer diameter.The inner diameter of the Amplatz sheath was 26 Fr.Resection of the tumour was performed with a 23.5 Fr Olympus resectoscope.Only the inner shaft without the continuous flow outer shaft was used.A slightly elevated pressure in the collecting system was created by the narrow space between the resectoscope and the Amplatz sheath and a water pressure of approximately 80 cm at the inflow site.This caused the dilatation needed during resection.Handling of the resection loop was done with great care to avoid touching non tumour afflicted parts of the pelvic wall,hence the need for a slight dilatation.It was necessary to estimate the depth of each cut using the effects on the tumour base of moving the tumour with the loop,without the application of cutting power.Each tumour chip was removed from the collectingsystem individually.In most cases the peripelvic fatty tissue became visible indicating that the base of the tumour was resected.Careful coagulation was performed of the entire tumour afflicted area after resection.The power settings were the same as those used for bipolar bladder tumour resection(280 W cutting and 80 W coagulating).Because the chance of an arterial lesion is greater with PCTR than with PCNL an angiography suite with staff was always standby during a PCTR procedure in order to perform an emergency(selective)embolization in case of an incontrollable bleeding.
After the procedure a 24 Fr nephrostomy tube was placed.Some patients received post operative antegrade instillation of mitomycin C(MMC)or Bacille Calmette Guerin (BCG).All patients were treated by a single surgeon only.
2.4.Follow up
Patients were evaluated every 3-4 months for 2 years and then annually for 5 years.Follow-up visits included a history,urine cytology,cystoscopy and upper tract imaging using retrograde ureteropyelography or a computed tomography-intravenous urography(CT-IVU).In most patients a flexible ureterorenoscopy was performed at 3 months and then yearly for 2 years.In case of recurrence the follow-up was changed accordingly.Tumour recurrence was defined as tumour in the ipsilateral kidney or ureter after complete initial resection(s).
2.5.Statistical analysis
All data were analyzed using SPSS version 23(IBM,North Castle,New York,USA).Recurrence free survivals(RFS)was calculated using the Kaplan-Meier analysis and Cox proportional hazards models were used for predictors of recurrence or stage progression to RNU.P values less than 0.05 were considered statistically significant.
3.Results
During a 24-year period,44 primary percutaneous resections were performed.Macroscopic radical resection occurred in 40 patients(90.9%).In two of these 40 patients a second PCTR was necessary in order to obtain a radical resection. This procedure was done during the same hospital stay.
An incomplete resection occurred in four patients. These were excluded for further analysis.The tumour afflicted part of the pelvic wall was too large for a safe or complete resection.Two of these patients received palliative treatment due to age and co-morbidity and two had a RNU.The delay of the RNU did not alter the cancer specific survival(CSS)of these patients,who are still alive without tumour recurrence.
Table 1 shows the patient demographics.The median age at diagnosis was 68 years(mean 69 years).Anatomical or functional mono kidney was present in 10 patients (25.0%).A prior history of urothelial cancer was noted in 15 patients(37.5%),of whom three patients had a cystectomy and six patients had nephroureterectomy.A Charlson comorbidity index score of 0 was only present in three patients(7.5%)and a score of or more than 3 was present in 14patients(35.0%).Of the 40 patients with a radical resection 20 patients were referred.The follow-up was performed by the referral centres causing omissions in follow-up data. The median follow-up was 53 months(3-217 months).
