Prevalence of multi-drug resistant uropathogenic Escherichia coli in Potohar region of Pakistan
?
Prevalence of multi-drug resistant uropathogenic Escherichia coli in Potohar region of Pakistan
Tel: +92 51 2896153040
E-mail: iqbal78@hushmail.com
Peer review under responsibility of Hainan Medical University.
Foundation Project: Partly supported by the research grant of Higher Education Commission Pakistan (No-3782) and internal research funds of Qauid-i-Azam University Islamabad.
#These authors contributed equally to this work.
Ihsan Ali1,#, Zara Rafaque1,#, Safia Ahmed1, Sajid Malik2, Javid Iqbal Dasti1*1Department of Microbiology, Qauid-i-Azam University, Islamabad, Pakistan
2Department of Animal Sciences, Qauid-i-Azam University, Islamabad, Pakistan
ARTICLE INFO
Article history:
Received 3 Aug 2015
Receivedinrevisedform26 Aug,2nd revised form 28 Aug 2015
Accepted 20 Sep 2015
Available online 10 Nov 2015
Keywords:
Fluoroquinolone-resistant
Uropathogenic E. coli
Multi-drug resistant
Extended spectrum beta-lactamase
ABSTRACT
Objective: To scrutinize patterns of multi-drug-resistant uropathogenic Escherichia coli (UPEC) strains and particularly of fluoroquinolone-resistance this is an alternative choice for the treatment of urinary tract infections.
Methods: Bacterial samples (n = 250) were collected from out-patients from August 2012 to August 2014 Islamabad. Antibiotic susceptibility profiling and determination of minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations were performedaccordingtotheguidelinesof Clinicaland Laboratory Standards Institute(CLSI, 2012). Genes, qnrA, qnrB and qnrS were identified by DNA amplification and sequencing. Results: The highest percentage of UPEC isolates were resistant to co-trimoxazole (82%) followed by cephalothin (80%), 2nd Gen, 3rd Gen and 4th Gen cephalosporins, respectively. Resistance against gentamicin, amikacin remained 29% and 4%. For other drugs including nitrofurantoin, tetracycline, carbapenem and beta-lactam inhibitors remained below 10%. Altogether, 59% of the isolates were resistant to at least three antibiotics including one fluoroquinolone. Overall, MICs for ciprofloxacin remained (MIC≥256 μg/mL) and for levofloxacin (MIC≥16 μg/mL and 32 μg/mL). No significant differences were observed regarding MIC values of extended spectrum β-lactamase (ESBL) and non-ESBL producers. For qnrS and qnrB positive isolates MICs remained above 32 μg/mL. Prevalence of UPEC was significantly higher among females and 40% of the isolates were ESBL producers.
Conclusions: Higher percentages of ESBL producing UPEC were associated with urinary tract infections. Moreover, the majority of these isolates were multi-drug resistant and fluoroquinolone-resistant.
1. Introduction
Uropathogenic Escherichia coli (E. coli) (UPEC) is one of the most common cause of urinary tract infections (UTIs) worldwide. A substantial amount of surveillance data indicates the prevalence of trimethoprim-sulfamethoxazole (TMP-SMX) resistant-UPEC strains [1–3]. Eventually,fluoroquinolone has become an alternative therapeutic choice for the treatment [3]. However, recently the prevalence of fluoroquinolone-resistance among uropathogenic strains has also been reported in different regions [1–3]. Particularly, in the Asian-Pacific region and in India, the prevalence of extended spectrum β-lactamases (ESBL) producing fluoroquinolone-resistant uropathogenic strains is significantly higher [4].
Fluoroquinolone-resistance is conferred by different molecular mechanisms including chromosomal mutations in DNA gyrase and/or topoisomerase IV, porins and/or efflux pumps genes. In addition, members of the pentapeptide repeat family of proteins encode plasmid-mediated quinolones resistance. The first member of this family of proteins is known as QnrA, which was identified in USA two decades earlier [5]. Several other genetic determinants belonging to this pentapeptide repeat family of proteins include QnrB, QnrC, QnrD and QnrS which were identified in subsequent years [5]. These molecular factors have wide distribution and were mostly reported inESBL producing Enterobacteriaceae [4]. Exceptionally, some of the homologues of Qnr-like proteins were also reported in other bacterial species including Mycobacterium, Enterococcus, Listeria, colistridia and recently, a new member of the same protein family was identified in Stenotrophomonas maltophilia, annotated as Smqnr[4].
Global epidemiological studies indicate the prevalence of multi-drug resistant (MDR) ESBL producing Enterobacteriaceae both in the community acquired and healthcare facility associated infections [6–10].
Previously, from Asian regions, several studies focused on the prevalence of ESBL producing UPEC, which is known to be associatedwith90%ofthecommunityacquiredandapproximately 50% of the nosocomial UTIs worldwide[11]. In Pakistan, over the last decade, an increase in resistance against quinolones has been witnessed in other bacterial species[12–16]. However, not much is known about the fluoroquinolone-resistance in ESBL producing UPEC strains and its co-relation with plasmid encoded qnr genes.
