Formulation and evaluation of semisolid jelly produced by Musa acuminata Colla (AAA Group) peels
?
Formulation and evaluation of semisolid jelly produced by Musa acuminata Colla (AAA Group) peels
Tel: +60 3 2203 1347, +60 19 986 1519
E-mail: mariamfirdhaus@utm.my
Peer review under responsibility of Hainan Medical University.
Foundation Project: Supported by the Research University Grant Scheme of Tier 1 provided by Universiti Teknologi Malaysia (Grant No. PY/2014/03649).
Noor Azwani Mohd Rasidek, Mariam Firdhaus Mad Nordin*, Kamyar Shameli
Malaysia-Japan International Institute of Technology, Universiti Teknologi Malaysia Kuala Lumpur, Jalan Semarak,
54100 Kuala Lumpur, Malaysia
ARTICLE INFO
Article history:
Received 19 Jun 2015
Received in revised form 6 Jul, 2nd revised form 9 Jul,
3rd revised form 6 Aug 2015
Accepted 21 Sep 2015
Available online 14 Nov 2015
Keywords:
Antioxidant activity
Banana peel
Jelly formulation
Musa acuminata Colla
Total phenolic content
ABSTRACT
Objective: To study the jelly formulation produced by Musa acuminata Colla (AAA Group) peels and evaluate its antioxidant properties which are related to the product quality.
Methods: The formulations of peel jelly were established under two-level full factorial designs within two blocks and one center point. Regarding response optimizer, the amount of sugar and citric acid was obtained; hence, the peel jellies were produced. The evaluation of antioxidant properties was conducted by using total phenolic content (TPC) assay and 1,1 diphenyl-2-picrylhydrazyl (DPPH) free radical assay.
Results: The TPC of peel powder varied from 91.8 to 602.26 mg gallic acid equivalents/ 100 g dry weight, and 5%–7% peel jellies had phenolic content ranging from 29.38 to 48.31 mg gallic acid equivalents/100 g dry weight. The results of DPPH test indicated that at 10 mg/mL, the peel powder showed 89% DPPH inhibition, while 7% peel jelly prominently exhibited 84% DPPH inhibition. The correlation between DPPH IC50value and TPC of peel powder as well as peel jelly was quite reasonably high with correlation coefficient ranging from 0.843 7 to 0.995.
Conclusions: TPC can be used as an indicator in assessing the antioxidant activity of fruits and vegetables. The present investigation reveals that TPC is mainly responsible for DPPH free radical scavenging capacity.
1. Introduction
Banana is one of the world's most important and cheapest fruits that is extensively cultivated in tropical countries for its profitable applications in food industry [1]. It has been used as staple food which is eaten raw or can be processed to a range of products such as chips, juice, smoothie, weaning food and other beneficial products [2,3]. Banana can be classified as a dessert, plantain cooking and non-plantain cooking bananas[4].
In Malaysia, many assortments of banana varieties can be accessible in the market, yet the most popular varieties are Cavendish (Musa acuminata L. cv cavendishii) and Dream [Musa acuminata Colla (M. acuminata) AAA cv Berangan] banana [5]. These varieties are normally freshly consumed as edible types compared to other varieties.
Normally, before any forms of use of the banana, either in daily routine consumptions or food industrial applications, the peels from the fruit were peeled out from the fruit pulp and reused as animal feedstock or discarded. Those peels are largely involved as agricultural residues in banana processing industry [6,7]. These enormous amounts of by-products are excellent sources of highly valuable compounds for other industries. If recycled, they will prevent the loss of huge amount of untapped biomass besides adding to the environmental benefits [1]. In terms of nutritional contents, the banana peel is well known to contain vast quantity of dietary fiber, mainly hemicelluloses and pectin compound, which is a complex mixture of polysaccharides found in many fruits and some root vegetables [8]. It is also reported as an important source of phenolic compounds that devote to natural sources of antioxidant[4].
The astringent taste of unripe bananas is due to presence of phenolic compounds [2]. Phenolic compounds are believed to account for a major portion of antioxidant activity in manyplants. The correlation between total phenolic concentration and antioxidant has been widely studied in different foodstuffs, such as fruits and vegetables [9]. Additionally, the National Cancer Standard Institute declared that the banana peels were classified as non toxic to human cells [10].