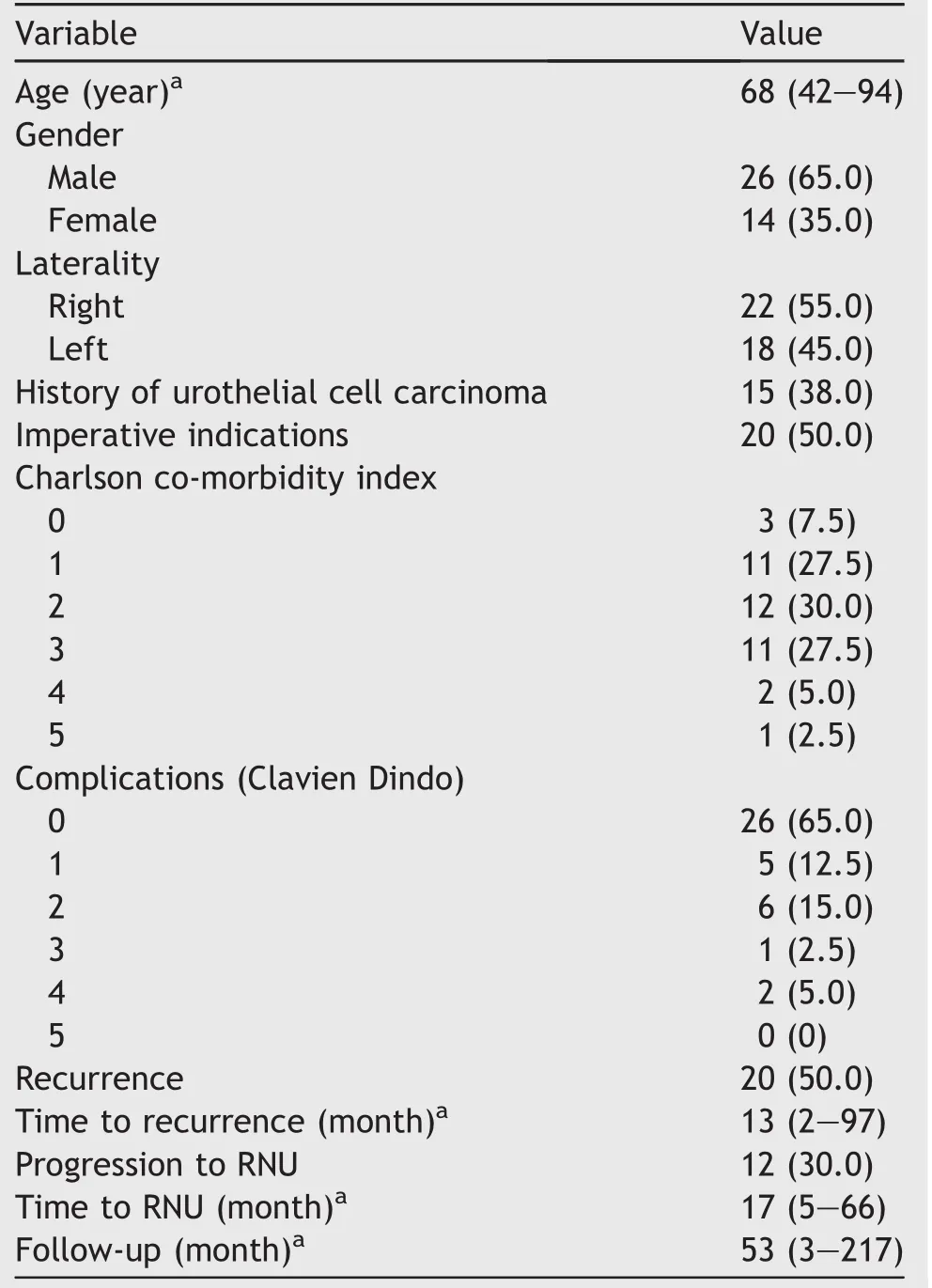
Table 1 Patient demographics,tumour characteristics and complications(n=40).
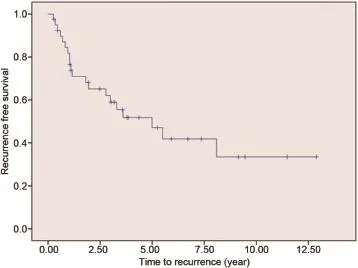
Figure 1 Kaplan-Meier curve of recurrence-free survival.