2. Materials and methods
2.1. Bacterial strains and antimicrobial agents
Bacterial isolates under scrutiny (n = 250)were collected from out-patients of the Pakistan Institute of Medical Sciences Islamabad during the period of August 2012 to August 2014. The experimental work was approved by the Ethical Committees of the hospital and in compliance with recommendations of the Ethical Committees Committee; privacy was maintained regarding patient data. Patients were of different age groups and from different geographical locations across the country. Available clinical history and associated symptoms were registered for the record. The identification of UPEC was performed according to the standard microbiological and biochemical protocols [17]. Antibiotic susceptibility profiling was performed by using Muller-Hinton agar (Oxide, England), according to the Clinical and Laboratory Standards Institute (CLSI) guideline provided in year 2013 [18]. The antibiotic discs were purchased from Bioanalyse (Turkey). The names and concentrations of antibiotic discs are as follows; TMP-SMX (1.25 μg TMP, 23.75 μg SMX), ciprofloxacin (5 μg), sparfloxacin (10 μg), norfloxacin (10 μg), levofloxacin (5 μg), cepfime (30 μg), ceftazidime (30 μg), cefotaxime (30 μg), cefixime (5 μg), cefuroxime (30 μg), cephalothine (30 μg), gentamicin (10 μg), amikacin (30 μg), and augmentin: amoxicillin-clavulanic acid (30 μg), nitrofurantoin (300 μg), sulzone: cefoperazone-sulbactum (105 μg), tigecycline (15 μg), minocycline (30 μg), meropenem (10 μg), aztreonam (30 μg), tazocin: tazobactum-piperacillin (110 μg). To confirm the prevalence of (ESBL) producing isolates, phenotypic tests were performed by using discs of amoxicillin-clavulanic acid, cefotaxime, ceftazidime and manobactum (aztreonam). For testing antibiotic susceptibility Muller-Hinton agar (Oxide, England) was used by following the Kirby Bauer disc-diffusion method as described in CLSI guidelines[18].
2.2. Determination of MICs and MBCs for levofloxacin and ciprofloxacin
Antibacterial activity of levofloxacin and ciprofloxacin were determined by measuring minimum inhibitory concentrations (MICs). For these measurements micro-dilution broth method was used as described by CLSI guidelines [18]. Briefly, an adjusted inoculum of the test organism was inoculated into Muller-Hinton broth (Oxoid, Basingstoke, UK) containing two fold serial dilution of an initial antibiotic solution, each well contained approximately 5×105CFU/mL. Bacterial cultures were tested against several antibiotic dilutions: 256, 128, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25 and 0.125 μg/mL. The cultures were incubated for 18 h at 37°C, prior to make any observations and upon inhibition of visible growth, the lowest concentrations of antimicrobial agents responsible for inhibition were considered MICs. By definition, minimum bactericidal concentration (MBC) was considered to be the concentration of drug at which 99.9% reduction in the colony forming unit has been recorded when comparing with the original inoculums. Determination of MBCs was carried out by platting 0.01 mL from the tube having no visible growth on Muller-Hinton agar plate (Oxide, England) after 18–24 h of incubation at 37°C and was defined as the concentration that reduced the colony forming units up to 99.9% in comparison to original inoculums. The strain of E. coli ATCC 25922 was used as quality control. The breakpoints for the resistance against ciprofloxacin were 4 mg/L and for levofloxacin 8 mg/L according to the CLSI guidelines [18].
2.3. DNA extraction and amplification of qnrA, qnrB and qnrS genes
Bacterial DNA extraction was performed by phenolchloroform method [19] and the qualitative analysis of the DNA was performed by directly visualizing on agarose gel (Sigma, Germany). After confirmation, eighty-five quinolones resistant strains were screened for the presence of qnrA, qnrB and qnrS genes by using previously reported sets of primers and PCR conditions [19].
2.4. DNA sequencing of qnrA, qnrB and qnrS genes
The amplified products were purified by using (Gel Band Purification Amersham, USA) DNA sequencing was done by automated DNA sequencing system (ABI 3130, Perkin–Elmer Applied Biosystems, Foster city, California). The electropherogram of sequenced DNA was analyzed by Chromas 2.23 (Technelysium, Queensland, Australia). Protein and nucleotide sequence similarity were analyzed by using BLAST (Basic Local Alignment Search Tool 138.0). DNA sequences were submitted to GenBank database which were assigned following accession numbers KR698789, KR698790, KR698791, KR698792, KR698793.
2.5. Statistical analysis
The antibiotic resistance data was recorded in MS-Excel and the statistical analysis of the data was performed through GraphPad Prism, version 5 and statistical significance between different parameters was checked by Chi-square test, t-test and ANOVA at a cut-off value of P<0.05.
3. Results
3.1. Prevalence of antibiotic resistance among UPEC
Altogether, 148 samples were confirmed as UPEC, antibiotic resistance was checked against cephalosporins (1st Gen, 2ndGen, 3rd Gen and 4th Gen) aminoglycosides, carbepenems, quinolones, nitrofurantoin, monobactams, sulfonamides, glycylcyclines, tetracycline and beta-lactamase inhibitors. The highest percentage of isolates was resistant to co-trimoxazole (82%) and cephalosporin (80%), 1st Gen, cephalothin that was followed by other generation of cephalosporin (Table 1). Seventy-one percent of the tested isolates were resistant to amoxicillin-clavulanic acid. Resistance against gentamicin remained 29% while against amikacin 4%. The percentage of resistance against other antibiotics including nitrofurantoin, tetracycline, carbapenem and β-lactam inhibitors remained below 10% (Table 1). A total of 59% of the isolates showed resistant to at least three antibiotics including fluoroquinolone and were therefore named as fluoroquinolone MDR (Figure 1a, b). The overall rate of resistance towards fluoroquinolone remained 61% (n = 91). Individually, the rate of resistance against ciprofloxacin, sparfloxacin, levofloxacin and norfloxacin remained 60%, 59%, 58% and 57%, respectively. Overall, the prevalence of UPEC was significantly higher among female patients in comparison to the male patients (Figure 1b). However, distribution of the fluoroquinolone-resistant isolates was found to be significantly higher among the male patients P<0.05 (Figure 2a). Observed patterns of fluoroquinoloneresistant MDR isolates are shown among different age groups of the patients (Figure 1a). Overall, higher numbers of isolate were MDR (Figures 3 and 4). The distribution of the MDR isolates among different genders and age groups is shown in (Figure 5a, b). Out of all tested isolates 40% of the isolates (n = 59) were ESBL producers while remaining (n = 89) isolates were non-ESBL producers.