Pectin gel can be made without adding any commercial pectin. The gelling properties are allowed with correct amount of sugar, optimum acidity and sufficient pectin.
Other than that, at all stages of maturity, the banana peels are mostly richer in dietary fiber and pectin contents compared with other fruit peels. Therefore, these peels are applicable for variety of utilizations in the health care and food industries. This is possible to generate new potentials of agricultural by-products [10,11].
For this reason, the objective of the present study is to formulate the M. acuminata (AAA Group) peel jelly without adding commercial pectin in the identical conditions and ingredients. Furthermore, after long cooking process, the antioxidant activity was evaluated on developed peel jellies to demonstrate the relationship between phenolic contents and 1,1-Diphenyl-2-picrylhydrazyl (DPPH) scavenging activities. Ideally, the banana peel has brought a new potential source as base ingredient in jelly making in addition to its nutritional value.
2. Materials and methods
2.1. Sample peel sources
The study used M. acuminata cv Berangan peels at yellowgreen stage of ripening. The bananas harvested from a local orchard and ripening storage were observed at room temperature until reached the required stage (yellow-green color). All data of samples were recorded in the sampling protocol.
2.2. Powdered peel samples
The fresh peels were cut into small pieces and frozen dried for 48 h. The samples were grounded by using commercial grinder into 300 μm mesh size, then stored at?18°C in airtight containers for further analysis.
2.3. Design of experiment
The design of experiment of peel jellies was generated by using two-level full factorial designs within two blocks and one center point and established by Minitab software version 16.0.2 (Minitab Inc.). The jelly formulation consisted of sugar (19.0–19.3 g) and citric acid (0.207–0.209 g). The concentration and volume of peel extracts were also considered in the formulation. The parameters of physicochemical properties of jellies were pH and total soluble solid (TSS). Both of response properties were used to fulfill the response optimization plot in a way of reaching at the optimum jelly condition.
2.4. Peel jelly processing
The jelly formulation was prepared at the extract concentrations of 5%, 6% and 7%. Prepared peel extracts were boiled for 5 min, and accurately 19.15 g of sugar was added. The mixture was then stirred completely and cooked further for 15 min until reaching the gelling temperature. After 20 min of cooking process, 0.207 g of citric acid was added to the mixture and stirred continuously. The mixture was heated again for 30 min at 80°C until reaching the desirable consistency. The jelly consistency in°Brix value was measured by using a digital handheld refractometer. The dispersion mixture was then poured into a jar and cooled for 48 h at room temperature to allow gel to set up. The produced jelly was then frozen dried, powdered and stored at?18°C for further analysis.
2.5. Peel extracts for evaluation of phenolic contents and antioxidant activity
The extraction procedure was conducted as method described by Lee et al. with some modifications [10]. Briefly, 2 g of powdered peel and peel jelly were extracted twice with continuous stirring at room temperature for 1 h by using 100 mL methanol (80:20, v/v) and 100 mL acetone (70:30, v/ v), respectively. The collected extracts were centrifuged at 4 000 r/min for 15 min. The supernatants were transferred into volumetric flasks and 80% methanol was added to make a total volume of 200 mL.
2.6. Chemicals and instruments
The analytical graded chemicals and instruments involved in formulation and antioxidant analysis consisted of citric acid (anhydrous), absolute methanol, acetone, sodium carbonate, ascorbic acid, gallic acid and Folin–Ciocalteau reagent that were purchased from Merck (Darmstadt, Germany). DPPH was from Sigma Chemical Co. (St Louis, MO, USA). Digital handheld refractometer (Atago, Japan), pH meter (HANNA 2211), digital hotplate, and UV spectrophotometer (Genesys 20) were also used.