Twenty patients(50.0%)developed an upper tract recurrence during follow-up(Fig.1).The median time torecurrence in this series was 13 months(2-97 months).Five patients could be treated by conservative treatment(flexible URS with laser coagulation or secondary PCTR).RNU was performed in 12 patients(30.0%).The median time to RNU was 17 months(5-66 months).One patient had an RNU which did not show any tumour.In this patient the indication to RNU was made based on imaging.URS was not possible due to urinary diversion surgery.Two patients did not receive any more curative treatment due to comorbidity or age.Both patients had a history of urothelial cell cancer which resulted in a cystectomy in one and nephroureterectomy in the other.One patient died of metastatic disease.The other patient is still alive 63 months after percutaneous resection.This patient also developed a tract metastasis which was locally resected. Two patients died of other causes before secondary treatment could be performed.During the follow-up a total of 19 patients(47.5%)died.
Of the 23 patients referred from other centres,20 had an imperative indication for conservative treatment.A patient flowchart is shown in Fig.2.In 15 patients there was a medical history of urothelial cancer.This had lead to a nephroureterectomy in six patients,cystectomy in four patients and the other patients had multiple transurethral resections of a bladder tumour.
Complications were seen in 14 patients(35.0%).A complication with a Clavien Dindo score of 3 or more occurred in three patients(7.5%),two due to bleeding,one due to myocardial infarction.Selective embolization was necessary in one patient.There were no surgery related deaths.
High grade tumours were found in three patients(7.5%) and low grade in 37 patients(92.5%).Of the four patients who were not radically resected,one patient had a high grade tumour.Urine cytology was performed in 34 patients and was malignant in only one patient.This was a patient with a high grade tumour.
Statistical analysis showed that multifocality is a significant risk factor for developing recurrence,but not for stage progression to RNU.No significance was shown for age,gender,grade,medical history of urothelial cell carcinoma(UCC)or solitary kidney for recurrence or stage progression to RNU(Tables 2 and 3).
4.Discussion
In this study the results of 40 patients treated with percutaneous resection for UTUC are presented.We acknowledge the small number of patients.This small number of patients is due to rarity of UTUC and the aggressive nature at presentation which limits the chance of conservative treatment even further,while RNU is a safe alternative with a good oncological outcome.Patients with imperative indications do not have this alternative,which can cause a bias in indications for PCTR.
In our study we did not see a relation between imperative cause and recurrence or stage progression to RNU.
In general the management of patients with UTUC often challenges one of the foremost urologic principals,which is to preserve as many nephrons as possible.For elderly patients with co-morbidity and a direct risk for CKD this is clear,but should not nephron sparing surgery be performed in every suitable patient(with a normal contralateral kidney)?There are no studies in which risk actors for OS,CSS, RFS and morbidity have been established for endoscopic treatment.Cutress et al.[9]reviewed the relevant literature published until 2011.All studies were retrospective.
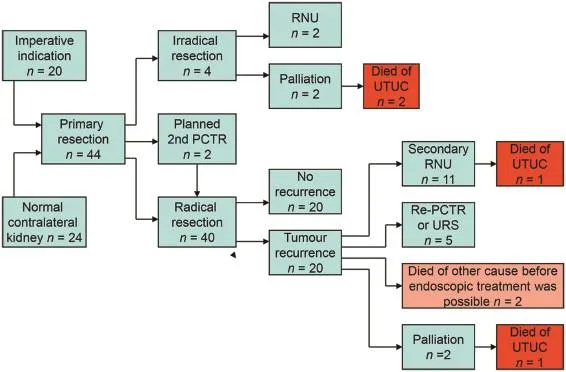
Figure 2 Patient flowchart.UTUC,upper tract urothelial cell carcinoma;URS,ureterorenoscopy;RNU,radical nephroureterectomy;PCTR,percutaneous tumour resection.