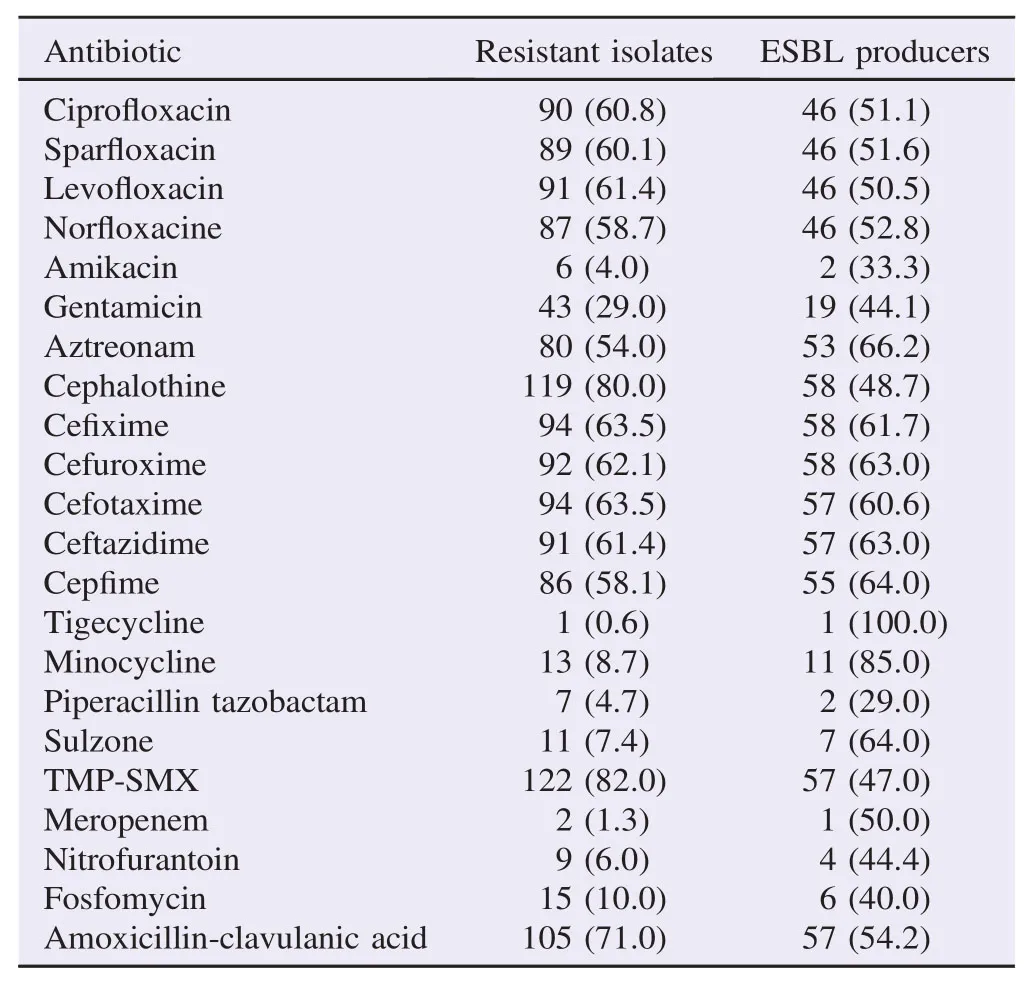
Table 1Numbers and percentages of antibiotic resistant UPEC isolates. n (%).
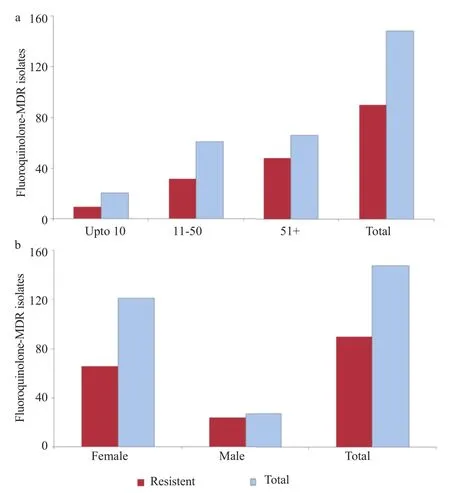
Figure 1. Age (a) and gender (b) wise prevalence of fluoroquinolone-MDR UPEC.
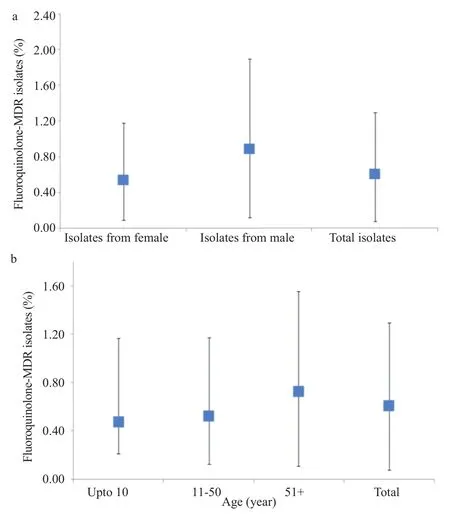
Figure 2. Age (a) and gender (b) wise distribution of fluoroquinolone-MDR UPEC.