2.7. Total phenolic assay
The phenolic content was assessed via the Folin–Ciocalteau method. Briefly, 0.5 mL of powdered peels or peel jelly or commercial fruit jelly were introduced into test tubes and mixed with 2.5 mL of a 10 fold diluted Folin–Ciocalteau reagent followed by 2 mL of 7.5% sodium carbonate. The tubes were kept for 30 min in the dark room. All concentrations were prepared in the range of 0.1–10 mg/mL. The triplicate absorbance was read at 760 nm spectrometrically. Total phenolic content (TPC) was calculated by using the following formula:
TPC=Gallic acid equivalents(GAE)(mg/mL)×V/m
where, V was the volume (mL) of extract and m was the weight (g) of pure plant extract. Total phenolics were expressed as mg GAE per 100 g extract.
2.8. Free radical scavenging activity
The DPPH radical scavenging activity was determined as method described by Jain et al. with slight modifications[11]. In brief, 0.1 mL of powdered peel or peel jelly or commercial fruit jelly at 0.1–10 mg/mL was added to 3 mL of 0.002% methanolic solution of DPPH. The reaction mixtures were incubated for 30 min in dark room and the absorbance at 517 nm was read against methanol as a blank. Standard ascorbic acid wasprepared at 0.1–10 mg/mL and the linear regression for sample references was generated. All tests were performed in triplicate, and DPPH radical scavenging capacity was calculated by the formula:
% Scavenging activity=1?(Abs/Abc)×100
where, Abs and Abc represented the average absorbance of samples and absorbance of methanolic DPPH as control at various concentrations, respectively.
3. Results
3.1. Response optimization plot
The response surface design was established in central composite design by using Minitab software version 16.0.2 (Minitab Inc.), within the collected responses of pH and TSS values as shown in Table 1. The response surface design was used to designate the range of ingredients contents in peel jelly with predicted value of responses, while the response optimizer was used to meet the desired contents of ingredients.
Figure 1 showed the optimization plot of effect of each factor (columns) on the responses or composite desirability (rows). The vertical red lines in the graph represented the current factor settings. The numbers displayed at the top of a column showed the current factor level settings (in red) and horizontal blue lines with numbers represented the responses for current factor level. The factors comprised in jelly formulation were sugar and citric acid, which responded to TSS and pH values. The goal setting of both responses, TSS and pH, were at the middle point, which are 72°Brix and 3.3 respectively. In present data, the current amounts of sugar and citric acid were 19.15 and 0.207 g, respectively.
Moreover, the predicted responses of pH and TSS contributed to desirability at 0.747 and 0.959, respectively. The composite desirability of both predicted values was 0.846 15. The predicted response indications of physicochemical properties of peel jelly were used tentatively to define the textural characteristics of the end product.
3.2. TPC
The antioxidant compounds reacted with Folin–Ciocalteau reagent and thus the concentrations of phenolic groups were measured [9].
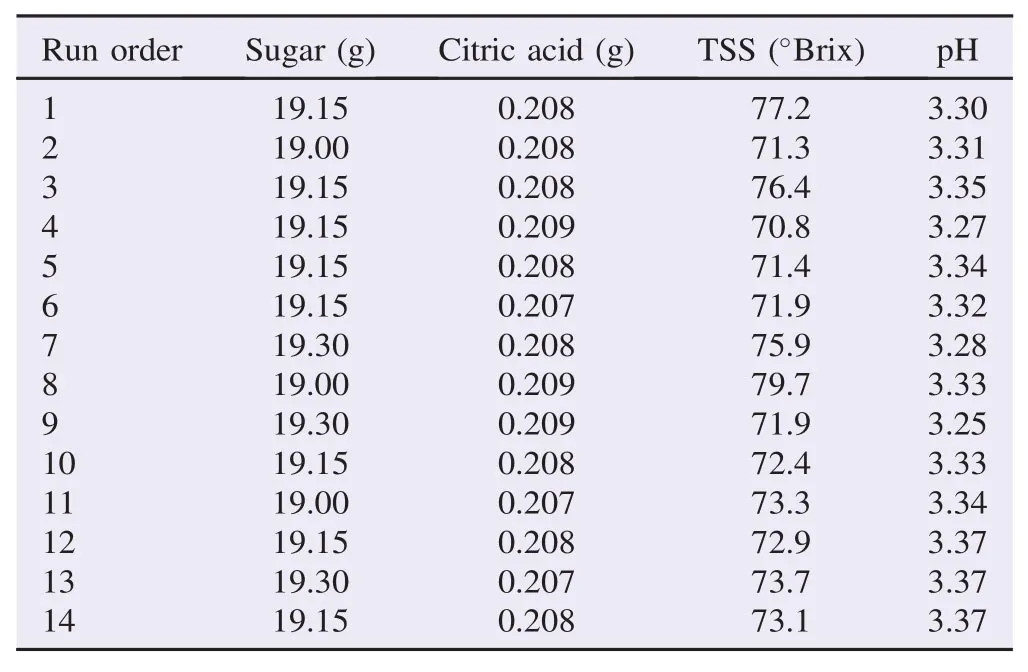
Table 1Formulation batches of peel jellies.