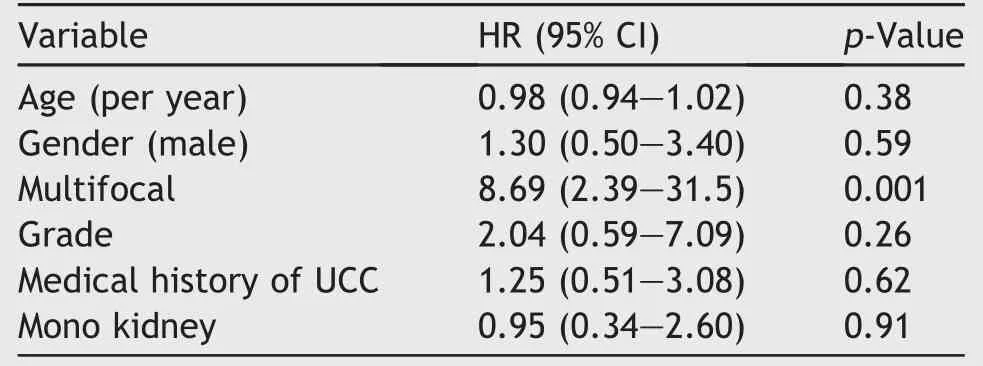
Table 2 Cox proportional hazard analysis model predicting recurrence.
We believe that PCTR is much more suitable for resecting tumour tissue than URS,the latter in general allowing only for laser coagulation.
Our retrospective study data were biased because the follow-up scheme could not be reliably controlled in all of the referring centres due to the lack of a generally applied follow-up protocol.
Of the 40 radically resected patients,25 patients retained their kidney at follow-up without residual or metastatic disease.Eleven patients had a secondary RNU after local tumour recurrence.Pathology revealed only four patients with high grade tumour and/or muscular invasion, suggesting that the other seven patients could have been treated conservatively with re-PCTR or URS.
4.1.Size and multifocality
According to the present European Guidelines multifocality and tumour size>1 cm are contra indications for endoscopic treatment.PCTR is only mentioned as a treatment option in patients who have a low risk,non-invasive tumour in the lower pole of the collecting system due to the diff iculty reaching it ureteroscopically[11].In some studies it has been suggested that multifocality is a risk factor[14,15] but statistical significance in endoscopic series was not achieved[10,16,17].In this study it was found that multifocality is a significant risk factor for recurrence,but not for stage progression to RNU.
We believe that multifocality or tumour size is irrelevant if radical resection can be obtained.
In RCC partial nephrectomies have followed a pattern of increasing tumour sizes that could be removed.The future will tell if a similar pattern with UTUC will occur.
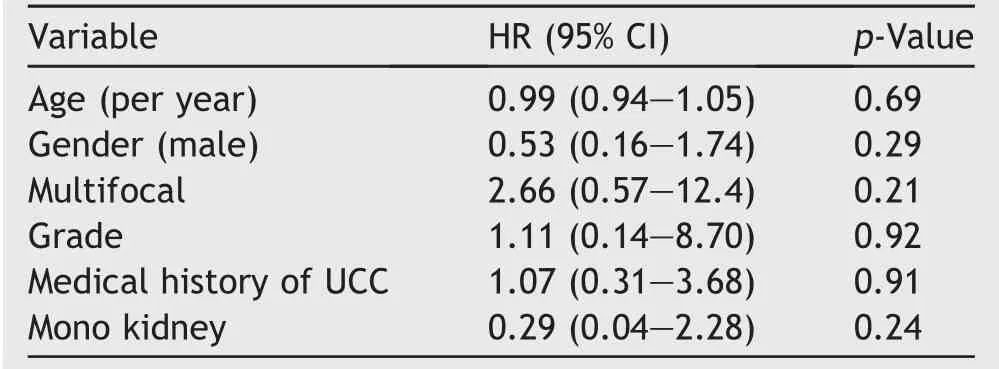
Table 3 Cox proportional hazard analysis model predicting stage progression to RNU.