Figure 3. Overall prevalence of MDR-UPEC in male and female patients.
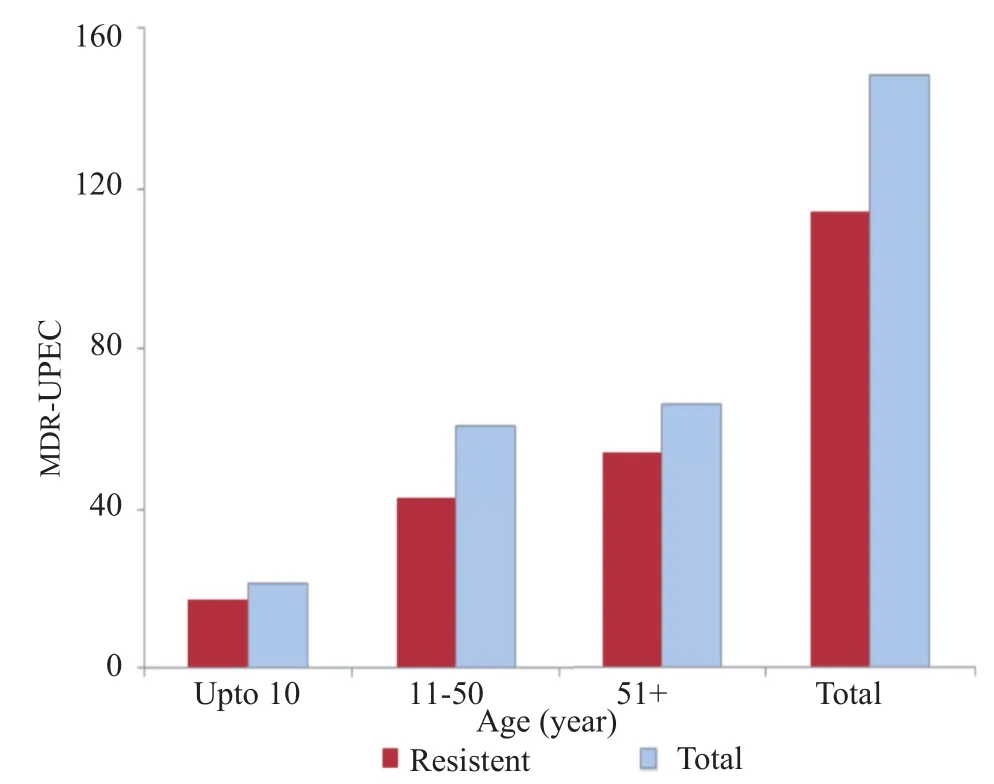
Figure 4. Overall prevalence of MDR-UPECs in different age groups.
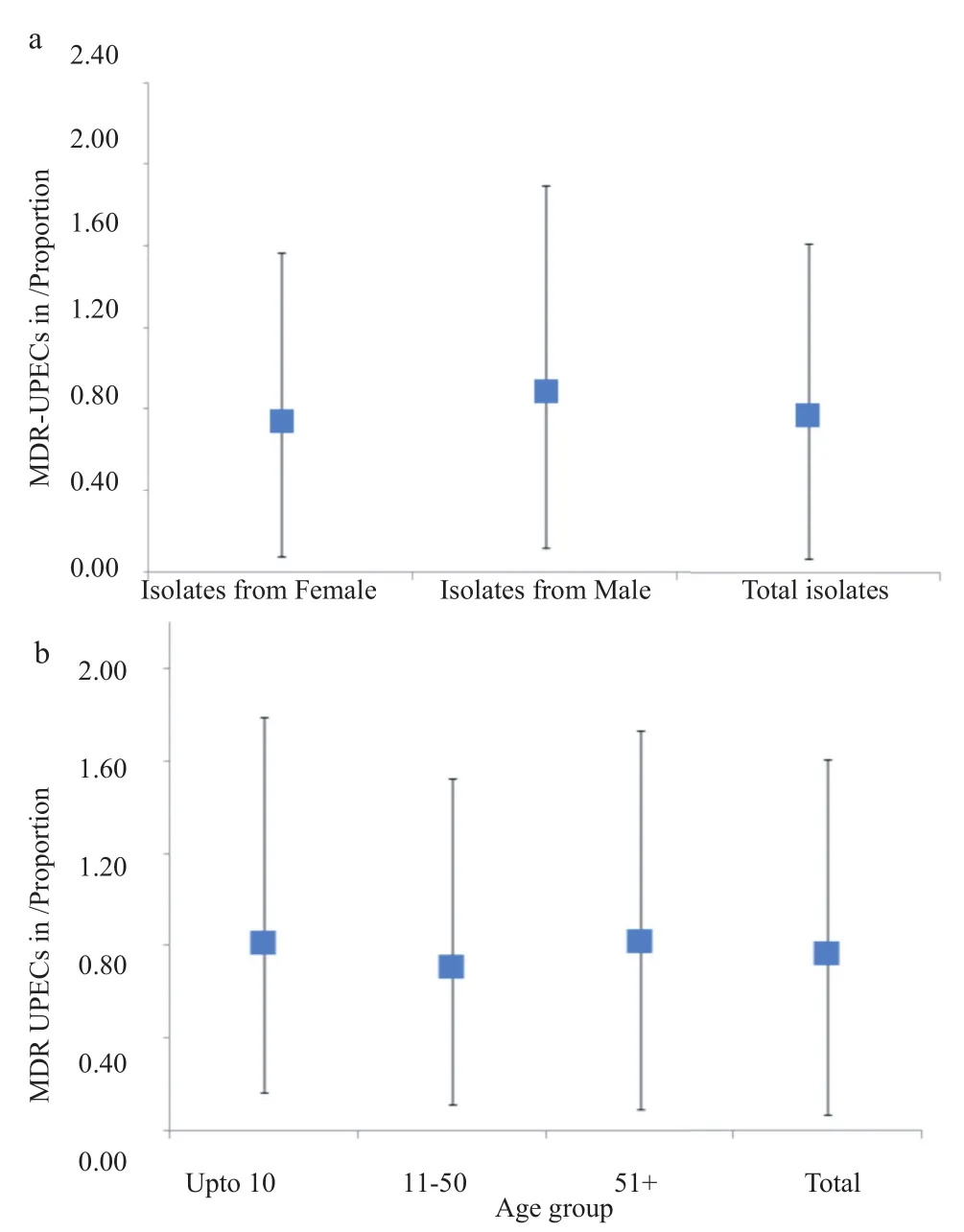
Figure 5. Overall distribution of MDR-UPEC among male and female patients (a) and among different age groups (b).