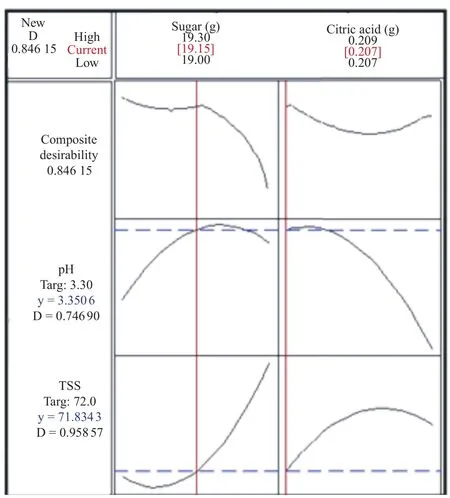
Figure 1. Optimization plot of M. acuminata cv Berangan peel jelly.D: Desirability which indicates the settings seem to achieve favorable results for all responses as a whole; Targ: Target which indicates most desirable response value, where if a response is equal to the target, its desirability is one. The composite desirability indicates that the jelly formulations are more effective at desired pH (0.747) and TSS (0.959).
The absorbance of gallic acid was determined and presented as phenolic contents by using regression Y = 0.354x–0.076 with R2= 0.995, and TPC was expressed as mg GAE/100 g. The TPC of peel powder and developed peel jelly was recorded in Table 2. At the concentrations of 0.1–10.0 mg/mL, the TPC of peel powder varied from 91.800 to 602.260 mg GAE/100 g, while the TPC of peel jelly of 5%–7% varied from 29.38 to 48.31 mg GAE/100 g. Conversely, the commercial fruit jelly showed TPC from 30.23 to 39.55 mg GAE/100 g. Thus, the TPC of peel jelly showed a potential to have an antioxidant activity even after a long cooking process.
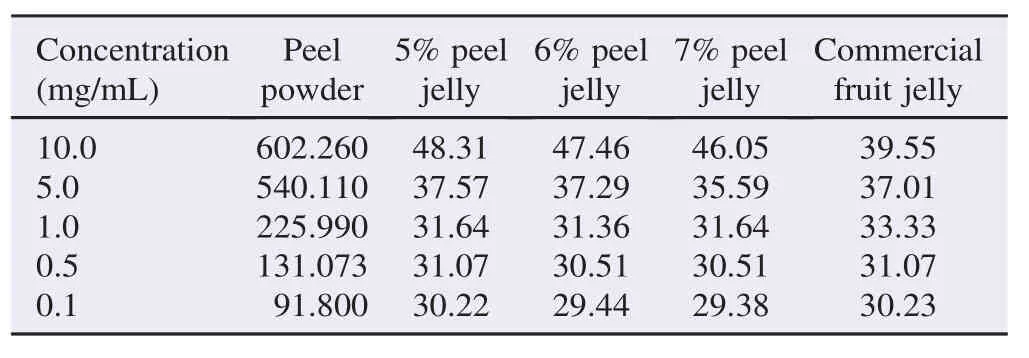
Table 2TPC at different concentrations. GAE mg/100 g of dry weight.
3.3. Antioxidant activity
The antioxidant activity was determined by using DPPH method, one of the most effective methods for evaluating radical-scavengers[12].