4.2.Patient selection
In our experience,the tumour appearance on imaging is the determining factor for eligibility for endoscopic treatment in general.The extent of the tumour could not always be properly established however.A diagnostic URS can give extra information on the extent of tumour,but cannot always preclude that the tumour afflicted area is too large to be completely resected in one session.However,URS is not always possible in patients with urinary diversion and imaging or percutaneous inspection will be the only tools to see if PCTR is possible.
In our study we only had three high grade tumours suggesting a correlation between non-infiltrative tumour appearance on imaging and low grade tumours.
Doubts about the non-invasiveness on imaging can result in an increased chance thatthe tumourcannotbe completely resected,even when thorough inspection after resection showsno residualtumour.Microscopic invasion can obviously not be seen on imaging.The fact however that the entire afflicted part of the pelvic wall should be either completely resected or completely coagulated(as opposed to what can be seen in bladder tumour resections)makes microscopical invasion less relevant.There were four patients in our group of whom the UTUC could ultimately not be radically resected.Imperative indicationsbiased properpatientselection in allofthese cases,while imaging wasobviously notsufficient.
In our series the presence of tumour in the peripelvic fatty tissue on pathological examination would mean that tumour was left behind,leading to an RNU.However this never happened in our cohort,underlining the value of imaging in patient selection.
4.3.Follow-up
The longest time to recurrence was 97 months.Long-term follow-up is therefore mandatory.We still do not know if the follow-up for patients successfully treated with PCTR should be 5 or 10 years.We find that the invasive nature of the follow-up scheme is a burden for some patients due to the possible need of multiple URS procedures with anaesthesia.In our series a first URS was performed 3 months after PCTR.The findings at this inspection determined the frequency of URS procedures during follow-up.The prognostic value of multifocality and tumour grading is still debatable[10],but it can be used to“intensify”the followup.The earlier tumour recurrences are found the smaller they are,which improves the chance of a curative ureteroscopic coagulation,avoiding a more invasive PCTR.
Tumour progression in the case of a recurrence is rare [18]as can be seen in our series.One of 40 patients(2.5%) died of metastasis after progression to RNU.We suggest the use of a follow-up protocol as described above,which evaluated in the course of the years.Retrograde urography as imaging modality is preferred over CT urography if possible,the former having the lowest X-ray exposure.
4.4.Preoperative biopsies
We believe that,even when there are no imperative indications,tumour grade should not influence the decisionwhether or not to proceed with PCTR as long as the tumour as a whole can be completely resected.The chance of recurrence appears to be higher.The question remains if a re-do endoscopic treatment or delayed RNU alters the CSS or OS[19-21].The answer to this question can only be acquired by a randomized controlled trial for patients suitable for endoscopic treatment.
Preoperative biopsies should not alter the treatment plan and preoperative URS should therefore be done in order to confirm the findings on imaging,assess the tumour area and to exclude unexpected findings,not primarily for biopsies.Pathology reports on tumour stage and grade are more reliable with tissue obtained after PCTR than with ureteroscopically achieved biopsy material and therefore the decision on proceeding with RNU can be more soundly made.The disadvantage of a possible second operation does not outweigh the chance of renal preservation with diminished chances of CKD later in life.In Fig.3 an algorithm is shown,used in our clinic.Patient counselling influences the choice of treatment.If the patient is eligible for PCTR a proper and uniform counselling of the treatment options is important.Some patients will choose RNU as first treatment option.Others have chosen RNU after the first recurrence and even after successful PCTR,due to the burden of invasive surveillance.