3.2. Measurement of MICs
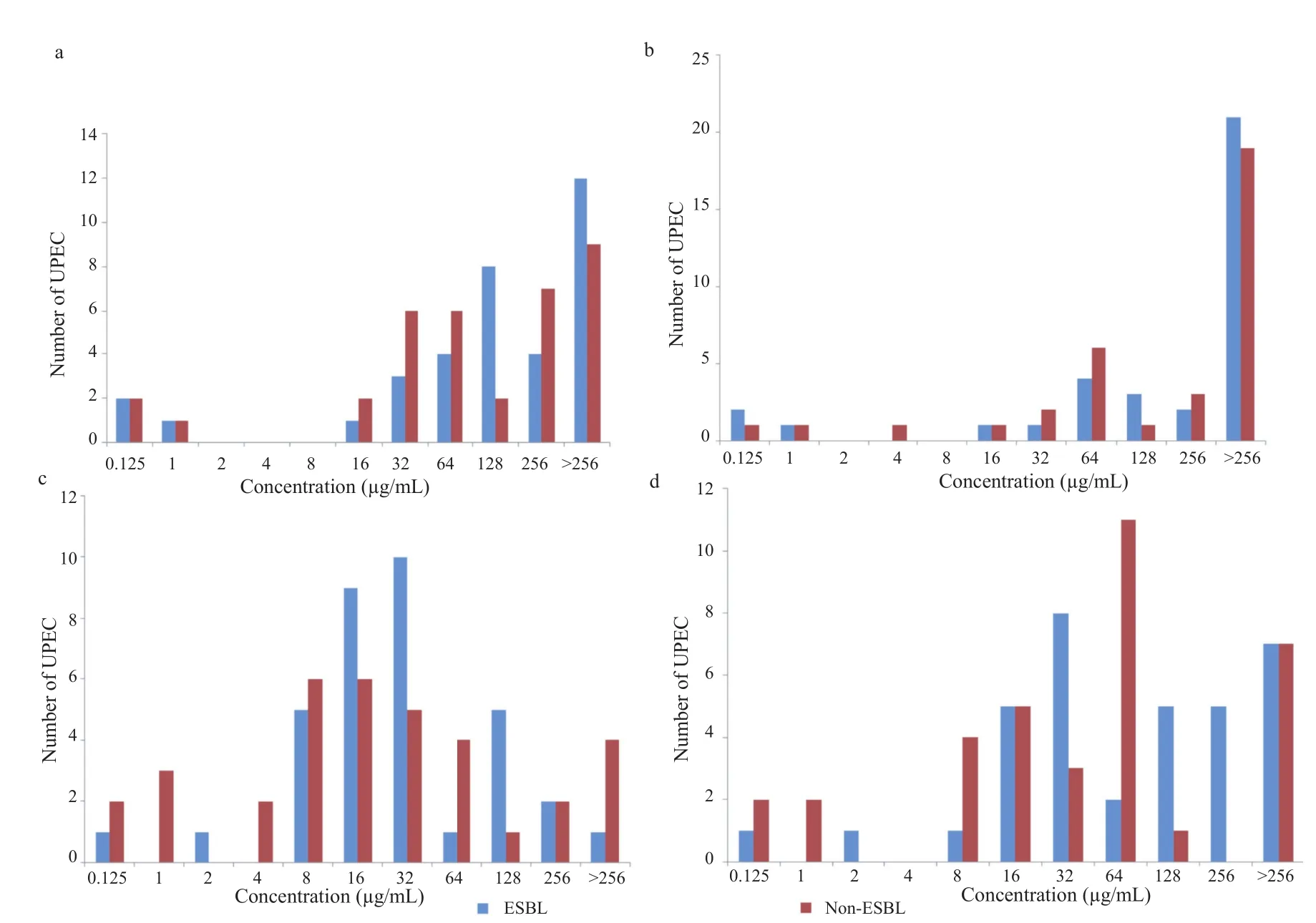
Figure 6. Ciprofloxacin MICs (a) and MBCs (b), and levofloxacin MICs (c) and MBCs (d) of ESBL and non-ESBL UPEC.