The scavenging activity of the methanolic extracts of peel powder and peel jelly and IC50value were showed in Tables 3 and 4 respectively. At the concentration of 0.1 mg/mL, the peel powder with IC50value of 1.56 mg/mL showed 20% scavenging activity, while at the highest concentration of 10 mg/mL, it showed the greatest scavenging activity reached at 89%. The 5%–7% peel jelly showed IC50values from 3.11 to 3.95 mg/mL, and the scavenging activity at the lowest concentration ranged from 7% to 11%. Otherwise, the 5%–7% peel jelly at 10 mg/mL increased scavenging activity from 74% to 84%. That remarkable scavenging activity was quite high as compared with commercial jelly with IC50of 4.28 mg/mL and scavenging activity of 36% which was almost half of scavenging activity of 7% peel jelly. In addition, at the same concentrations, the scavenging activities of 5% and 6% peel jelly were also slightly higher than that of commercial jelly.
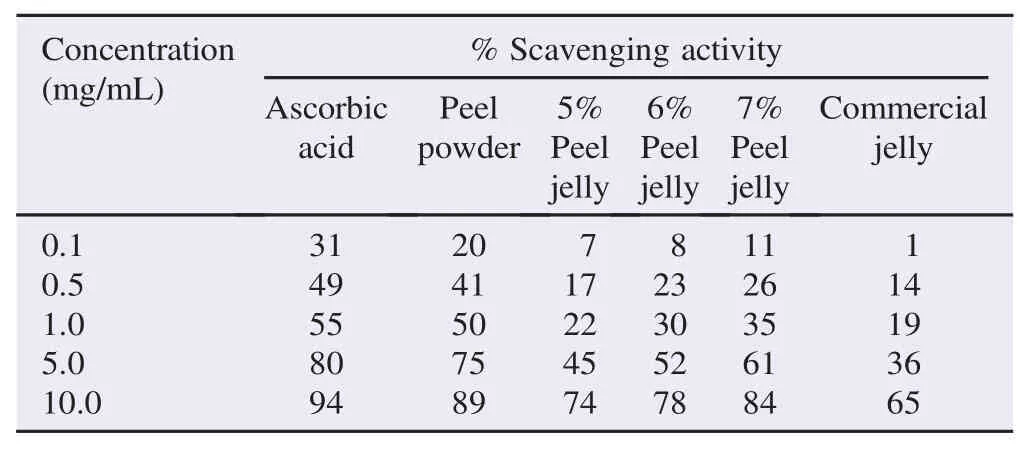
Table 3Percentage of scavenging activity at different concentrations.
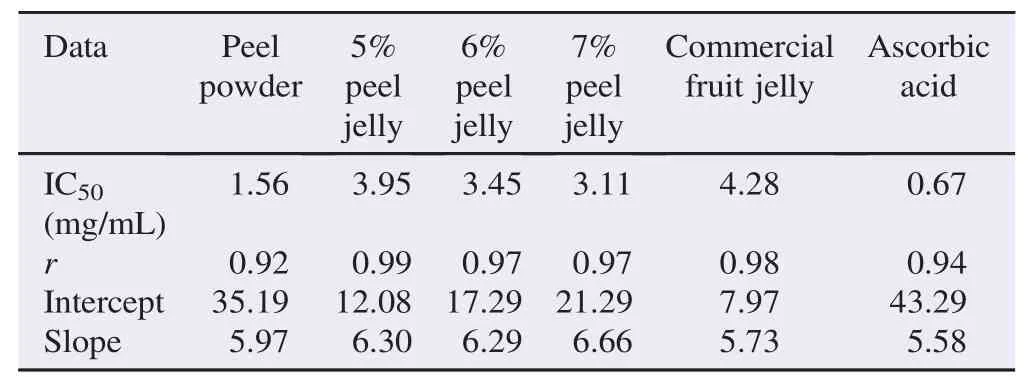
Table 4IC50values of banana peel powder and jellies.
3.4. Correlation between TPC and antioxidant activity
Figure 2 showed the relationship between TPC and antioxidant activity of peel powders and peel jelly. Only 7% peel jelly was chosen for this comparing because it showed the highest scavenging activity. The correlation of DPPH IC50and TPC was quite reasonably high for peel powder with R2= 0.934 0, while for 7% peel jelly R2value was 0.899 3. The strong relationship between TPC and scavenging activity might be due to the combined effect of various phenolic compounds and their high hydrogen atom donating abilities [13].