4.5.BCG or MMC
The influence of postoperative instillation with BCG or MMC in the collective system has not been assessed in our study. We did not systematically instillate the upper tract due to the risk of complications after resecting part of the pelvic wall and therefore allowing infiltration of peripelvic fatty tissue.In one patient with a solitary kidney we found what appeared to be seeding recurrence in the ureter after PCTR,necessitating frequent URS treatments,ultimately leading to strictures and finally RNU.
The question arises whether the urothelial cell in the upper urinary tract behaves the same as in the lower urinary tract.If so than the same risk calculators for the bladder would be sufficient for the kidney.A single antegrade MMC instillation after resection would be advisable to reduce the chance for recurrence.In our institution a single antegrade admission of MMC after each PCTR after sealing of the collecting system(no leakage on antegrade contrast imaging)has been applied in the last 2 years.
After a curative resection of a high grade tumour adjuvant BCG in 6 weekly admissions has been used in our institution.However this scheme was abandoned because of the high co-morbidity.Motamedinia et al.[10]found that adjuvant BCG did not mitigate the risk of recurrence,RNUor death regardless of tumour grade in their series.We recommend a single admission of MMC because of the low morbidity and the proven efficacy preventing bladder tumour recurrences.Future studies will have to prove the efficacy with UTUC as well.
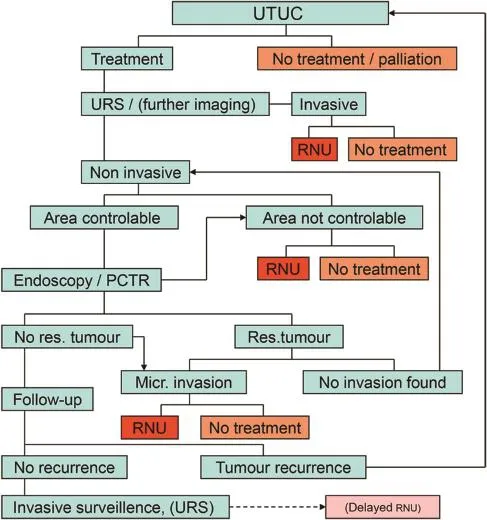
Figure 3 Treatment algorithm for UTUC in Zuyderland MC in the Netherlands.UTUC,upper tract urothelial cell carcinoma;URS, ureterorenoscopy;RNU,radical nephroureterectomy;res.,residual;PCTR,percutaneous tumour resection;Micr.,microscopic.
4.6.Influence of surgical technique
Dilatation of a percutaneous tract in a tumour afflicted calyx increases the risk for residual tumour in this calyx. This should be avoided if possible.In order to achieve safe access it is therefore important to use tumour free calices. The direction of kidney entrance should be such that an optimal view of the tumour afflicted site can be achieved. This means that all dorsal and lateral calices are entry candidates leading to a great variety in flank entry sites. Obviously these are often above the 12th or 11th rib because the position of the percutaneous tract is then parallel to the renal axis,allowing for the largest exposure. Ultrasonic guidance while establishing the percutaneous tract allows a wide range of entry sites without compromising other organs.
The main limiting factor in PCTR in the case of noninvasive appearing tumours is the surface area afflicted with tumour.We have not resected more than about 30%of the pelvic wall at a time because of the risk of uncontrollable bleeding.This means that in some cases multifocality and tumour size can be an issue if the afflicted part of the pelvic wall is too large.In all of the six cases where the primary resection could not be radical,this was due to the size of the tumour afflicted area of the pelvic wall.
Performing PCTR we have used a 23.5 Fr resectoscope and tried to resect as much tumour tissue as possible without damaging large and uncontrollable blood vessels. The entire pelvic wall is either resected or,if unable to appreciate the depth of resection,completely coagulated. One has to bear in mind that coagulation effects are deeper than the thickness of the pelvic wall.The procedure is done with controlled water pressure and flow for sustained visibility.The availability of bipolar equipment seems to have increased the controllability,however this has not been studied here;the settings were the same as with transurethral bladder tumour resections.The limited space within the collecting system calls for optimal visualisation and approach of the tumour area,necessitating an optimal angle of the percutaneous tract.It can therefore be necessary to perform more than one PCTR in order to achieve total removal of all tumour.In our series this was the case with 2 patients,and it did not alter the outcome, CSS and OS.