Two equal subset of the selected isolates each (n = 35) ESBL producers and (n = 35) non-ESBL producers were tested to determine MIC of ciprofloxacin and levofloxacin, two most commonly prescribed fluoroquinolone antibiotics. Bacterial isolates were tested against different concentrations of antibiotics ranging from 0.125 μg/mL to 256 μg/mL. Overall, MIC values for ciprofloxacin remained significantly higher (MIC≥256 μg/mL) for both ESBL producers and non-ESBL producers (Figure 6a). In case of levofloxacin however, MIC values for the majority of the isolates fall between (MIC≥16 μg/mL and 32 μg/mL as shown in Figure 6c). Similarly, MBCs for both antibiotics (ciprofloxacin and levofloxacin) were determined for both ESBL producers and non-ESBL producers. Overall, the majority of isolates both ESBL producers and non-ESBL producers showed MBC value≥256 μg/mL for ciprofloxacin (Figure 6b). In contrast to ciprofloxacin for levofloxacin, MBC value remained 64 μg/mL for the majority of ESBL producers and 32 μg/mL for non-ESBL producing isolates (Figure 6d). Overall, the differences between ESBL producers and non-ESBL producers regarding their MICs and MBCs values were not statistically significant. The mean distribution of MIC-ciprofloxacin, MBC-ciprofloxacin, MIC-levofloxacin and MBC-levofloxacin is shown (Figure 7). An independent comparison between mean values of MIC-ciprofloxacin and MBC-ciprofloxacin, MIC-levofloxacin and MBC-levofloxacin confirmed significant differences (P<0.0001). Spearman's rank correlation was established between MIC and MBC values of ciprofloxacin and levofloxacin that showed that these differences were not more than fourfold in any case (Figure 8a, b). Similarly, the difference between MIC-ciprofloxacin and MIC-levofloxacin were statistically significant (P<0.0001).
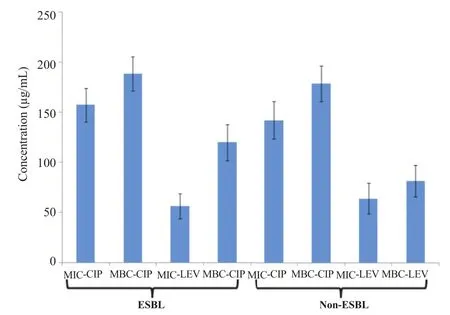
Figure 7. Mean distribution of MICs and MBCs of ciprofloxacin and levofloxacin.
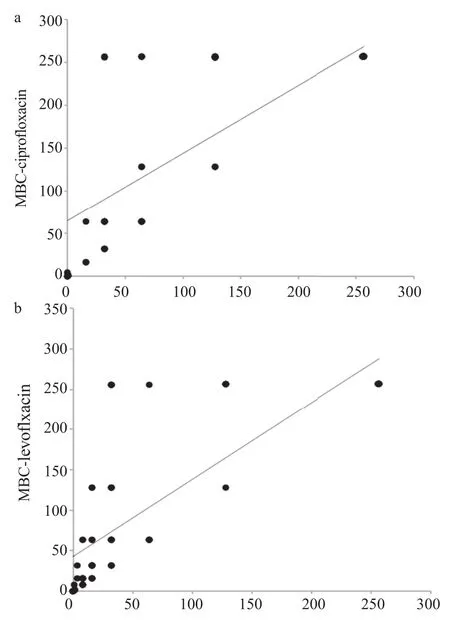
Figure 8. Spearman's rank correlation established between MIC and MBC values of ciprofloxacin (a) and levofloxacin (b).
3.3. Detection and DNA sequencing of qnrA, qnrB and qnrS genes
All the fluoroquinolone-resistant isolates (n = 91) were screened for the presence of plasmid-mediated quinolones resistance genes out of which 2 (2.35%) were found to carry qnrB gene while three (3.53%) were found to carry qnrS gene. None of the sample was positive for qnrA gene. Overall, MICs for qnrS and qnrB positive isolates remained above 32 μg/mL.
4. Discussion
In this study, the highest percentage of isolates was resistant to TMP-SMX which was widely used as a first treatment choice for UTI infections [20]. For this class of antibiotics, similar trends have previously been noticed [1,2]. Due to the higher percentage of resistance, in some countries use of TMP-SMX has become limited. For example, German national guidelines do not recommend this agent as a first choice for the treatment of uncomplicated cystitis [21]. Since TMP-SMX resistance is associated with the development of concomitant resistance to other antibiotics thereof, limited use of TMPSMX may help to sustain its effectiveness over the long run. Other than TMP-SMX, uncomplicated acute cystitis in female patients is treated with nitrofurantoin, however it is not recommended for the patients with pyelonephritis[22]. Overall rate of resistance against nitrofurantoin remained low in this study, therefore, our finding reiterates that nitrofurantoin is a suitable empiric choice for the treatment of acute cystitis in this region.