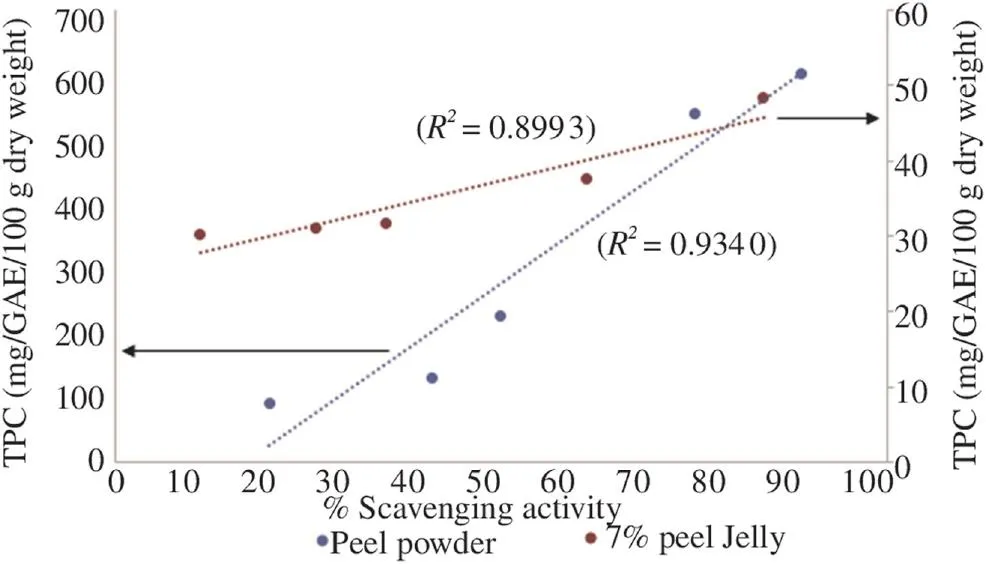
Figure 2. Linear correlation between DPPH IC50and TPC of peel powder and 7% peel jelly.
The results in this analysis revealed that sample studied were excellent free radical inhibitors or scavenger by acting as antioxidants.
4. Discussion
4.1. Response optimization
The jelly formulation from pectin substances is a well-known source since pectin has been long unambiguously established as a genuine gelling agent in diverse food formulations, notably, preserves, jellies, and marmalades. The presented formulation of peel jellies were developed by using the optimization plots with fulfilling the jelly ingredients specification such as amount of sugar and citric acid. Other than that, this optimized condition can act as a guidance to continue to change formulation ingredients until fit the needs for jelly properties.
4.2. TPC
In the study of Lee et al., they reported the TPC of banana peel powder as 929 mg GAE/100 g, which was higher than those detected in apple, plum and pear peels [10]. The TPC value of M. acuminata cv Berangan peel powder in the present study was slightly lower than that in the study of Lee et al., which differs about 327 mg GAE/100 g [10]. Even so, in their study, the banana varieties were not mentioned. However, Jain et al. reported that the highest TPC of local seeded banana peels in ethyl acetate extract was 1.26–19.46 mg GAE/g extract [11]. Meanwhile, Shian et al. and Sulaiman et al. also reported the TPC of a variety of bananas similar to the banana variety in this study (around 58.6–767.3 mg GAE/100 g and 13–263 mg GAE/100 g respectively) [14,15]. In the present study, the developed peel jelly was made from 5% to 7% of extracts which were extracted from 2 to 3 g of peel powder on a dry basis. The 5% peel jelly revealed a higher TPC reaching at 48.31 mg GAE/100 g compared to 6% and 7% peel jelly and commercial jelly containing seaweed extract which only showed TPC of 39.55 mg GAE/100 g. On the other side, the peel jelly contained a higher amount of phenolic than that reported by Lee et al. where the phenolic content in their peel jelly produced from 20 g of peel powder was 185.8 mg GAE/ 100 g. However, it actually contained 198 mg GAE/100 g of phenolics, which was 7% higher than the calculated value of Lee et al. [10]. Thus, the actual TPC in our peel jelly product is probably higher than recorded data.