4.7.Learning curve
The PCTR technique,the great variety of the collecting system anatomy and the low incidence of UTUC make that the learning curve may take an entire career.This fact makes exchange of experiences in patient selection,technique,adjuvant treatment and follow-up between centres the more important.A wider application of PCTR will of course increase the numbers and shorten the learning curve.
A well designed,multi-centre study is necessary to formulate a more definite answer to the question so nicely stated by Motamedinia et al.[10]:when deciding on appropriate treatment of macroscopically non-invasive UTUC,what is lost by electing endoscopic management over immediate RNU?Can we minimise this possible loss by refining selection criteria and improving imaging modalities?The future will show if agreement on algorithms like the one used in our institution is possible(Fig.3).
5.Conclusion
In the management of UTUC endoscopic treatment, particularly PCTR has shown to be an effective and safe treatment option.In this series we preserved 70%of the kidneys reducing the risk of CKD.Selection criteria and patient counselling should be based primarily on urinary tract imaging and secondarily on other factors such as grade,co-morbidity,costs,surveillance burden,etc.The selection criteria could be further refined.A study based on randomisation of centres as a whole could be an option. The long learning curve calls for intensive exchange and cooperation between centres world wide.
Conflicts of interest
The authors declare no conflict of interest.
[1]Huffman JL,Bagley DH,Lyon ES,Morse MJ,Herr HW, Whitmore Jr WF.Endoscopic diagnosis and treatment of upper tract urothelial tumors.A preliminary report.Cancer 1985;55: 1422-8.
[2]Streem SB,Pontes EJ.Percutaneous management of upper tract transitional cell carcinoma.J Urol 1986;135:773-5.
[3]Roupre?t M,Babjuk M,Compe′rat E,Zigeuner R,Sylvester RJ, Burger M,et al.European guidelines on upper tract urothelial carcinomas:2013 update.Eur Urol 2013;63:1059-71.
[4]IKNL,Cancer Centre of the Netherlands.Dutch cancer figures, urinary tract 1990-2015.http://www.cijfersoverkanker.nl.
[5]Margulis V,Shariat SF,Matin SF,Kamat AM,Zigeuner R, Kikuchi E,et al.Outcomes of radical nephroureterectomy:a series from the Upper Tract Urothelial Carcinoma Collaboration.Cancer 2009;115:1224-33.
[6]Go AS,Chertow GM,Fan D,McCulloch CE,Hsu CY.Chronic kidney disease and the risk of death,cardiovascular events and hospitalization.N Engl J Med 2004;351:1296-305.
[7]Huang WC,Levey AS,Serio AM,Snyder M,Vickers AJ,Raj GV, et al.Chronic kidney disease after nephrectomy in patients with renal cortical tumours:a retrospective cohort study. Lancet Oncol 2006;7:735-40.
[8]Raman JD,Lin YK,Kaag M,Atkinson T,Crispen P,Wille M, et al.High rates of advanced disease,complication and decline of renal function after radical nephroureterectomy. Urol Oncol 2014;32:47.e9-14.
[9]Cutress ML,Stewart GD,Zakikhani P,Phipps S,Thomas BG, Tolley DA.Ureteroscopic and percutaneous management of upper tract urothelial carcinoma(UTUC):systematic review. BJU Int 2012;110:614-28.
[10]Motamedinia P,Keheila M,Leavitt DA,Rastinehad AR, Okeke Z,Smith AD.The expanded use of percutaneousresection for upper tract urothelial carcinoma:a 30-year comprehensive experience.J Endourol 2015;20:1-6.