Along with other antibiotics, resistance against fluoroquinolone is of particular interest because it is frequently recommended for the treatment of complicated cystitis in female patients. In this study, the overall prevalence of fluoroquinolone-resistant isolates is higher in female patients. Vice versa, given the small numbers of male UTI cases, overall distribution of fluoroquinolone-resistant isolates is higher among male patients. Fluoroquinolones are also used as a first choice for the treatment of UTIs in men, mainly because of certain advantages of this antibiotic over co-amoxiclav, particularly in terms of its pharmacokinetic properties [23,24]. Taken together, in this study 59% of the isolates were fluoroquinolone-resistant, indicating considerable constraints on treatment options particularly for complicated cystitis. ESBL producing fluoroquinolone-resistant uropathogenic strains have significantly been increased in the Asian-Pacific region and particularly in India [4]. Upon scrutiny, 40% of the isolates were ESBL producers in this study and equal numbers of both ESBL producers and non-ESBL producers were tested to determine MICs of ciprofloxacin and levofloxacin, both are most commonly prescribed fluoroquinolone across Pakistan. For ESBL producers, resistance to monobactams and all four generations of cephalosporin ranged from 54% to 80.4% while resistance to co-amoxiclav remained 71%. Furthermore, the majority of isolates were resistant to all three classes of antibiotics. It is noteworthy, that frequent uses of cephalosporin are linked with the selection of ESBL producers in different regions [25].
Upon measurement of MICs and MBCs of ciprofloxacin and levofloxacin, no significant differences were observed regarding ESBL and non-ESBL producing isolates, though MICs values for both groups remained significantly higher. In case of both antibiotics, MICs and MBCs differences did not exceed more than three folds, generally for bactericidal drugs like fluoroquinolone, MBCs do not exceed more than fourfold of MICs. Overall, ciprofloxacin (MIC≥256 μg/mL) MICs values were significantly higher. In case of levofloxacin, MIC values remained between MIC≥16 μg/mL and 32 μg/mL. Fluoroquinolones have particular pharmacodynamic properties as they exhibit bactericidal activity which is concentrationdependent and have extended post antibiotic effects. However, it is important to note that determination of MICs and MBCs in vitro may provide insights about the antimicrobial activity of drugs, however such findings have the least significance regarding the treatment of infected patients, mainly because fluctuating level of drug in the serum of the treated patients and the numbers of bacteria at site of infection in vivo may pose completely different scenario. Furthermore, during in vitro measurement of MICs or MBCs bacterial growth is not static that is normally the case in vivo. Nevertheless, in vitro determination of MICs and MBCs based on breakpoints differentiates two populations (i.e. susceptible and resistant).
In this study, qnrB and qnrS genes were associated with low level of fluoroquinolone-resistance. Previously, several independent reports confirmed that Qnr-positive ESBL producer isolates conferred low level resistance to quinolones particularly because the key function of the Qnr proteins is to protect the gyrase proteins. However, resistance mediated by these molecular factors may facilitate co-selection of plasmid encoded resistance to other antibiotics[26–28]. Conclusively, the problem of antibiotic resistance has gained unprecedented momentum worldwide, therefore important measures are mandatory to ensure rational and well-informed use of antibiotics as are taken in European Union. Overall, this report indicates a higher prevalence of MDR and ESBL UPEC in this region and prevalence of qnr genes in UPEC is linked with the moderate level of fluoroquinolone-resistance.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The work was partly supported by the research grant of Higher Education Commission Pakistan (No.-3782) and internal research funds of Qauid-i-Azam University Islamabad.
References
[1] Sanchez GV, Master RN, Karlowsky JA, Bordon JM. In vitro antimicrobial resistance of urinary Escherichia coli isolates among U.S. outpatients from 2000 to 2010. Antimicrob Agents Chemother 2012; 56: 2181-3.
[2] Sanchez GV, Baird AM, Karlowsky JA, Master RN, Bordon JM. Nitrofurantoin retains antimicrobial activity against multidrugresistant urinary Escherichia coli from US outpatients. J Antimicrob Chemother 2014; 69: 3259-62.
[3] Karlowsky JA, Hoban DJ, Decorby MR, Laing NM, Zhanel GG. Fluoroquinolone-resistant urinary isolates of Escherichia coli from outpatients are frequently multidrug resistant: results from the North American urinary tract infection collaborative alliancequinolone resistance study. Antimicrob Agents Chemother 2006; 50: 2251-4.
[4] Dalhoff A. Global fluoroquinolone resistance epidemiology and implications for clinical use. Interdiscip Perspect Infect Dis 2012; http://dx.doi.org/10.1155/2012/976273.
[5] Wang D, Wang H, Qi Y, Liang Y, Zhang J, Yu L. Novel variants of the qnrB gene, qnrB31 and qnrB32, in Klebsiella pneumoniae. J Med Microbiol 2011; 60: 1849-52.
[6] Ma XJ, Wu YH, Li LY, Xu Q, Hu BJ, Ni YX, et al. First multicenter study on multidrug resistant bacteria carriage in Chinese ICUs. BMC Infect Dis 2015; http://dx.doi.org/10.1186/s12879-015-1105-7.
[7] Raji MA, Jamal W, Ojemeh O, Rotimi VO. Sequence analysis of genes mediating extended-spectrum beta-lactamase (ESBL) production in isolates of Enterobacteriaceae in a Lagos Teaching Hospital, Nigeria. BMC Infect Dis 2015; http://dx.doi.org/10.1186/ s12879-015-1005-x.
[8] Wegner C, Hubner NO, Gleich S, Thalmaier U, Kruger CM, Kramer A. One-day point prevalence of emerging bacterial pathogens in a nationwide sample of 62 German hospitals in 2012 and comparison with the results of the one-day point prevalence of 2010. GMS Hyg Infect Control 2013; http://dx.doi.org/10.3205/ dgkh000212.
[9] Xiao YH, Giske CG, Wei ZQ, Shen P, Heddini A, Li LJ. Epidemiology and characteristics of antimicrobial resistance in China. Drug Resist Updat 2011; 14: 236-50.