Similar result was noted by Carvalho et al. in an increase of phenolic compounds from 21 to 102 mg GAE/100 g for sapota pulp jelly [16]. However, Poiana et al. [17] found a loss of polyphenolic compounds in low sugar fruit jams, and Carvalho et al. suggested that there was a difference in thermal stability among the fruits [16]. Therefore, the TPC in peel jelly may increase through the cooking process during production. In addition, higher phenolic content in previous studies was caused by many factors. For example, types of solvents used for extraction significantly affected the amount of phenolics [18], as the TPC increased in acetone: water extraction system and contributed to high antioxidant capacity of extracts. Other reasons contributing to increase in TPC amount include different combinations of time, temperature in extraction and UV spectrophotometer types used in absorbance analysis, andclimacteric factors such as origin, cultivar, harvest, storage time as well as drying and extraction method[9,15].
4.3. Antioxidant activity
The peel powder and peel jelly prominently showed a good DPPH scavenging activity reaching 89% inhibition. Baskar et al. indicates that the‘Mondhan’banana variety exhibits a good antioxidant activity with DPPH inhibition of 98.19% at 10 mg/ mL [19]. However, Lee et al. and Babbar et al. found that concentrations of 0.4 and 5.0 mg/mL showed the inhibition of 79%–83%, which was slightly lower than that of synthetic antioxidant, butylated hydroxytoluene[9,10].
Furthermore, the correlation between DPPH IC50value and TPC of peel powder and peel jelly was quite reasonably high, since they gave a high correlation coefficient as stated previously. This finding was supported by Maizura et al. who reported the correlation between DPPH and TPC (R2= 0.86) when herb and plant extract were used [20]. The strong relationship between TPC and free radical scavenging activity might be due to the combined effect of various phenolic compounds and their high hydrogen atom donating abilities [13]. Thus, both forms of banana peels showing the high scavenging activity with low IC50value possess a potential as optional antioxidant sources.
The utilization of M. acuminata cv Berangan peels for jelly formulation without adding any pectin was successful. The extracts with higher concentration contained higher phenolic content and scavenging activities, which reflect their potential as antioxidant sources. Therefore, the developed peel jellies exhibit a good potential that can be used for many applications as natural antioxidants, functional foods, and nutraceuticals subjected to proper investigations.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors would like to express their appreciation to the Research University Grant Scheme of Tier 1 with grant No. PY/ 2014/03649 provided by Universiti Teknologi Malaysia, and Universiti Malaysia Kelantan for providing research facilities.
References
[1] Padam BS, Tin HS, Chye FY, Abdullah MI. Banana by-products: an under-utilized renewable food biomass with great potential. J Food Sci Technol 2014; 51(12): 3527-45.
[2] Mohapatra D, Mishra S, Sutar N. Banana and its by-product utilisation: an overview. J Sci Ind Res 2010; 69: 323-9.
[3] Mohapatra D, Mishra S, Singh CB, Jayas DS. Post-harvest processing of banana: opportunities and challenges. Food Bioprocess Technol 2011; 4(3): 327-39.
[4] Passo Tsamo CV, Herent MF, Tomekpe K, Happi Emaga T, Quetin-Leclercq J, Rogez H, et al. Phenolic profiling in the pulp and peel of nine plantain cultivars (Musa sp.). Food Chem 2015; 167: 197-204.
[5] Ramli S, Alkarkhi AFM, Yong YS, Easa AM. Physicochemical properties of banana flour as influenced by variety and stage of ripeness: multivariate statistical analysis. J Agric Sci Technol 2010; 4(1): 69-78.
[6] Gonz′alez-Montelongo R, Lobo MG, Gnz′alez M. Antioxidant activity in banana peel extracts: testing extraction conditions and related bioactive compounds. Food Chem 2010; 119(3): 1030-9.