[11]Roupre?t M,Babjuk M,Compe′rat E,Zigeuner R,Sylvester RJ, Burger M,et al.European guidelines on urothelial carcinomas of the upper urinary tract.2015.limited update march 2015.
[12]Roupre?t M,Zigeuner R,Palou J,Boehle A,Kaasinen E, Sylvester R,et al.European guidelines for the diagnosis and management of upper tract urothelial cell carcinomas:2011 update.Eur Urol 2011;59:584-94.
[13]Plancke HR,Strijbos WE,Delaere KP.Percutaneous endoscopic treatment of urothelial tumours of the renal pelvis.Br J Urol 1995;75:736-9.
[14]Palou J,Piovesan LF,Huguet J,Salvador J,Vicente J, Villavicencio H.Percutaneous nephroscopic management of upper tract transitional cell carcinoma:recurrence and long term follow-up.J Urol 2004;172:66-9.
[15]Williams AK,Kassouf W,Chin J,Rendon R,Jacobsen N, Fairey A,et al.Multifocality rather than tumour location is a prognostic factor in upper tract urothelial carcinoma.Urol Oncol 2013;31:1164-5.
[16]YafiFA,Novara G,Shariat SF,Gupta A,Matsumoto K, Walton TJ,et al.Impact of tumour location versus multifocality in patients with upper tract urothelial carcinoma treated with nephroureterectomy and bladder cuff excision: a homogeneous series without perioperative chemotherapy. BJU Int 2012;110:E7-13.
[17]Ouzzane A,Colin P,Xylinas E,Pignot G,Ariane MM,Saint F, et al.Ureteral and multifocal tumours have worse prognosis than renal pelvic tumours in urothelial carcinoma of the upper urinary tract treated by nephroureterectomy.Eur Urol 2011; 60:1258-65.
[18]Rastinehad AR,Ost MC,Vanderbrink BA,Greenberg KL,El-Hakim A,Marcovich R,et al.A 20-year experience with percutaneous resection of upper tract transitional carcinoma: is there an oncologic benefit with adjuvant bacillus Calmette Guerin therapy?Urology 2009;73:27-31.
[19]Hendin BN,Streem SB,Levin HS,Klein EA,Novick AC.Impact of diagnostic ureteroscopy on long term survival in patients with upper tract urothelial cell carcinoma.J Urol 1999;161:783-5.
[20]Yakoubi R,Colin P,Seisen T,Le′on P,Nison L,Bozzini G,et al. Radical nephroureterectomy versus endoscopic procedures for the treatment of localised upper tract urothelial carcinoma:a meta analysis and a systematic review of current evidence from comparative studies.Eur J Surg Oncol 2014;40: 1629-34.
[21]Gadzinski AJ,Roberts WW,Faerber GJ,Wolf JS.Long term outcomes of nephroureterectomy versus endoscopic management for upper tract urothelial carcinoma.J Urol 2010; 183:2148-53.
Received 31 March 2016;received in revised form 27 April 2016;accepted 28 April 2016 Available online 27 May 2016
*Corresponding author.
E-mail address:W.Strijbos@Zuyderland.nl(W.E.Strijbos).
Peer review under responsibility of Second Military Medical University.
http://dx.doi.org/10.1016/j.ajur.2016.04.003
2214-3882/?2016 Editorial Office of Asian Journal of Urology.Production and hosting by Elsevier B.V.Ltd.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Urology2016年3期
Asian Journal of Urology2016年3期
- Asian Journal of Urology的其它文章
- Robot-assisted laparoscopic radical cystectomy with complete intracorporeal urinary diversion
- Current status of laparoscopic and robotassisted nerve-sparing radical cystectomy in male patients
- Stents for malignant ureteral obstruction
- Techniques to resect the distal ureter in robotic/laparoscopic nephroureterectomy
- Thulium laser treatment for bladder cancer
- Narrow band imaging for bladder cancer