[10] Park SH, Byun JH, Choi SM, Lee DG, Kim SH, Kwon JC, et al. Molecular epidemiology of extended-spectrum β-lactamase-producing Escherichia coli in the community and hospital in Korea: emergence of ST131 producing CTX-M-15. BMC Infect Dis 2012; http://dx.doi.org/10.1186/1471-2334-12-149.
[11] Kucheria R, Dasgupta P, Sacks S, Khan M, Sheerin N. Urinary tract infections: new insights into a common problem. Postgrad Med J 2005; 81: 83-6.
[12] Jabeen K, Nizamuddin S, Irfan S, Khan E, Malik F, Zafar A. Increasing trend of resistance to penicillin, tetracycline, and fluoroquinolone resistance in Neisseria gonorrhoeae from Pakistan (1992–2009). J Trop Med 2011; http://dx.doi.org/10.1155/2011/ 960501.
[13] Ahmed I, Jabeen K, Inayat R, Hasan R. Susceptibility testing of extensively drug-resistant and pre-extensively drug-resistant Mycobacterium tuberculosis against levofloxacin, linezolid, and amoxicillin-clavulanate. Antimicrob Agents Chemother 2013; 57: 2522-5.
[14] Nasiri MI, Naqvi SB, Zaidi AA, Saeed R, Raza G. Comparative study on resistant pattern of clinical isolates against levofloxacin and cefepime. Pak J Pharm Sci 2013; 26: 415-9.
[15] Yasmin F, Akhtar N, Hameed A. In vitro synergistic effect of ciprofloxacin with aminoglycosides against multidrug resistant-Pseudomonas aeruginosa. Pak J Pharm Sci 2013; 26: 1041-4.
[16] Habeeb MA, Haque A, Iversen A, Giske CG. Occurrence of virulence genes, 16S rRNA methylases, and plasmid-mediated quinolone resistance genes in CTX-M-producing Escherichia coli from Pakistan. Eur J Clin Microbiol Infect Dis 2014; 33: 399-409.
[17] Marhova M, Kostadinova S, Stoitsova S. Antimicrobial resistance profiles of urinary Escherichia coli isolates. Biotechnol Biotechnol Equip 2009: 616-20.
[18] Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 23rd informational supplement. M100–S23. Wayne: Clinical and Laboratory Standards Institute; 2013.
[19] Ali I, Kumar N, Ahmed S, Dasti JI. Antibiotic resistance in uropathogenic E. coli strains isolated from non-hospitalized patients in Pakistan. J Clin Diagn Res 2014; 8: DC01-4.
[20] Go?ebiewski M, Kern-Zdanowicz I, Zienkiewicz M, Adamczyk M, Zylinska J, Baraniak A, et al. Complete nucleotide sequence of the pCTX-M3 plasmid and its involvement in spread of the extendedspectrum beta-lactamase gene bla CTX-M-3. Antimicrob Agents Chemother 2007; 51: 3789-95.
[21] Wagenlehner FM, Schmiemann G, Hoyme U, Funfstuck R, Hummers-Pradier E, Kaase M, et al. [National S3 guideline on uncomplicated urinary tract infection: recommendations for treatment and management of uncomplicated community-acquired bacterial urinary tract infections in adult patients]. Urol A 2011; 50: 153-69. German.
[22] Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for microbiology and infectious diseases. Clin Infect Dis 2011; 52: e103-20.
[23] Wagenlehner FM, Weidner W, Naber KG. Therapy for prostatitis, with emphasis on bacterial prostatitis. Expert Opin Pharmacother 2007; 8: 1667-74.
[24] Charalabopoulos K, Karachalios G, Baltogiannis D, Charalabopoulos A, Giannakopoulos X, Sofikitis N. Penetration of antimicrobial agents into the prostate. Chemotherapy 2003; 49: 269-79.
[25] Dancer SJ. The problem with cephalosporins. J Antimicrob Chemother 2001; 48: 463-78.
[26] Wang M, Tran JH, Jacoby GA, Zhang Y, Wang F, Hooper DC. Plasmid-mediated quinolone resistance in clinical isolates of Escherichia coli from Shanghai, China. Antimicrob Agents Chemother 2003; 47: 2242-8.
[27] Vien le TM, Minh NN, Thuong TC, Khuong HD, Nga TV, Thompson C, et al. The co-selection of fluoroquinolone resistance genes in the gut flora of vietnamese children. PLoS One 2012; http://dx.doi.org/10.1371/journal.pone.0042919.
[28] Hernandez A, Sanchez MB, Martinez JL. Quinolone resistance: much more than predicted. Front Microbiol 2011; http://dx.doi.org/ 10.3389/fmicb.2011.00022.
Original article http://dx.doi.org/10.1016/j.apjtb.2015.09.020
*Corresponding author:Javid Iqbal Dasti, PhD, Department of Microbiology, Qauid-i-Azam University, Islamabad, Pakistan.
 Asian Pacific Journal of Tropical Biomedicine2016年1期
Asian Pacific Journal of Tropical Biomedicine2016年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- The antibacterial activity of selected plants towards resistant bacteria isolated from clinical specimens
- Evaluation of antibacterial activity and synergistic effect between antibiotic and the essential oils of some medicinal plants
- Comparative studies of elemental composition in leaves and flowers of Catharanthus roseus growing in Bangladesh
- Formulation and evaluation of semisolid jelly produced by Musa acuminata Colla (AAA Group) peels
- Antibiotic resistance profile and RAPD analysis of Campylobacter jejuni isolated from vegetables farms and retail markets
- Urban air pollution, climate and its impact on asthma morbidity