[7] Borges SV, Valente WA, Figueiredo LP, Dias MV, Pereira PP, Pereira AG, et al. Quality evaluation of banana skin extract jellies. Food Sci Technol Int 2011; 17(2): 177-83.
[8] Alkarkhi AFM, Ramli S, Yong YS, Easa AM. Comparing physicochemical properties of banana pulp and peel flours prepared from green and ripe fruits. Food Chem 2011; 129(2): 312-8.
[9] Babbar N, Oberoi HS, Uppal DS, Patil RT. Total phenolic content and antioxidant capacity of extracts obtained from six important fruit residues. Food Res Int 2011; 44(1): 391-6.
[10] Lee EH, Yeom HJ, Ha MS, Bae DH. Development of banana peel jelly and its antioxidant and textural properties. Food Sci Biotechnol 2010; 19(2): 449-55.
[11] Jain P, Bhuiyan MH, Hossain KR, Bachar SC. Antibacterial and antioxidant activities of local seeded banana fruits. Afr J Pharm Pharmacol 2011; 5(11): 1398-403.
[12] Nagarajaiah SB, Prakash J. Chemical composition and antioxidant potential of peels from three varieties of banana. Asian J Food Ago-Ind 2011; 4(1): 31-46.
[13] Yan SW, Asmah R. Comparison of total phenolic contents and antioxidant activities of tumeric leaf, pandan leaf and torch ginger flower. Int Food Res J 2010; 17: 417-23.
[14] Shian TE, Abdullah A, Musa KH, Maskat MY, Abd Ghani M. Antioxidant properties of three banana cultivars (Musa acuminata ‘Berangan’,‘Mas’and‘Raja’) extracts. Sains Malays 2012; 41(3): 319-24.
[15] Sulaiman SF, Md Yusoff NA, Eldeen IM, Seow EM, Sajak AAB, Supriatno, et al. Correlation between total phenolic and mineral contents with antioxidant activity of eight Malaysian banana (Musa sp.). J Food Compost Anal 2011; 24(1): 1-10.
[16] Carvalho VS, Damiani C, Asquieri ER, Orsi DC, Nishi ACF. Development and antioxidant capacity of sapota pulp jelly (Quararibea cordata VISCHER). Cienc Agrotecnol 2012; 36(3): 341-7.
[17] Poiana MA, Alexa E, Mateescu C. Tracking antioxidant properties and color changes in low-sugar bilberry jam as effect of processing, storage and pectin concentration. Chem Cent J 2012; 6: 4.
[18] Gonz′alez M, Gonz′alez V. Sample preparation of tropical and subtropical fruit biowastes to determine antioxidant properties. Anal Methods 2010; 2: 1842-66.
[19] Baskar R, Shrisakthi S, Sathyapriya B, Shyampriya R, Nithya R, Poongodi P. Antioxidant potential of peel extracts of banana varieties (Musa sapientum). Food Nutr Sci 2011; 2: 1128-33.
[20] Maizura M, Aminah A, Wan Aida WM. Total phenolic content and antioxidant activity of kesum (Polygonum minus), ginger (Zingiber officinale) and turmeric (Curcuma longa) extract. Int Food Res J 2011; 18: 529-34.
Original article http://dx.doi.org/10.1016/j.apjtb.2015.09.022
*Corresponding author:Mariam Firdhaus Mad Nordin, Malaysia-Japan International Institute of Technology, Universiti Teknologi Malaysia Kuala Lumpur, Jalan Semarak, 54100 Kuala Lumpur, Malaysia.
 Asian Pacific Journal of Tropical Biomedicine2016年1期
Asian Pacific Journal of Tropical Biomedicine2016年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- The antibacterial activity of selected plants towards resistant bacteria isolated from clinical specimens
- Evaluation of antibacterial activity and synergistic effect between antibiotic and the essential oils of some medicinal plants
- Comparative studies of elemental composition in leaves and flowers of Catharanthus roseus growing in Bangladesh
- Prevalence of multi-drug resistant uropathogenic Escherichia coli in Potohar region of Pakistan
- Antibiotic resistance profile and RAPD analysis of Campylobacter jejuni isolated from vegetables farms and retail markets
- Urban air pollution, climate and its impact on asthma morbidity
